Lisinopril in the treatment of arterial hypertension in patients with pathology of the digestive system
The prevalence of arterial hypertension (AH) in Russia reaches 40% in men and 50% in women. In 83.3% of patients, hypertension is combined with diseases of the digestive system, including 30% with liver pathology.
To correct blood pressure (BP) in patients with hypertension, antihypertensive drugs of various pharmacological groups are used, among which the most frequently prescribed drugs are angiotensin-converting enzyme (ACE) inhibitors [7].
Over the past 30 years, ACE inhibitors have been called the “cornerstone of the treatment of cardiovascular diseases” (E. Braunwald, 1991) and the “gold standard of therapy” (J. Cohn, 1998) [10].
The results of numerous international studies have shown that these drugs are the most effective, reducing mortality from cardiovascular diseases, having organoprotective effects, and therefore are recommended as first-line antihypertensive drugs for long-term treatment of patients with hypertension.
Currently, the best known ten ACE inhibitors are captopril, enalapril, benazepril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril and trandolapril. Five of them (captopril, enalapril, lisinopril, ramipril, trandolapril) have been shown to reduce mortality in large studies [21, 23, 24, 28–32].
The Scandinavian study (STOP-2) compared the effectiveness of ACE inhibitors (lisinopril or enalapril 10 mg per day) with other antihypertensive drugs (beta-adrenergic blockers, hydrochlorothiazide in combination with amiloride or felodipine) in the treatment of 6614 patients with hypertension for 54 months [ 22].
This study showed that ACE inhibitors significantly reduced the risk of heart failure.
The ALLHAT study included 33,357 hypertensive patients followed for an average of 4.9 years [1, 17]. The first group of patients was prescribed chlorthalidone (up to 25 mg per day), the second - amlodipine (up to 10 mg per day), the third - lisinopril (up to 40 mg per day). During therapy with lisinopril, stroke (“end point”) occurred less frequently than when using a diuretic.
The TPOPHY study compared the effectiveness of monotherapy with hydrochlorothiazide and lisinopril in overweight hypertensive patients. Monotherapy with an ACE inhibitor ensured blood pressure control in 60%, and monotherapy with a diuretic in 43% of patients. In the group receiving lisinopril, in more than half, a dose of 10 mg/day was sufficient, and only one in four needed to be prescribed 40 mg/day. To achieve the target blood pressure level during diuretic therapy, almost every second person required the prescription of 50 mg/day of hydrochlorothiazide, which is associated with the likelihood of life-threatening arrhythmias.
In the GISSI-3 study, patients receiving lisinopril had a significantly reduced risk of death and cardiovascular disease [9].
Therapy with ACE inhibitors in patients with hypertension and diabetes mellitus significantly reduces the risk of target organ damage. In the EUCLID study in 530 patients with type 1 diabetes mellitus, lisinopril had a nephroprotective effect and reduced the risk of progression of retinopathy.
The multicenter, randomized, double-blind ATLAS trial showed that treatment with high doses (33.2 mg per day) of lisinopril was associated with a significant reduction in the risk of death or hospitalization by 12% [27].
The antihypertensive effect of ACE inhibitors is associated with:
- inhibition of the renin-aldosterone-angiotensin system in tissues and the vascular wall;
- inhibition of the conversion of inactive angiotensin I into the active vasoconstrictor angiotensin II and a decrease in aldosterone secretion;
- increased plasma renin activity;
- accumulation of bradykinin due to inhibition of kininase II;
- dilatation of renal vessels with increased natriuresis;
- increased synthesis of prostaglandins PGI2 and PGE2 [18, 20].
The release of PGI2 and PGE2 has vasodilatory, diuretic and natriuretic effects. Treatment with ACE inhibitors also reduces the formation of other vasoconstrictor and antinatriuretic substances, such as norepinephrine, arginine vasopressin, and endothelin-1 [12].
Hemodynamic effects when using ACE inhibitors are manifested:
- a decrease in total vascular resistance due to an indirect vasodilating effect, which leads to a decrease in blood pressure by 15–25%;
- relaxation of volume vessels with a decrease in filling pressure of the left ventricle;
- increased minute blood volume;
- an increase in renal blood flow due to dilatation of efferent arterioles in the glomeruli [11].
Classification of ACE inhibitors. Despite the common mechanism of action, ACE inhibitors differ in chemical structure, the presence of additional functional groups in the molecule, the nature of the prodrug, activity and pharmacokinetic profile, which is very important to consider when treating patients with various pathologies of the digestive organs [3].
The most popular chemical classification, according to which drugs are divided into four main classes depending on which chemical group in their molecule binds to the zinc ion in the active centers of the angiotensin I-converting enzyme:
- preparations containing a sulfhydryl group;
- drugs containing a carboxyl group;
- preparations containing a phosphinyl group;
- drugs containing the hydroxamic group [15].
Analysis of literature data shows that according to the duration of the antihypertensive effect, ACE inhibitors can be divided into two groups:
- medium duration - captopril;
- long-acting - enalapril, lisinopril, quinapril, which in most cases provide round-the-clock control over blood pressure levels when taken once a day.
Taking into account data on physicochemical properties and pharmacokinetic characteristics, ACE inhibitors are divided into three classes:
1. Lipophilic ACE inhibitors (captopril), which themselves have pharmacological activity, but undergo further transformations in the liver to form pharmacologically active disulfides, which are eliminated by renal excretion.
2. Lipophilic prodrugs (pharmacologically inactive) become active diacid metabolites after metabolic transformation in the liver (enalapril to enalaprilat), which are then transformed into inactive compounds [13]. In patients with liver pathology, both of these processes are impaired, and with a decrease in blood flow in the liver, there is a delay in the conversion of the prodrug to its active form during the first passage through it.
Accordingly, in liver diseases, drugs that require transformation to acquire activity have a weaker effect [6, 14].
Prodrugs are more lipophilic than their pharmacologically active metabolites, which allows them to ensure rapid and complete absorption when taken orally.
ACE inhibitors of this class should be divided into three subgroups depending on the predominant route of elimination of their active diacid metabolites:
- subclass A - drugs with predominantly renal elimination;
- subclass B - drugs with two main elimination routes;
- subclass C - drugs with predominantly hepatic elimination.
3. Hydrophilic drugs (lisinopril), which are not metabolized in the patient’s body. They circulate in the blood in a form unbound to plasma proteins and are eliminated unchanged through the kidneys. Their concentration in the blood plasma is determined by the dose taken orally, as well as the rate of absorption and the rate of excretion through the kidneys [16, 19, 26].
Only four ACE inhibitors (captopril, libenzapril, lisinopril and ceronapril) directly have biological activity. All other ACE inhibitors themselves are inactive substances or pro-drugs, i.e., they exhibit their effect after biotransformation in the liver and the formation of active metabolites [25].
The degree of blocking of the tissue renin-angiotensin system by various ACE inhibitors varies. Drugs that are characterized by less lipophilicity cause less accumulation of bradykinin in tissues, which is associated with the appearance of a side effect - dry cough.
Lisinopril is a well-studied ACE inhibitor, the benefit of which has been proven in the treatment of patients with hypertension.
Lisinopril is an active pharmacological compound.
Lisinopril was the third ACE inhibitor (after captopril and enalapril) of the drugs in this group that entered clinical practice.
Lisinopril has a prolonged antihypertensive effect. The onset of the antihypertensive effect is 1–3 hours after oral administration, the peak of action is after 6 hours, the duration of action is 24 hours with a stable effect after 2–4 weeks of treatment [4, 5].
The antihypertensive effect lasts more than a day. In case of abrupt cessation of therapy with lisinopril, there is no sudden increase in blood pressure, as well as a significant increase in blood pressure values before the start of treatment.
Lisinopril causes dilatation of arterioles and veins, which leads to a decrease in blood pressure by approximately 15% due to a decrease in total peripheral vascular resistance. Lisinopril does not cause reflex tachycardia due to stimulation of the vagus nerve and a decrease in the sensitivity of carotid sinus baroreceptors due to improved compliance and dilatation of the carotid artery.
Pharmacokinetics
After oral administration, the bioavailability of lisinopril is 25–29%. The functional state of the liver does not affect bioavailability. Eating does not change the absorption of the drug from the gastrointestinal tract. In the human body it is not metabolized and is excreted unchanged in the urine. In blood plasma, lisinopril does not bind to proteins. The half-life is 12.6 hours. The drug undergoes glomerular filtration, is secreted and reabsorbed in the tubules. The maximum concentration is achieved 6 hours after taking a single dose, and the steady-state level of concentration with regular use is achieved after 2-3 days.
For hypertension, the initial dose is 10 mg/day for a single dose, followed by a possible gradual increase to 40 mg/day.
Thus, when treating patients with hypertension with pathology of the digestive organs, the doctor has the opportunity to choose a drug from various classes of ACE inhibitors, depending on their pharmacokinetic characteristics.
In our work, we assessed the effectiveness of an ACE inhibitor (lisinopril) in the treatment of patients with hypertension with various pathologies of the digestive organs.
Purpose of the study
To evaluate the pharmacodynamics of lisinopril in various diseases of the digestive system in patients with hypertension.
Materials and research methods
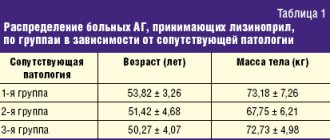
The study included 60 patients with hypertension in combination with steatosis (group 1), liver cirrhosis (group 2), duodenal ulcer (group 3), 20 people in each group, respectively.
