Home | About us | Delivery | Advertisers | Login | Registration
Delivery on Sundays and holidays does not work!
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2022 Pharmacy 84.
Trimethoprim
Stylab / Catalog / Antibacterial drugs / Trimethoprim
STYLAB offers test systems for the analysis of trimethoprim in meat and other animal tissues, as well as in urine, blood plasma and feed by ELISA.
| Enzyme-linked immunosorbent assay (ELISA), strip plate | Trimethoprim (TMP) ELISA Kit |
Trimethoprim is a bacteriostatic antibiotic of the diaminopyrimidine group, which also includes baquiloprim . It is on the WHO Model List of Essential Medicines. In medicine, this antibiotic is used mainly to treat bacterial urinary tract infections caused by both gram-positive and gram-negative microorganisms. In veterinary medicine, trimethoprim is used more widely: for the treatment of diseases of the respiratory system, gastrointestinal tract, genitourinary system and other organs caused by bacteria or mycoplasmas. Preparations containing this antibiotic are prescribed to poultry, calves, pigs, sheep, horses, as well as dogs and cats. It should not be used to treat adult ruminants. Trimethoprim is also not prescribed to laying hens because it can accumulate in the eggs. In the Russian Federation, as of 2022, 29 veterinary drugs containing this antibiotic are registered.
The mechanism of action of trimethoprim is based on its ability to inhibit the enzyme folate reductase. As a result, the inactive form of folic acid cannot be restored to the active form. This leads to disruption of the synthesis of DNA, RNA and protein, and consequently, cells cannot divide and die. Some microorganisms are capable of developing resistance to trimethoprim. In this regard, in medications, trimethoprim is often combined with sulfonamides , which also interfere with the synthesis of folic acid. This combination of drugs has a bactericidal effect. in combination with sulfamethoxazole or dapsone, is used to treat Pneumocystis pneumonia in people with HIV.
In humans and other mammals, trimethoprim is well absorbed in the gastrointestinal tract. High concentrations of this antibiotic are created in the lungs and urinary tract. Then, 10-20% of ingested trimethoprim is metabolized in the liver to form at least 5 metabolites: trimethoprim-1-oxide, trimethoprim-3-oxide, 4-hydroxytrimethoprim, 3-hydroxytrimethoprim and α-hydroxy-trimethoprim. The remaining antibiotic is excreted unchanged by the kidneys. The half-life of trimethoprim in humans ranges from 8.6 to 17 hours. This antibiotic is able to cross the placental barrier and affect the embryo, and therefore it is not prescribed to pregnant women. In addition, small amounts of trimethoprim pass into breast milk.
The acute toxicity of trimethoprim to mammals is low. For rats, when taken orally, the LD50 exceeds 5300 mg/kg body weight, for mice it is 2764 mg/kg body weight. The symptoms of trimethoprim overdose and its side effects have been well studied. These include nausea, vomiting, diarrhea, changes in taste perception, skin rash, increased photosensitivity of the skin and itching, changes in blood composition (thrombocytopenia, leukopenia, increased potassium levels in the blood). There are known cases of hypersensitivity to this antibiotic in humans, as well as allergic reactions. Since trimethoprim inhibits the synthesis of folic acid, it is not prescribed if there is a deficiency of this vitamin in the body. For the same reason, trimethoprim can lead to the development of pernicious (megaloblastic) anemia. This disease, caused by a lack of folic acid, causes anemia and damage to the nervous system. In rare cases, trimethoprim may cause kidney and liver problems.
In a study in rats receiving 300 mg/kg body weight of trimethoprim for 13 weeks, the animals showed changes in bone marrow function. A similar result was demonstrated in monkeys. In addition, they observed fatty changes in the liver. In high doses, trimethoprim has teratogenic and embryotoxic effects, especially when used in the first trimester of pregnancy. In this case, developmental disorders of the cardiovascular, genitourinary and nervous systems are most likely. The latter include anencephaly and spina bifida. In studies on rabbits, an increase in the number of stillbirths was also observed. In an experiment on rats - underdevelopment of the lower jaw, cleft palate, underdevelopment of the eyes and limbs. Interestingly, the use of trimethoprim in humans at therapeutic doses did not lead to fetal developmental disorders.
Trimethoprim can be released into the environment either through production emissions or through runoff from farms and cities. In soil and sediments in water, it slowly biodegrades: the half-life of this antibiotic is about 100 days with access to oxygen and about 75 days without it. Trimethoprim has little ability to bioconcentrate in aquatic organisms.