Titration of lisinopril dosages was carried out weekly under the control of 24-hour blood pressure monitoring (ABPM). Based on complaints, medical history and examination (blood tests, esophagogastroduodenoscopy, ultrasound examination of the abdominal organs), the presence of pathology in the liver and upper digestive tract was established. Patients with duodenal ulcer with normal liver function constituted the comparison group (Table 1).
To assess the effectiveness of lisinopril, ABPM was carried out using the ABRM-02 monitor using the oscillometric method of measuring blood pressure in a free motor mode. Registration was carried out on the “non-working” arm in the absence of blood pressure asymmetry. If blood pressure asymmetry is more than 5 mm Hg. Art. the study was conducted on the arm with higher scores. Blood pressure measurements were carried out for 24 hours every 15 minutes from 6.00 to 22.00 hours and every 30 minutes from 22.00 to 6.00 hours.
In order to clarify the daily blood pressure profile and assess the hypotensive effect of lisinopril, average blood pressure values were determined based on ABPM data. Normally, during the daytime, blood pressure should not exceed 140 and 90 mmHg. Art., at night - 120 and 80 mm Hg. Art. As an indicator of pressure load, we assessed the time index (TI) - the percentage of time during which blood pressure exceeds the critical level for certain time periods (in accordance with the recommendations of the American Society of Hypertension, a VI of more than 30% indicates the presence of elevated blood pressure) [3].
Statistica 5.0 program was used for statistical data processing. For each indicator, the mean value and standard deviation from the mean value were calculated. The statistical significance of changes in indicators was determined using Fisher's test. Differences were considered statistically significant at p < 0.05.
Results and its discussion
According to ABPM data, a persistent increase in blood pressure was initially detected in all examined patients; no significant differences were found between the groups.
The effectiveness of therapy was assessed after 1, 2, 3 and 4 weeks according to the VI ABPM level: good - with VI less than 30%, unsatisfactory - with VI more than 30%.
If lisinopril was insufficiently effective, the dose was gradually increased to 20 mg (Tables 2, 3).
As can be seen from table. 2 and 3, during therapy with lisinopril at a dose of 10 mg/day, a decrease in average daily blood pressure and blood pressure values was noted in all three groups. When treated with lisinopril, a good antihypertensive effect was obtained in 50% of patients with liver cirrhosis. In 60% of patients with duodenal ulcer, a good effect was achieved when taking 10 mg/day, in 30% of patients - 20 mg/day.
Insufficient effectiveness of lisinopril therapy was observed in 15% of cases in patients with liver cirrhosis and in 10% in patients with duodenal ulcer, even when the dose of the drug was doubled.
Conclusion
The results of this study indicate that the effectiveness of lisinopril monotherapy did not depend on the severity of liver changes in patients with hypertension.
In this regard, the use of antihypertensive drugs that are not metabolized in the liver, which can provide adequate blood pressure control for 24 hours in hypertensive patients with gastrointestinal pathology, becomes especially relevant [2, 8].
Thus, lisinopril is a highly effective antihypertensive drug in the treatment of patients with various pathologies of the digestive system.
Literature
- Belenkov Yu. N., Mareev V. Yu., Ageev F. T. Angiotensin-converting enzyme inhibitors in the treatment of cardiovascular diseases (quinapril and endothelial dysfunction). M., 2001. 86 p.
- Drapkina O. M., Mayevskaya M. V., Korneeva O. N., Tutnov D. A., Ivashkin V. T. Clinical study of the effectiveness and safety of dapril (lisinopril) in liver pathology and concomitant arterial hypertension // Russian Medical News . 2004, No. 2, p. 39–42.
- Kobalava Zh. D. Kotovskaya Yu. V., Khirmanov V. N. Blood pressure in research practice. Ed. V. S. Moiseeva, R. S. Karpova. M.: Reafarm, 2004. 384 p.
- Komissarenko I. A., Lazebnik L. B., Mikheeva O. M. Features of the metabolism of antihypertensive drugs in patients with pathology of the digestive organs // Cardiovascular therapy and prevention. Appendix 1. 2009. 8 (6). P. 239.
- Komissarenko I. A., Mikheeva O. M., Drozdov V. N., Petrakov A. V., Silvestrova S. Yu. The use of angiotensin-converting enzyme inhibitors in patients with arterial hypertension against the background of liver pathology // Consilium medicum. 2007. T. 9. No. 11. P. 72–75.
- Kushakovsky M. S. Hypertension. St. Petersburg: Sotis, 1995. pp. 243–253.
- Lazebnik L. B., Drozdov V. N. Diseases of the digestive organs in the elderly. Anacharsis, 2003, pp. 37–39.
- Lazebnik L. B., Mikheeva O. M., Komissarenko I. A., Drozdov V. N., Petrakov A. V., Silvestrova S. Yu. Features of treatment of patients with hypertension with ACE inhibitors for pathology of the digestive organs // Experimental and Clinical gastroenterology, 2007. No. 4. pp. 47–55.
- Mazur N. A. Efficacy of non-lipophilic angiotensin-converting enzyme inhibitors in the treatment of cardiovascular diseases // Russian Journal of Cardiology. 2003. No. 4 (42). pp. 76–79.
- Mareev V. Yu. Application of ACE inhibitors in the treatment of cardiovascular diseases in the 21st century. Why is it beneficial to choose fosinopril? Heart diseases. Guide for doctors. Ed. R. G. Oganova, I. G. Fomina. M.: Litterra, 2006. pp. 3–8.
- Metelitsa V.I. Handbook of clinical pharmacology of cardiovascular drugs. St. Petersburg - M.: Binom. 2nd ed. 2002. 925 p.
- Preobrazhensky D.V., Sidorenko B.A. Treatment of arterial hypertension. M., 1999. pp. 126–136.
- Savenkov M.P., Ivanov S.N., Botsoeva M.A., Mikhailusova M.P. Correction of high blood pressure in the morning with the help of ACE inhibitors // Gedeon Richter in the CIS. 2001. No. 4 (8). pp. 27–30.
- Savenkov M.P., Ivanov S.N., Solomonova L.A., Savenkova A.M. Morning begins with dawn and increased blood pressure // Russian Medical Journal. 2006. T. 14, No. 10. P. 734–736.
- Storozhakov G.I. ACE inhibitors: place in the treatment and prevention of cardiovascular diseases // Consilium medicum. Extra edition. 2002, January, p. 3–4.
- Tkhostova E. B. Clinical effectiveness of lisinopril in patients with cardiovascular diseases // Gedeon Richter in the CIS. 2001. No. 4 (8). pp. 23–25.
- ALLHAT Authors. Major Outcomes in High-Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme inhibitor or Calcium channel blocker VS Diuretic. JAMA, December 18, 2002, 288, 23, 2981–2996.
- Campbell DJ, Kladis A., Duncan AM Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides // Hypertens. 1994, 23: 439–449.
- Choodoff L. Lisinopril: a new ACE inhibitor for the treatment of hypertension and congestive heart failure // Mt. Sinai. J. Med. 1990. Vol. 57. P. 169–171.
- Furberg CD, Pitt B. Are all angiotensin-converting enzyme inhibitors interchangeable? // Am Coll Cardiol. 2001, 37, 1456–1460.
- Gruppo Italiano per to Studio della Sopravvivenza nell'Infarto Miocardico (GISSI-3). Effects of lisinopril and transdermal glyceryl trinitrate singly and together on six-week mortality and ventricular function after acute myocardial infarction // Lancet. 1994; 343:1115–1122.
- Hansson L., Lindholm LH, Ekbom T. et al. Randomized trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 Study // Lancet. 1999, 354, 1751–1756.
- ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group (ISIS-4). A randomized factorial trial assessing early oral captopril, oral mononitrate and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction // Lancet. 1995; 345:669–685.
- Kober L. et al. for the Trandolapril Cardiac Evaluation (TRACE) Study Group // N Engl J Med. 1995: 333: 1670–1676.
- Lancaster SG, Todd PA Lisinopril: a preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in hypertension and congestive heart failure // Drugs. 1988, 35: 646–669.
- Opie HL Angiotensin-converting enzyme inhibitors. Wiley-Liss-Authors Publishing. New York, 1992.
- Packer M., Poole-Wilson PA, Armstrong PW et al. Comparative effects of low and high doses of angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group // Circulation. 1999; 100(23):2312–2318.
- Pfeffer MA et al. On behalf of the SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival And Ventricular Enlargement trial // N Engl J Med. 1992; 327:669–677.
- The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure // Lancet. 1993; 342:821–828.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) // N Engl J Med/1987; 316:1429–1435.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients // N Engl J Med. 2000; 342:145–153.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart future // N Engl J Med. 1991; 325; 293–302.
L. B. Lazebnik, Doctor of Medical Sciences, Professor O. M. Mikheeva, Doctor of Medical Sciences, Professor I. A. Komissarenko, Doctor of Medical Sciences, Professor
State Budgetary Educational Institution of Higher Professional Education MGMSU Ministry of Health and Social Development of Russia, Central Research Institute of Gastroenterology of the City Health Department, Moscow
Contact information for authors for correspondence
Lisinopril: a universal drug in the arsenal of a cardiologist
It became possible to effectively influence the cardiovascular continuum with the introduction of diuretics and selective beta-blockers into clinical practice. The next stage was the emergence of angiotensin-converting enzyme inhibitors (ACEIs) and calcium antagonists (CAs), which contributed to further advances in the treatment of CVD. With good reason, the last quarter of the twentieth century can be called the “era of ACE inhibitors.” Today, five main classes of antihypertensive drugs - thiazide diuretics, ACE inhibitors, angiotensin receptor blockers (ARBs) and β-blockers (BABs) - are suitable for initiation and maintenance of antihypertensive treatment in monotherapy or in combination [2 ,3]. When choosing one or another antihypertensive drug, it is necessary to remember that it must not only adequately reduce blood pressure to the target level, control it throughout the day, improving the blood pressure profile, but also meet a number of other requirements: the drug must reduce the reabsorption of Na+ and water, and not increase dysfunction endothelium, do not activate the sympathetic nervous system, have organoprotective properties, and be metabolically neutral. These requirements are fully met by ACE inhibitors, of which there are today more than 30 original drugs and their generics. Their pharmacological action is due to their influence on the functional state of the renin–angiotensin–aldosterone system (RAAS). ACE inhibitors have a highly selective action: they suppress the conversion of angiotensin I to angiotensin II without directly interacting with other components of the RAAS. Summarizing the data on the properties and mechanism of action of ACE inhibitors, we can dwell on the main protective effects of this group of drugs: 1. Cardioprotective effects (restoring the balance between the need and supply of myocardium O2, reducing pre- and afterload of the left ventricle (LV), reducing volumes and weight, slowing LV remodeling, decreased sympathetic stimulation, antiarrhythmic effect). 2. Vasoprotective effects (potentially direct antiatherogenic effect, antiproliferative and antimigration effect on smooth muscle cells, monocytes, neutrophils; improved endothelial function, antiplatelet effect, increased endogenous fibrinolysis, improved arterial compliance and tone). Among the many representatives of the ACEI class, lisinopril deserves special attention. Lisinopril (Diroton®, produced by the pharmaceutical company) is an ACE inhibitor with an extremely wide spectrum of action and original properties, which allows it to be used in a wide variety of situations. In addition, a large evidence base has been accumulated on the effectiveness and safety of lisinopril based on the results of a number of clinical studies. Lzinopril (Diroton) is the only hydrophilic ACEI, practically does not bind to plasma proteins and is not distributed in adipose tissue. The chemical structure of lisinopril contains a carboxyl group, which binds the zinc-containing domain of ACE. Unlike most ACE inhibitors, lisinopril is not a prodrug. Absorbed into the gastrointestinal tract, it does not undergo further metabolic transformations and is excreted unchanged by the kidneys. Lisinopril clearance correlates with creatinine clearance, so as creatinine clearance decreases, lisinopril excretion also decreases. In patients with renal failure, the elimination of the drug is slowed down, and therefore dose adjustment is required. The drug has fairly variable bioavailability - from 26 to 60%. Food intake does not affect the bioavailability of the drug. Its action begins 1 hour after oral administration, the peak effect develops after 4–6 hours, and the duration of action reaches 24 hours, which provides a convenient administration regimen - once a day [4]. The severity of the inhibitory effect of lisinopril on ACE activity was studied in vitro on rabbit lungs. The affinity constant of ACE for lisinopril was comparable to that of enalaprilat and captopril, but the dissociation half-life of the drugs was 105, 27 and 9 minutes, respectively. These data indicate a greater affinity of lisinopril for ACE [5]. Unlike most other ACE inhibitors, lisinopril does not contain a sulfhydryl group, which is the cause of a number of side effects (neutropenia and proteinuria) [6]. Lisinopril is used to treat patients with hypertension, congestive heart failure, and after acute myocardial infarction (AMI) [7]. Moreover, it was recently approved in a number of countries (Great Britain, Spain, Belgium) for the treatment of diabetic nephropathy, and in Mexico, Portugal and New Zealand for the treatment of patients with diabetic retinopathy [8]. Efficacy of lisinopril in the treatment of hypertension In 2002, the results of the ALLHAT (Antihypertensive and Lipid–Lowering treatment to prevent Heart Attack Trial) study were published [9], which assessed mortality from coronary artery disease and the incidence of myocardial infarction in elderly patients. In the ALLHAT study, 15,255 patients received chlorthalidone at a dose of 12.5–25 mg, 9,048 patients received amlodipine at a dose of 2.5–10 mg, and 9,054 patients received lisinopril at a dose of 10–40 mg per day. If the target blood pressure level could not be achieved, then at the next stage a second drug was added (atenolol - 25-100 mg, reserpine - 0.05-0.2 mg once a day or clonidine - 0.1-0.3 mg twice a day day). If there was no effect, hydralazine was added at the third stage - 25-100 mg twice a day. None of the three drugs was shown to be superior in their ability to prevent the primary composite endpoint of myocardial infarction and cardiovascular mortality. The analysis of overall mortality also did not reveal any benefits of any drug. Lisinopril was slightly inferior to chlorthalidone in its ability to prevent strokes, hospitalization for angina, and worsening heart failure. However, lisinopril was significantly superior to amlodipine in preventing decompensated heart failure in whites; in black patients, the effectiveness of lisinopril and amlodipine did not differ significantly. A comparison of the effectiveness of two ACEIs, enalapril and lisinopril, was carried out using blood pressure monitoring to control the effectiveness of therapy. The target BP was set to 140/90 mm Hg, and the dose of both drugs was titrated to achieve this BP level. Hydrochlorothiazide was added if necessary. Both drugs significantly reduced blood pressure, but the effect of lisinopril was more pronounced. The average doses of drugs at the end of the study were 18 mg enalapril and 8 mg hydrochlorothiazide in one group, and 17 mg lisinopril and 6 mg hydrochlorothiazide in the second group. With the same administration regimen (once daily), lisinopril has a longer duration of action. The safety of the drugs was comparable [10]. A relatively small study (65 patients with DBP 95–115 mm Hg) compared the effectiveness and tolerability of lisinopril and the b-blocker nebivolol. Lisinopril was prescribed at a dose of 20 mg once daily, nebivolol - 5 mg once daily. Both drugs caused a significant decrease in blood pressure and were well tolerated by patients [11]. A Norwegian multicenter study examined the antihypertensive efficacy, tolerability, and impact of lisinopril (mean dose 18.8 mg) and nifedipine (mean dose 37.4 mg) on quality of life in 828 patients with mild to moderate hypertension. Lisinopril was more effective in lowering blood pressure and was better tolerated by patients. Both drugs had an equally good effect on the quality of life of patients [12]. A study comparing the effectiveness of lisinopril (20 mg) and the ARB telmisartan (80 mg) included 32 previously untreated patients with hypertension. The effectiveness of the drugs was the same both according to routine office blood pressure measurements and according to 24-hour blood pressure monitoring [13]. Lisinopril demonstrated comparable efficacy to the ARB valsartan. The large randomized trial PREVAIL (Ehe Blood Pressure Reduction and Tolerability of Valsartan in Comparison with Lisinopril study) included 1213 patients with grade 1–3 hypertension (SBP 160–220 mmHg and DBP 95–110 mmHg. ). Patients were randomized to receive valsartan 160 mg or lisinopril 20 mg per day. After four weeks, if there was insufficient effectiveness, hydrochlorothiazide was added to therapy. The total duration of treatment was 16 weeks. 1100 patients completed the full course of treatment; 51 patients in the valsartan group and 62 patients in the lisinopril group discontinued treatment due to side effects of therapy. The decrease in blood pressure was identical in both treatment groups – 31.2/15.9 mmHg. and 31.4/15.9 mmHg. respectively [14]. Lisinopril for obesity Two large studies - the NHS (non-smoking women) and the Seventh–Day Adventist Study (non-smoking, non-alcoholic vegetarian men) revealed a direct correlation between body mass index and cardiovascular mortality [15]. According to the Framingham study, every 4.5 kg of weight increases systolic blood pressure by 4.4 mmHg. in men and by 4.2 mm Hg. among women. The multicenter, double-blind, randomized, placebo-controlled study TROPHY conducted a comparative study of the effectiveness of 12-week treatment of 232 obese and hypertensive patients with lisinopril and hydrochlorothiazide (HCT). ABPM data showed that lisinopril and hydrochlorothiazide effectively reduced blood pressure throughout the day compared to placebo (p < 0.001). However, a decrease in DBP below 90 mm Hg. noted in 60% of patients treated with lisinopril and only in 43% of patients treated with hydrochlorothiazide (p<0.05). It is important that the majority of patients (57%) taking lisinopril remained on a dose of 10 mg throughout the treatment period, while the majority of patients receiving HCTZ (71%) required an increase in dose to 25–50 mg per day , which is associated with extremely adverse metabolic effects. Both drugs had no significant effect on insulin levels and lipid profiles, but plasma glucose levels at 12 weeks differed significantly (p<0.001) in the lisinopril (–0.21 mmol/L) and hydrochlorothiazide (+0.31 mmol/L) groups [16 ]. It must be recalled that lisinopril is the only hydrophilic ACE inhibitor with a duration of action of 24–30 hours that is not distributed in adipose tissue. These properties allow us to consider it a drug of choice in the treatment of obese patients with hypertension. Lisinopril and nephroprotection The nephroprotective effect of lisinopril has been demonstrated at various stages of diabetic nephropathy, regardless of the presence of hypertension. The multicenter 2-year placebo-controlled trial EUCLID (Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria) examined the early administration of lisinopril on the progression of diabetic nephropathy and retinopathy in 530 patients with type 2 diabetes (T2DM). ) without hypertension with normoalbuminuria (85% of patients) and microalbuminuria (15%). The level of microalbuminuria (MAU) in the lisinopril group at the end of observation was 18.8% lower than in the placebo group. The maximum effect was found in patients who already had nephropathy at the beginning of the study: in patients with initial normoalbuminuria, the decrease in urinary albumin excretion compared to placebo was 12.7% (1.0 mcg/min.), while in patients with initial normoalbuminuria MAU – 49.7% (34.2 mcg/min.). Thus, the EUCLID study demonstrated the ability of ACE inhibitors to slow down both the development and progression of the initial stage of diabetic nephropathy. At the same time, the greatest nephroprotective properties were manifested precisely at the MAU stage. The EUCLID trial also assessed the effect of lisinopril therapy on the development and progression of diabetic retinopathy (DR) [17]. In the lisinopril group, a 50% reduction in the risk of progression of DR was detected (OR=0.5) compared with placebo, while the maximum protective effect of ACE inhibitors on the development and progression of DR (OR=0.34) was observed in patients with compensation of carbohydrate metabolism - with HbA1c level less than 7%. In one of the largest studies using lisinopril in patients with type 2 diabetes, which included 3463 patients with initial and severe diabetic nephropathy (DN) and hypertension, the administration of lisinopril even for a short period (3 months) showed not only the high antihypertensive effectiveness of the drug, but also an improvement in nitrogen excretion function of the kidneys - in almost 50% of patients with initially elevated creatinine levels, this indicator stabilized. Studies also noted a positive effect of lisinopril on indicators of metabolic control (levels of glycated hemoglobin and blood lipids) and good tolerability of therapy - side effects developed in only 2.2% of patients [18]. Lisinopril and cardioprotection The 2-year ELVERA study (Effects of amlodipine and lisinopril on Left Ventricular mass) examined the effect of lisinopril and amlodipine on myocardial mass and left ventricular diastolic function in elderly patients with hypertension who did not receive antihypertensive therapy. The study included 166 patients with hypertension (DBP 95–115 mm Hg and SBP 160–220 mm Hg) aged 60 to 75 years: 81 patients received amlodipine at a dose of 2–10 mg per day, 85 patients received lisinopril at a dose of 10–20 mg per day. Myocardial mass index (MMI) decreased by 25.7 g/m2 in the amlodipine group and by 27 g/m2 in the lisinopril group. The SAMPLE study [19] included 206 patients with hypertension and LVH. During therapy with lisinopril at a dose of 20 mg/day. in combination with HCTZ (12.5–25 mg/day) and without it, an adequate reduction in blood pressure and a decrease in LV MMI by 15.8% were observed. The effectiveness of early use of lisinopril in acute myocardial infarction (AMI) has also been proven. The results of the GISSI-3 study showed that if treatment with lisinopril begins on the first day of AMI with stable hemodynamics, there is a significant reduction in overall mortality. Nitrates did not improve these indicators. Mortality rates and combined endpoints by month 6 were significantly lower (p=0.03) in the group of patients treated with lisinopril [20]. The SMILE-2 (Survival of Myocardial Infarction) study directly compared two ACE inhibitors for AMI: zofenopril at a dose of 30–60 mg and lisinopril at a dose of 5–10 mg per day. Both drugs were prescribed to patients who received thrombolytic therapy for AMI. ACE inhibitor therapy began no later than 12 hours after completion of thrombolysis and lasted 42 days. A total of 1024 patients were included in the study. There were no significant differences in the risk of cardiovascular complications in both treatment groups [20]. Lisinopril for CHF The randomized ATLAS study compared the effectiveness and tolerability of long-term therapy with low (2.5–5 mg) and high (32.5–35 mg) doses of lisinopril in 3164 patients with class II–IV CHF and an ejection fraction of no more than 30% . During observation, in the group of patients receiving high doses of lisinopril, there was a decrease in mortality from all causes by 8% and mortality from cardiovascular causes by 10%. In addition, therapy with high doses of lisinopril led to a significant reduction in the need for hospitalization due to decompensated CHF (by 24%) [22]. In addition, the ATLAS study established an extremely favorable economic effect when using high doses of the drug - the cost of treatment was $2 billion/year lower [23]. One of the most worthy representatives of lisinopril on the Russian market, completely satisfying the price-quality ratio, is lisinopril - Diroton. In Volgograd, under the guidance of prof. S.V. Nedogoda [24] conducted a 6-month open randomized study comparing the clinical and pharmacoeconomic effectiveness of lisinopril - Diroton (5 mg N 28) and Lizoril (10 mg N 30) in 40 patients with hypertension. It was proven that Diroton reduced both SBP and DBP to a slightly greater extent than Lizoril (p>0.05), but at the same time, the cost-effectiveness ratio of Diroton was 1.6 times better than that of Lizoril. Thus, lisinopril (Diroton) is an extremely effective and economical antihypertensive drug with organoprotective properties, which is convenient for doctors to work with in a wide variety of clinical situations. References 1. MRFIT research group. Multiple Risk Factor intervention Trial. Risk factor changes and mortality results. JAMA 1982;248:1465–77 2. The Task Force for the management of arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension. J Hypertens 2007; 25:1105–1187. 3. VNOK. Prevention, diagnosis and treatment of arterial hypertension. Russian recommendations (second revision). Cardiovascular Therapy and Prevention 2004. Appendix 4. 4. Semple PF et al. Onset of action of captopril, enalapril, enalaprilic acid and lisinopril in normal man // Cardiovascular Drugs and Therapy. – 1987– Vol. 1. – P. 45–50. 5. Bull HG Inhibition of rabbit lung ACE by lisinopril, enalapril and captopril. // J. of Biological Chem. –1985–Vol.260 – P.2952–2962. 6. Chodoff L. Lisinopril: a new ACE inhibitor for the treatment of hypertension and congestive heart failure // Mt. Sinai. J. Med. – 1990. – Vol. 57. – P. 169–171. 7. Lisinopril. Mosby's GenRx – The complete reference for generic and brand drugs. 9th edn., Mosby, Inc., St. Louis, Missouri, 1999. 8. AstraZeneca. 'Zestril' prescribing information. . Available from: https://www.zestrilinfo.com/info/info.htm. 9. Davis BR, Culter JA, Gordon DJ Antihypertensive and lipid–lowering treatment to prevent heart attack trial. Am. J. Hypertens. – 1996. –Vol.9. –P.342–60. 10. Diamant M, Vincent HH Lisinopril versus enalapril: evaluation of through: peak ratio by ambulatory blood pressure monitoring. J Hum Hypertens. – 1999/ – Jun;13(6):405–12. 11. Rosei EA, Rizzoni D, Comini S et al. Evaluation of the efficacy and tolerability of nebivolol versus lisinopril in the treatment of essential arterial hypertension: a randomized, multicentre, double-blind study. Blood Press Suppl. – 2003, May;1:30–5 12. Os I, Bratland B, Dahlof B at al. Lisinopril or nifedipine in essential hypertension? A Norwegian multicenter study on efficacy, tolerability and quality of life in 828 patients. J Hypertens. – 1992, Feb;10(2). 13. Stergiou GS, Efstathiou SP, Roussias LG et. Al. Blood pressure– and pulse pressure–lowering effects, through:peak ratio and smoothness index of telmisartan compared with lisinopril. J Cardiovasc Pharmacol. – 2003, Oct;42(4):491–6. 14. Malacco E, Santonastaso M, Vari NA et al. Comparison of valsartan 160 mg with lisinopril 20 mg, given as monotherapy or in combination with a diuretic, for the treatment of hypertension: the Blood Pressure Reduction and Tolerability of Valsartan in Comparison with Lisinopril (PREVAIL) study. Clin Ther. – 2004. – Jun;26(6):855–65. 15. Lindsted K. Study Seventh–Day Adventist. Int J Obesity, 1991. 16. Reisin E et al. Lisinopril versus HCTZ in obese hypertensive patients: a multicenter placebo–controlled trial. Treatment in obese Patients with Hypertension (TROPHY) Study Group. Hypert 1997: Jul 30: 140–145 17. Euclid Study Group. Effect of Lisinopril On ProgReSSion of Retinopathy in Normotensive Peopler with Type 1 Diabetes. Lancet 1998; 351: 28–31. 18. Parting hh. Effects of Ace Inhibitors On Renal Function InCipient and Overtic Nephropathy. // J Diabetes Complications. 1996; 10 (3): 133–135. 19. Mancia G., Zanchetti A. et al. Study on Monitoring of Blood Pressure and Lisinopril EvalUation. Circulation, 1997; 95 (6); 1464–70. 20. Latini R., Nicolosi G., Maggioni Ap. et al. The Beneficial Effect of Lisinopril on Left Ventricular Remodeling After a First Myocardial IS Modulated by Age. The Gissi -3 Echo Database (abstract) no. 775–11. .1 Am Coll Cardiol 1996; 27 (2) SUPPL. A: 281 A 21. Ambrossioni E., Borgii S., Magnani V. et al. The Effect of the Angiotensin - Convertmg - Enzmie Inhibitor Zofenopril On Mortaly and Morbidity Anterior MyCardial Infarction. New Engi J Med. 1995; 332: 2: 80–85. 22. Packer M., Poole - Wilson P., Armstrong P. et al. Comparative Effects of Low - Dose Versus High - Dose Lisinopril on Survival and Major Events in Chronic Heart Failure: The Assessement OF TREATMENTH LISINOPRI ). Europ. Heart J., 1998; 19 (SUPPL.): 142 (abstract). 23. Packer m et al. Comparative Effects of Low and High Doses of the Acei Lisinopril, On Morbidity and Mortaly in Chronic Heart Failure. Circulation 1999; 100: 1–7. 24. Ostroumova O.D., Nedogoda S.V., Mamaev V.I., Shorikova E.G., pharmacoeconomic aspects of the effectiveness of angiotensic enzyme inhibitors in arterial hypertension and heart failure, rmzh volume 11, No. 5, p. 262– 267
Lisinopril Stada tablets 5 mg 30 pcs ➤ instructions for use
Orally, regardless of food intake, 1 time per day in the morning, preferably at the same time. The dose is selected individually.
To ensure the dosage regimen of the drug at a dose of 2.5 mg, it is recommended to use tablets of 2.5 mg or 1/2 tablet of 5 mg with a score.
For arterial hypertension (in patients not receiving other antihypertensive drugs):
the initial dose is 5-10 mg 1 time per day, depending on the patient’s condition. If there is no effect, the dose is increased every 2 weeks by 5 mg to an average therapeutic dose of 20-40 mg/day (a dose above 40 mg/day usually does not lead to a further decrease in blood pressure). The usual maintenance dose is 20 mg/day. The maximum daily dose is 40 mg.
The therapeutic effect usually develops within 2-4 weeks from the start of treatment, which should be taken into account when increasing the dose. If the therapeutic effect is insufficient, the drug can be used in combination with other antihypertensive drugs.
If the patient is receiving diuretic therapy, then these drugs should be stopped 2-3 days before starting lisinopril. If it is impossible to discontinue diuretics, the initial dose of lisinopril should not exceed 5 mg/day. In this case, after taking the first dose, medical supervision is recommended for several hours, taking into account the maximum hypotensive effect after 6 hours (a pronounced decrease in blood pressure may develop).
For renovascular hypertension or other conditions associated with increased activity of the renin-angiotensin-aldosterone system (RAAS) (hypovolemia, diet with limited salt intake, cardiac decompensation or severe hypotension):
the initial dose is 2.5-5 mg per day under strict medical supervision (monitoring blood pressure, kidney function, potassium levels in the blood serum). The maintenance dose, while continuing strict medical supervision, should be determined depending on the dynamics of blood pressure.
For renal failure
Due to the fact that lisinopril is excreted through the kidneys, the initial dose is determined depending on creatinine clearance. Further, in accordance with the therapeutic effect and tolerability, a maintenance dose should be established under conditions of frequent monitoring of renal function, potassium and sodium levels in the blood serum.
| Creatinine clearance, ml/min | Initial dose, mg/day |
| 31-80 | 5-10 |
| 10-30 | 2,5-5 |
| less than 10** | 2,5 |
*including patients on hemodialysis
For persistent arterial hypertension, long-term maintenance therapy of 10-15 mg/day is indicated.
For chronic heart failure
The initial dose is 2.5 mg 1 time per day, followed by an increase in dose by 2.5 mg after 1-2 weeks to a maintenance daily dose of 5-20 mg, depending on the dynamics of blood pressure. The maximum daily dose is 20 mg.
In elderly patients (over 65 years old)
a more pronounced and prolonged antihypertensive effect is often observed, which is associated with a decrease in the rate of elimination of lisinopril (it is recommended to start treatment with a dose of 2.5 mg per day).
Acute myocardial infarction (as part of combination therapy):
in early (in the first 24 hours) treatment of patients with stable hemodynamic parameters (systolic blood pressure at least 100 mm Hg) to maintain these parameters and prevent left ventricular dysfunction and heart failure - on the first day the dose is 5 mg, then 5 mg after 1 day, 10 mg after two days and then 10 mg 1 time per day as maintenance therapy. In patients with acute myocardial infarction, the drug should be used for at least 6 weeks.
Patients with low systolic blood pressure (120 mmHg or lower) at the beginning of treatment or during the first 3 days after acute myocardial infarction should receive the drug at a dose of no more than 2.5 mg per day.
In case of decreased blood pressure (systolic blood pressure is less than or equal to 100 mm Hg), the daily dose of 5 mg can be temporarily reduced to 2.5 mg if necessary. In case of prolonged pronounced decrease in blood pressure (systolic blood pressure below 90 mm Hg for more than 1 hour), the drug should be discontinued.
For diabetic nephropathy
10 mg of lisinopril is used once a day. The dose, if necessary, can be increased to 20 mg 1 time per day in order to achieve diastolic blood pressure values below 75 mm Hg. Art. in the sitting position for patients with type 1 diabetes mellitus and below 90 mmHg. Art. in the sitting position in patients with type 2 diabetes mellitus.
Hydrochlorothiazide + Lisinopril-Akrikhin tab 12.5 mg + 20 mg N28 (Akrikhin)
Hydrochlorothiazide. Not recommended combinations of drugs. Lithium preparations. With the simultaneous use of hydrochlorothiazide and lithium preparations, the renal clearance of lithium is reduced, which can lead to an increase in the concentration of lithium in the blood plasma and an increase in its toxicity. If concomitant use of hydrochlorothiazide is necessary, the dose of lithium preparations should be carefully selected, the concentration of lithium in the blood plasma should be regularly monitored and the dose of the drug should be adjusted accordingly. Combinations of drugs that require special attention. Drugs that can cause polymorphic ventricular tachycardia of the “pirouette” type. Hydrochlorothiazide should be used with extreme caution simultaneously with drugs such as: class IA antiarrhythmic drugs (quinidine, hydroquinidine, disopyramide, procainamide); class III antiarrhythmic drugs (dofetilide, ibutilide, bretylium tosylate), sotalol, dronedarone, amiodarone; other (non-antiarrhythmic) drugs, such as: neuroleptics: phenothiazines (chlorpromazine, cyamemazine, levomepromazine, thioridazine, trifluoperazine, fluphenazine), benzamides (amisulpride, sultopride, sulpride, tiapride), butyrophenones (droperidol, haloperidol); pimozide, sertindole; antidepressants: tricyclic antidepressants, selective serotonin reuptake inhibitors (citalopram, escitalopram); antibacterial agents: fluoroquinolones (levofloxacin, moxifloxacin, sparfloxacin, ciprofloxacin); macrolides (erythromycin for intravenous administration, azithromycin, clarithromycin, roxithromycin, spiramycin), co-trimoxazole; antifungals: azoles (voriconazole, itraconazole, ketoconazole, fluconazole); antimalarials (quinine, chloroquine, mefloquine, halofantrine, lumefantrine); antiprotozoal drugs (pentamidine for parenteral administration); antianginal agents (ranolazine, bepridil); antitumor agents (vandetanib, arsenic trioxide, oxaliplatin, tacrolimus); antiemetics (domperidone, ondansetron); drugs that affect gastrointestinal motility (cisapride); antihistamines (astemizole; terfenadine; mizolastine); other drugs (anagrelide, vasopressin, difemanil methyl sulfate, ketanserin, probucol, propofol, sevoflurane, terlipressin, terodiline, cilostazol); due to an increased risk of ventricular arrhythmias, especially polymorphic ventricular tachycardia of the “pirouette” type (risk factor - hypokalemia). The potassium level in the blood plasma should be determined and, if necessary, adjusted before starting combination therapy with hydrochlorothiazide with the above drugs. It is necessary to monitor the patient’s clinical condition, blood plasma electrolyte levels and ECG parameters. In patients with hypokalemia, it is necessary to use drugs that do not cause polymorphic ventricular tachycardia of the “pirouette” type. Medicines that can prolong the QT interval. The simultaneous use of hydrochlorothiazide with drugs that can prolong the QT interval should be based on a careful assessment for each patient of the relationship between the expected benefit and the potential risk (possible increased risk of developing torsade de pointes (TdP). When using such combinations, it is necessary to regularly record an ECG (to detect prolongation of the QT interval), as well as monitor potassium levels in the blood. Drugs that can cause hypokalemia: amphotericin B (with intravenous administration), gluco- and mineralocorticosteroids (with systemic use), tetracosactide (ACTH), glycyrrhizic acid (carbenoxolone, drugs containing licorice root), laxatives that stimulate intestinal motility Increased risk of developing hypokalemia when used simultaneously with hydrochlorothiazide (additive effect). Regular monitoring of potassium levels in the blood plasma is necessary and, if necessary, its correction. During therapy with hydrochlorothiazide, it is recommended to use laxatives that do not stimulate intestinal motility. Cardiac glycosides. Hypokalemia and hypomagnesemia caused by the action of thiazide diuretics increase the toxicity of cardiac glycosides. When using hydrochlorothiazide and cardiac glycosides simultaneously, you should regularly monitor the concentration of potassium in the blood plasma, ECG readings, and, if necessary, adjust therapy. Combinations of drugs that require attention. Other antihypertensive drugs. Potentiation of the antihypertensive effect of hydrochlorothiazide (additive effect). It may be necessary to adjust the dose of concomitantly used antihypertensive drugs. It is recommended to stop taking hydrochlorothiazide 2-3 days before starting ACE inhibitor therapy to prevent the development of symptomatic hypotension. If this is not possible, then the initial dose of ACE inhibitors should be reduced. Ethanol, barbiturates, antipsychotics (neuroleptics), antidepressants, anxiolytics, narcotic analgesics and general anesthesia. The antihypertensive effect of hydrochlorothiazide may be enhanced and orthostatic hypotension may be potentiated (additive effect). Non-depolarizing muscle relaxants (for example, tubocurarine). The effect of non-depolarizing muscle relaxants may be enhanced. Adrenergic agonists (pressor amines). Hydrochlorothiazide may reduce the effect of adrenergic agonists such as epinephrine (adrenaline) and norepinephrine (norepinephrine). Nonsteroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors and high doses of acetylsalicylic acid (>3 g/day). NSAIDs may reduce the diuretic and antihypertensive effects of hydrochlorothiazide. With simultaneous use, there is a risk of developing acute renal failure due to a decrease in glomerular filtration rate. Hydrochlorothiazide may enhance the toxic effects of high doses of salicylates on the central nervous system. Oral hypoglycemic agents and insulin. Thiazide diuretics affect glucose tolerance (hyperglycemia may develop) and reduce the effectiveness of hypoglycemic agents (dose adjustment of hypoglycemic agents may be required). Hydrochlorothiazide and metformin should be used together with caution due to the risk of lactic acidosis due to renal impairment caused by hydrochlorothiazide. Beta blockers, diazoxide. Concomitant use of thiazide diuretics (including hydrochlorothiazide), beta-blockers or diazoxide may increase the risk of hyperglycemia. Medicines used to treat gout (probenecid, sulfinpyrazone, allopurinol). Dose adjustment of uricosuric drugs may be required as hydrochlorothiazide increases serum uric acid concentrations. Thiazide diuretics may increase the incidence of hypersensitivity reactions to allopurinol. Amantadine. Thiazide diuretics (including hydrochlorothiazide) may reduce the clearance of amantadine, lead to increased plasma concentrations of amantadine and increase the risk of adverse effects. Anticholinergic drugs (cholinergic blockers). Anticholinergic drugs (eg, atropine, biperiden) increase the bioavailability of thiazide diuretics by reducing gastrointestinal motility and the rate of gastric emptying. Cytotoxic (antitumor) drugs. Thiazide diuretics reduce the renal excretion of cytotoxic drugs (for example, cyclophosphamide and methotrexate) and potentiate their myelosuppressive effects. Methyldopa. Cases of hemolytic anemia have been described with the simultaneous use of hydrochlorothiazide and methyldopa. Carbamazepine. Risk of developing symptomatic hyponatremia. When using hydrochlorothiazide and carbamazepine simultaneously, it is necessary to monitor the patient's condition and monitor the sodium content in the blood plasma. Cyclosporine. With simultaneous use of thiazide diuretics and cyclosporine, the risk of developing hyperuricemia and exacerbation of gout increases. Oral anticoagulants. Thiazide diuretics may reduce the effect of oral anticoagulants. Iodinated contrast agents. Dehydration while taking thiazide diuretics increases the risk of developing acute renal failure, especially when using high doses of iodinated contrast agents. Before using iodinated contrast agents, it is necessary to compensate for fluid loss. Calcium preparations. With simultaneous use, it is possible to increase the calcium level in the blood and develop hypercalcemia due to a decrease in the excretion of calcium ions by the kidneys. If simultaneous administration of calcium-containing drugs is necessary, the calcium level in the blood plasma should be monitored and the dose of calcium supplements should be adjusted. Anion exchange resins (colestyramine and colestipol). Anion exchange resins reduce the absorption of hydrochlorothiazide. Single doses of cholestyramine and colestipol reduce the absorption of hydrochlorothiazide in the gastrointestinal tract by 85% and 43%, respectively. Lisinopril. Dual blockade of the renin-angiotensin-aldosterone system (RAAS). In patients with atherosclerotic disease, heart failure, or diabetes mellitus with end-organ damage, concomitant therapy with an ACE inhibitor and an ARB II is associated with a higher incidence of hypotension, syncope, hyperkalemia, and deterioration of renal function (including acute renal failure) compared with only one drug that affects the RAAS. Dual blockade (for example, when combining an ACE inhibitor with an ARB II) should be limited to selected cases with careful monitoring of renal function, potassium levels and regular monitoring of blood pressure. The simultaneous use of ACE inhibitors with medicinal products containing aliskiren is contraindicated in patients with diabetes mellitus and/or with moderate or severe renal impairment (GFR less than 60 ml/min/1.73 m2 body surface area) and is not recommended in other patients. Concomitant use of ACE inhibitors with ARB II is contraindicated in patients with diabetic nephropathy and is not recommended in other patients. Potassium-sparing diuretics, potassium preparations, potassium-containing table salt substitutes and other drugs that can increase the potassium content in the blood serum. With simultaneous use of lisinopril with potassium-sparing diuretics (spironolactone, triamterene, amiloride, eplerenone), potassium preparations or potassium-containing substitutes for table salt and other drugs that can increase potassium levels in the blood serum (including angiotensin II receptor antagonists, heparin, tacrolimus, cyclosporine ; drugs containing co-trimoxazole [trimethoprim + sulfamethoxazole]), the risk of developing hyperkalemia increases (especially in patients with impaired renal function). Therefore, these combinations are prescribed with caution, under the monitoring of plasma potassium levels and renal function. In elderly patients and patients with impaired renal function, concomitant use of ACE inhibitors with sulfamethoxazole/trimethoprim was accompanied by severe hyperkalemia, which is believed to be caused by trimethoprim, therefore lisinopril should be used with caution with drugs containing trimethoprim, regularly monitoring the content of potassium in the blood plasma. Potassium-sparing diuretics. With simultaneous use of lisinopril with non-potassium-sparing diuretics, hypokalemia caused by their use can be reduced. Other antihypertensive drugs. When used simultaneously with vasodilators, beta-blockers, blockers of “slow” calcium channels, diuretics and other antihypertensive drugs, the severity of the antihypertensive effect of lisinopril increases. Lithium preparations. When lisinopril is used simultaneously with lithium preparations, the elimination of lithium from the body slows down (risk of increased cardiotoxic and neurotoxic effects of lithium). The simultaneous use of lisinopril with lithium preparations is not recommended. If it is necessary to use this combination, the concentration of lithium in the blood plasma should be regularly monitored. NSAIDs, including selective COX-2 inhibitors, and high doses of acetylsalicylic acid (> 3 g/day). NSAIDs (including selective COX-2 inhibitors) and acetylsalicylic acid in doses of more than 3 g/day reduce the antihypertensive effect of lisinopril. In some patients with impaired renal function (for example, elderly or dehydrated patients, including those taking diuretics) receiving NSAID therapy (including selective COX-2 inhibitors), concomitant use of ACE inhibitors or ARB II may cause further deterioration renal function, including the development of acute renal failure, and hyperkalemia. These effects are usually reversible. The simultaneous use of ACE inhibitors and NSAIDs should be used with caution (especially in elderly patients and in patients with impaired renal function). Patients should receive adequate fluids. It is recommended to carefully monitor renal function, both at the beginning and during treatment. The use of lisinopril in combination with acetylsalicylic acid as an antiplatelet agent is not contraindicated. Hypoglycemic drugs. Concomitant use of lisinopril and insulin, as well as oral hypoglycemic agents, can lead to the development of hypoglycemia. The greatest risk of development is observed during the first weeks of combined use, as well as in patients with impaired renal function. Tricyclic antidepressants/neuroleptics/general anesthetics/narcotics. When used simultaneously with tricyclic antidepressants, neuroleptics, general anesthesia, barbiturates, and muscle relaxants, an increase in the antihypertensive effect of lisinopril is observed. Alpha and beta adrenergic agonists. Alpha and beta adrenergic agonists (sympathomimetics), such as epinephrine (adrenaline), isoproterenol, dobutamine, dopamine, may reduce the antihypertensive effect of lisinopril. Baclofen. Enhances the antihypertensive effect of ACE inhibitors. Blood pressure should be carefully monitored and, if necessary, the dose of antihypertensive drugs should be adjusted. Ethanol. With simultaneous use of ethanol, lisinopril enhances the antihypertensive effect. Estrogens. Estrogens weaken the antihypertensive effect of lisinopril due to fluid retention. Allopurinol, procainamide, cytostatics, immunosuppressants, glucocorticosteroids (GCS) (for systemic use). The combined use of ACE inhibitors with allopurinol, procainamide, and cytostatics increases the risk of developing neutropenia/agranulocytosis. Gold preparations. With the simultaneous use of lisinopril and intravenous gold preparations (sodium aurothiomalate), a symptom complex has been described, including facial flushing, nausea, vomiting and decreased blood pressure. Selective serotonin reuptake inhibitors. Concomitant use of lisinopril with selective serotonin reuptake inhibitors can lead to severe hyponatremia. mTOR (mammalian Target of Rapamycin) inhibitors (for example, temsirolimus, sirolimus, everolimus). In patients taking ACE inhibitors and mTOR inhibitors simultaneously (temsirolimus, sirolimus, everolimus), an increased incidence of angioedema was observed. Dipeptidyl peptidase N type (DPP-IV) inhibitors (gliptins), for example, sitagliptin, saxagliptin, vildagliptin, linagliptin. An increased incidence of angioedema was observed in patients taking ACE inhibitors and dipeptidyl peptidase type IV inhibitors (gliptins) simultaneously. Estramustine. Increased incidence of angioedema when used simultaneously with ACE inhibitors. Neutral endopeptidase inhibitors (NEP). An increased risk of angioedema has been reported with concomitant use of ACE inhibitors and racecadotril (an enkephalinase inhibitor). When ACE inhibitors are used simultaneously with drugs containing sacubitril (neprilysin inhibitor), the risk of developing angioedema increases, and therefore the simultaneous use of these drugs is contraindicated. ACE inhibitors should be prescribed no earlier than 36 hours after discontinuation of drugs containing sacubitril. Prescription of drugs containing sacubitril is contraindicated in patients receiving ACE inhibitors, as well as within 36 hours after discontinuation of ACE inhibitors. Tissue plasminogen activators. Observational studies have shown an increased incidence of angioedema in patients taking ACE inhibitors following the use of alteplase for thrombolytic therapy of ischemic stroke. Pharmacokinetic interactions. Antacids and cholestyramine reduce the absorption of lisinopril in the gastrointestinal tract.
Efficacy and safety of lisinopril in clinical practice
Despite the successes achieved in the treatment of arterial hypertension (AH) recently, this disease remains one of the most common and socially significant in many countries of the world. Currently, 39% of men and 41% of women suffer from hypertension in Russia, and with age this figure increases to 80%. Meanwhile, hypertension, being a very serious disease, represents one of the risk factors for other diseases of the cardiovascular system. The results of epidemiological studies have shown a significant relationship between hypertension and coronary heart disease (CHD), cerebral stroke, chronic heart failure (CHF) and chronic renal failure (CRF). The need for long-term therapy of hypertension is beyond doubt and is aimed at reducing the prevalence of cardiovascular diseases and associated mortality. However, despite the many effective antihypertensive drugs, the treatment of arterial hypertension remains a very difficult problem for a number of reasons [8]. The experience of clinical trials is very slowly transferred to real practice, despite the great significance of the results obtained.
A number of large international studies involving patients with hypertension have shown that lowering blood pressure leads to a reduction in the incidence of cardiovascular diseases and mortality. In what has already become a “classic” study, NOT determined the so-called target blood pressure levels, which were later used as the basis for European and domestic recommendations for the treatment of hypertension (VNOK, 2007). According to these recommendations, in patients with hypertension and diabetes mellitus (DM) and/or renal impairment, blood pressure (BP) should be reduced below 130/80 mmHg. Art., in all other patients with hypertension (regardless of age, gender, duration of the disease, initial blood pressure figures, etc.) - below 140/90 mm Hg. Art. Such low target blood pressure levels minimize the risk of cardiovascular complications, but place special demands on the effectiveness of antihypertensive drugs.
Hypertension is a heterogeneous clinical syndrome. There are many mechanisms for raising and maintaining blood pressure: activation of neurohumoral systems (renin-angiotensin-aldosterone and sympathoadrenal), hypervolemia, and endothelial dysfunction. It has been proven that the endothelium is not only a barrier between blood and tissues, but primarily an active organ, the dysfunction of which is an essential component of cardiovascular diseases such as hypertension, atherosclerosis, coronary heart disease, and CHF. In order to determine the leading links in the pathogenesis of hypertension in each specific case, the use of complex and expensive biochemical and instrumental methods is required. Therefore, in everyday clinical practice, antihypertensive drugs for long-term therapy have to be selected empirically without taking into account the etiopathogenetic mechanisms of the disease, but taking into account the known data on the characteristics of the course of hypertension in different categories of patients. Also, when choosing antihypertensive therapy, concomitant diseases should be taken into account. At the present stage of development of medicine, the determining factor in choosing a drug is its effectiveness and safety based on the results of long-term randomized studies. Currently, thiazide (and thiazide-like) diuretics, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, calcium antagonists, AT1-angiotensin receptor blockers, I1-imidazoline receptor agonists, and central alpha2-adrenergic receptor agonists are used for the treatment of hypertension.
recommendations are presented on the choice of antihypertensive drug depending on various clinical situations, according to which ACE inhibitors are the drugs of choice.
Numerous clinical and experimental studies have shown that ACE inhibitors not only effectively reduce and control blood pressure levels, but also have a beneficial effect on the state of target organs in hypertension - the myocardium, blood vessels, kidneys (suppressing the activity of both plasma and tissue renin-angiotensin components). systems (RAS), ACE inhibitors can prevent and even reverse the development of changes occurring in target organs during hypertension). The condition of target organs in hypertension has important prognostic significance and determines the management of patients with hypertension. According to the recommendations of the WHO and the International Society of Hypertension, patients with hypertension should be stratified according to the degree of risk of developing cardiovascular complications [7]. The presence of signs of target organ damage determines that patients belong to a high-risk group, the incidence of cardiovascular complications in which is 20–30% over the next 10 years. This determines the leading position of ACE inhibitors among all currently known groups of antihypertensive drugs [4, 5].
The earliest echocardiographic sign of the formation of a hypertensive heart is a violation of the diastolic function of the left ventricular myocardium. Under the influence of angiotensin II and aldosterone, the synthetic function of fibroblasts increases, which leads to the accumulation of collagen fibers in the myocardium, an increase in its rigidity and a decrease in the compliance of the heart walls. Subsequently, there is an increase in the mass of the left ventricular myocardium. The development of myocardial hypertrophy in patients with hypertension has an unfavorable prognostic value, as it is associated with an increase in the risk of developing cardiovascular complications by 2–6 times [6]. In addition, further progression of hypertrophic and fibrotic changes in the myocardium can lead to a decrease in myocardial contractility and the development of systolic heart failure. Data were obtained indicating regression of left ventricular myocardial hypertrophy during treatment with lisinopril, as well as improvement in endothelial function. The mechanism of regression of left ventricular (LV) myocardial hypertrophy when using lisinopril is due to both hemodynamic and metabolic effects of the drug: inhibition of angiotensin (AII) formation, which has a negative effect on LV pressure and coronary blood flow, and prevention of the breakdown of bradykinin, which, on the contrary, increases pressure in the left ventricle, increases coronary blood flow and myocardial contractility. Thus, against the background of long-term therapy with ACE inhibitors, in particular lisinopril, there is a reverse development of myocardial hypertrophy and an improvement in the functional state of the LV [2].
Hypertension is the most important risk factor for the development of coronary atherosclerosis. The morphological basis for the decrease in coronary reserve in hypertension is the structural changes in the vessels of the microvasculature, in which the processes of eutrophic remodeling occur. There is an increase in the thickness of the muscle layer of arterioles: primarily due to the rearrangement of smooth muscle cells around the lumen of the vessel and, to a lesser extent, due to their hypertrophy, as a result of which the lumen and outer diameter of the vessel decrease. In addition, due to an increase in the number of fibrillar structures, the compliance of their walls decreases [1]. Hyperactivation of the renin-angiotensin-aldosterone system (RAAS) is a prerequisite for endothelial dysfunction [11]. According to immunohistochemistry, the main part of ACE is located directly on the membrane of endothelial cells. According to V. Dzau, 90% of the total volume of the RAAS is in organs and tissues (10% in plasma), among which the vascular endothelium ranks first.
An increase in the activity of ACE, located on the surface of endothelial cells, catalyzes the breakdown of bradykinin. A decrease in stimulation of endothelial bradykinin receptors leads, in turn, to a decrease in the production of endothelium-dependent relaxation factor - nitric oxide. As a result of the resulting structural and functional changes, the tendency of blood vessels to vasoconstriction increases and their ability to adequately respond to vasodilator stimuli is impaired.
The results of the clinical study “Clinical efficacy of Diroton (lisinopril) and enalapril in patients with mild and moderate arterial hypertension (AH) associated with chronic viral hepatitis” confirm the positive effect of ACE inhibitors on coronary microcirculation in patients with hypertension. Thus, in patients with hypertension with signs of myocardial ischemia and intact coronary arteries after long-term, for 1 year, therapy with the ACE inhibitor enalapril, along with an increase in coronary reserve, a decrease in ECG criteria for myocardial ischemia was noted according to stress tests. This effect persisted a week after discontinuation of lisinopril and, therefore, cannot be explained only by hemodynamic factors (decrease in blood pressure and decrease in myocardial oxygen demand) [9].
Hypertension is the cause of the development of 24% of cases of end-stage renal failure. With systemic hypertension, the tone of the afferent arterioles of the renal glomeruli increases, which protects the renal glomeruli from the development of intraglomerular hypertension. When autoregulation mechanisms fail, hydrostatic pressure in the renal glomeruli increases, proteinuria and glomerulosclerosis develop [12, 14]. Several clinical studies have noted the advantages of therapy with ACE inhibitors (lisinopril) compared to beta-blockers and nifedipine in terms of the effect on the rate of decline in glomerular filtration in patients with type 1 and type 2 diabetes mellitus [10]. In addition, ACE inhibitors reduce the permeability of the glomerular capillary wall, which, along with a decrease in hydrostatic pressure in the glomeruli of the kidneys, helps to reduce the daily excretion of albumin in the urine (by 30–86%). Considering the presence of distinct nephroprotective properties of ACE inhibitors, their use is recommended for all patients with overt or latent diabetic nephropathy, regardless of the type of diabetes mellitus and the level of systemic blood pressure.
Currently, much attention is paid to the metabolic neutrality of antihypertensive drugs. Therefore, an important advantage of lisinopril is its high effectiveness in patients with metabolic risk factors. The close relationship between metabolic disorders and cardiovascular diseases is beyond doubt. In patients with hypertension, disorders of carbohydrate metabolism (impaired carbohydrate tolerance, type 2 diabetes mellitus), lipid profile (hypertriglyceridemia, decreased cholesterol (HC) of high-density lipoproteins (HDL), increased cholesterol of low-density lipoproteins (LDL) and fat ( abdominal obesity) metabolism, hyperuricemia, changes in the fibrinolytic system. This symptom complex is currently combined into a cluster of risk factors and is included in the concept of “metabolic syndrome.” The metabolic syndrome is based on a decrease in tissue sensitivity to insulin - insulin resistance with the formation of compensatory hyperinsulinemia. hyperinsulinemia and hyperglycemia are considered as factors contributing to the pathogenesis of hypertension and atherosclerosis. Metabolic syndrome nowadays attracts close attention from clinicians of various specialties. This is due to the accumulation of data on the action of insulin on target organs. There are many studies examining the subtle mechanisms of the influence of insulin resistance and hyperinsulinemia on the level of HELL. It is believed that endothelial dysfunction plays an important role in the pathogenesis of hypertension associated with metabolic disorders. During therapy with ACE inhibitors, there are no adverse changes in carbohydrate, lipid metabolism, or uric acid levels. A beneficial effect on insulin resistance and some indicators of hemostasis is known (decrease in the level of tissue plasminogen activator inhibitor, increase in tissue plasminogen activator). The vasodilatory effects, antiproliferative, vasoprotective, antisclerotic properties of ACE inhibitors can be explained by endothelium-dependent reactions associated with the prevention of the breakdown of bradykinin, which is a powerful stimulator of the release of endothelium-dependent relaxing factors such as nitric oxide, hyperpolarizing factor and prostacyclin.
In the TROPHY trial, lisinopril (Diroton) demonstrated superior antihypertensive efficacy and safety over hydrochlorothiazide. The cardio- and vasoprotective effects of the drug were confirmed in experimental models and in clinical groups: regression of left ventricular remodeling (reduction of myocardial mass, reduction of perivascular fibrosis), antioxidant properties, antithrombotic activity, correction of endothelial dysfunction.
The open study involved 27 patients with mild and moderate hypertension (17 women and 10 men) aged 46 to 75 years with a combination of metabolic risk factors. In 88.9% of patients, various lipid disorders were recorded (increased LDL cholesterol, hypertriglyceridemia, decreased HDL cholesterol), 74.7% of patients had abdominal obesity, hyperuricemia was observed in 25.9% of cases, and disorders were diagnosed in 58.3% of patients. carbohydrate metabolism (impaired carbohydrate tolerance, fasting hyperglycemia). The duration of treatment was 12 weeks. At the first stage of the study, 2 people (7.4%) dropped out due to the development of side effects. Monotherapy with Diroton was started with a dose of 10 mg/day. If after 4 weeks the level of office diastolic blood pressure (DBP) was 90 mm Hg. Art. and higher, the dose of Diroton was increased to 20 mg [1]. During therapy, a positive clinical effect was observed in the form of improved well-being of patients, reduction of weakness, headaches, and palpitations. Complaints of headache remained after the end of therapy in only 4% of patients (60% before treatment). There was also a decrease in pain in the heart area, episodes of dizziness, and an improvement in the quality of life. Improving the characteristics of the initially altered daily blood pressure profile, without disturbing the normal biphasic rhythm, does not affect normal blood pressure variability and reduces increased variability, provides adequate blood pressure control in the early morning hours, that is, it meets all the basic requirements for a long-acting antihypertensive drug. The drug did not have a negative effect on carbohydrate, lipid and purine metabolism. During therapy, there were no significant fluctuations in morning and postprandial glycemia, lipid spectrum and uric acid. The data obtained demonstrated the safety and effectiveness of Diroton monotherapy in patients with mild and moderate arterial hypertension with metabolic risk factors.
As part of the DESIRE (Diroton Efficiency and Safety Investigation: Russian Estimate) program, the effectiveness and safety of long-term treatment with lisinopril in patients with arterial hypertension in combination with pathology of the bronchopulmonary system was studied. The open study involved 31 patients aged from 18 to 59 years, while 17 patients who made up the main group experienced exacerbations of chronic bronchitis or bronchial asthma for three or more years. One of the inclusion criteria was the ability to discontinue previous antihypertensive therapy at least 14 days before the study. Diroton was prescribed in tablets initially at a dose of 10 mg once in the morning or evening. If there was insufficient reduction in blood pressure during the control study at week 4, the dose was increased to 20 mg/day once, maintaining the same dosage time. The duration of treatment was 12 weeks. During Diroton therapy in patients with bronchopulmonary pathology, a significant decrease in average daily SBP/DBP was noted (from 150.04 ± 8.29/88.98 ± 4.3 to 130.65 ± 6.13/77.65 ± 3.6 mmHg, p < 0.01), as well as averaged SBP and DBP during the day and night. Diroton therapy was generally well tolerated. Adverse reactions noted during therapy did not require discontinuation of the drug in any of the groups. The majority of patients felt better during treatment: headaches decreased, tolerance to physical activity increased, and mood improved, which indicates an improvement in the quality of life of patients. Dry cough was noted in 5 (16.1%) patients; in none of the cases was drug discontinuation required. In this study, in patients with concomitant broncho-obstructive pulmonary diseases (chronic obstructive bronchitis, bronchial asthma), a clear connection between increased cough and the start of taking Diroton was noted in only one patient. None of the patients experienced shortness of breath, asthma attacks, or worsening night sleep. In the control group, the appearance of dry cough was noted in 4 patients (28%), 3 of them were women.
High efficiency and good tolerability are especially important in the presence of concomitant pathology of the bronchopulmonary system, since the combination of hypertension with obstructive pulmonary diseases is a fairly common clinical situation. This combination significantly increases the risk level in patients with arterial hypertension due to a negative effect on the condition of the heart muscle and vascular wall due to hypoxemia and hypoxia, chronic inflammation, sympathicotonia, secondary pulmonary hypertension and overload of the right heart and creates certain difficulties in selecting adequate antihypertensive therapy. A rare side effect, a dry cough is not equivalent to bronchial obstruction.
We conducted a study that showed the effectiveness of the use of the ACE inhibitor lisinopril (Diroton) for hypertension and silent myocardial ischemia (PSI) in patients with polycythemia vera (PV). 21 patients with hypertension and PV in a state of clinical and hematological remission were treated with a long-acting ACE inhibitor lisinopril (Diroton) at a dose of 10–40 mg once in the morning (the average dose was 17.1 ± 2.8 mg/day). During therapy with Diroton in patients with hypertension and polycythemia vera, more than 50% of treated patients noted a decrease in headaches and fatigue. A feature of lisinopril, as a hydrophilic compound, is the lack of hepatic metabolism. This makes it possible to use it in patients with liver diseases, which is important in the treatment of patients with concomitant pathology, in this case hypertension with PV. When studying the indicators of 24-hour blood pressure monitoring (ABPM), a significant decrease in systolic blood pressure (SBP) and DBP was obtained (Fig.).
Double product (DP), as a physiological indicator, is used for indirect judgment about metabolic processes in the heart and myocardial oxygen consumption. It more clearly shows the level of hemodynamic load than blood pressure or heart rate separately. The average DP value per day of treatment was 115.0 ± 2.0, against the background of 97.4 ± 2.4 mm Hg. Art. × beats/min, in the morning (from 4 to 10 o’clock) the average value of DP before therapy was 111.7 ± 3.3 and against the background of 91.7 ± 4.7 mm Hg. Art. × beats/min (p < 0.0001 in both cases). During therapy, the number of patients with the “dipper” and “non-dipper” type of daily blood pressure profile increased. There was not a single patient left with the “night picker” type of daily blood pressure profile. The initial normal biphasic daily rhythm of blood pressure was not changed by taking lisinopril, which indicates the physiological effect of the drug. Target blood pressure level is below 135/85 mm Hg. Art. was achieved in 65% of patients. During therapy with lisinopril, the average daily values of SBP decreased by 12.2%, DBP by 9.5%, PP by 25%, and DP by 15.4% (p < 0.0001) [3].
According to the results of 24-hour ECG monitoring in PV with concomitant hypertension, episodes of myocardial infarction were recorded in 25% of patients, of which 5% were without diagnosed coronary artery disease. During therapy with lisinopril, IM was detected in 10% of patients with previously diagnosed IHD, and the frequency of episodes significantly decreased. During treatment, a positive trend in the dynamics of blood rheological properties was observed. A decrease in the degree of erythrocyte aggregation, spontaneous platelet aggregation, and blood viscosity was revealed. Lisinopril has a positive effect on the activity of the sympatho-adrenal system, as evidenced by a decrease in adrenoreactivity during treatment to the upper limit of normal (46%).
Thus, clinical studies have proven the high effectiveness and safety of the use of the ACE inhibitor lisinopril (Diroton) for the treatment of patients with arterial hypertension, in patients with metabolic risk factors, since the drug does not have a negative effect on carbohydrate, lipid and purine metabolism, improves the quality of life, improves well-being of patients; the drug has been proven to be highly effective and well tolerated in the presence of concomitant pathology of the bronchopulmonary system; contributes to the normalization of the sympatho-adrenal system; In addition, lisinopril is an effective treatment for arterial hypertension, capable of controlling blood pressure levels in hypertensive patients with polycythemia vera when taken once a day in the morning, has a positive effect on the main characteristics of the initially disturbed circadian rhythm of blood pressure, reduces the number of episodes of silent myocardial ischemia, and has a positive effect on rheological properties of blood.
For questions regarding literature, please contact the editor.