In the Russian Federation and the countries of the EAEU, the content of trimethoprim in food raw materials and finished products is limited by TR CU 034/2013 “On the safety of meat and meat products.” According to this document, the maximum permissible levels of this antibiotic in meat, raw fat, kidneys and liver of all types of productive animals should not exceed 0.05 mg/kg. The European Union has regulation 37/2010, which sets the same MRLs for trimethoprim for products from all types of food-producing animals, with the exception of horses. In horse meat, as well as in the liver, kidneys and fat of horses, the content of trimethoprim should not exceed 0.1 mg/kg. According to this document, the content of trimethoprim in fish is also limited to 0.05 mg/kg.
STYLAB offers test systems for the analysis of trimethoprim in meat and other animal tissues, as well as in urine, blood plasma and feed using the ELISA method. They allow you to quickly screen large numbers of samples and do not require expensive equipment.
Literature
- Trimethoprim. Register of medicines in Russia.
- Trimethoprim. Pubchem
- Brogden, R.N.; Carmine, A.A.; Heel, R.C.; Speight, T.M.; Avery, G.S. Trimethoprim: a review of its antibacterial activity, pharmacokinetics and therapeutic use in urinary tract infections. Drugs. June 1982. 23 (6): 405–30.
- Andersen JT, Petersen M, Jimenez-Solem E, Broedbaek K, Andersen EW, Andersen NL, Afzal S, Torp-Pedersen C, Keiding N, Poulsen HE. Trimethoprim use in early pregnancy and the risk of miscarriage: a register-based nationwide cohort study. Epidemiology and Infection. 2013, 141(8): 1749–1755.
- Choi, Michael J.; Fernandez, Pedro C.; Patnaik, Asit; Coupaye-Gerard, Brigitte; D'Andrea, Denise; Szerlip, Harold; Kleyman, Thomas R. Trimethoprim-Induced Hyperkalemia in a Patient with AIDS. New England Journal of Medicine. 1993-03-11. 328(10):703–706.
←Return
AZITHROMYCIN (Azithromycin)
Antacids
Antacids do not affect the bioavailability of azithromycin, but reduce the maximum blood concentration by 30%, so the drug should be taken at least one hour before or two hours after taking these drugs and eating.
Cetirizine
Concomitant use of azithromycin with cetirizine (20 mg) for 5 days in healthy volunteers did not lead to pharmacokinetic interaction and a significant change in the QT interval.
Didanosine (dideoxyinosine)
The simultaneous use of azithromycin (1200 mg/day) and didanosine (400 mg/day) in 6 HIV-infected patients did not reveal changes in the pharmacokinetic indications of didanosine compared to the placebo group.
Digoxin and colchicine (P-glycoprotein substrates)
Concomitant use of macrolide antibiotics, including azithromycin, with P-glycoprotein substrates, such as digoxin and colchicine, leads to increased concentrations of P-glycoprotein substrate in the blood serum. Thus, with the simultaneous use of azithromycin and digoxin, it is necessary to take into account the possibility of increasing the concentration of digoxin in the blood serum.
Zidovudine
Concomitant use of azithromycin (single dose of 1000 mg and multiple doses of 1200 mg or 600 mg) has a minor effect on the pharmacokinetics, including renal excretion of zidovudine or its glucuronide metabolite. However, the use of azithromycin caused an increase in the concentration of phosphorylated zidovudine, a clinically active metabolite in peripheral blood mononuclear cells. The clinical significance of this fact is unclear.
Azithromycin interacts weakly with isoenzymes of the cytochrome P450 system. Azithromycin has not been shown to participate in pharmacokinetic interactions similar to erythromycin and other macrolides. Azithromycin is not an inhibitor or inducer of cytochrome P450 isoenzymes.
Ergot alkaloids
Given the theoretical possibility of ergotism, the simultaneous use of azithromycin with ergot alkaloid derivatives is not recommended.
Pharmacokinetic studies were conducted on the simultaneous use of azithromycin and drugs whose metabolism occurs with the participation of isoenzymes of the cytochrome P450 system.
Atorvastatin
Concomitant use of atorvastatin (10 mg daily) and azithromycin (500 mg daily) did not cause changes in atorvastatin plasma concentrations (based on an HMC-CoA reductase inhibition assay). However, in the post-marketing period, isolated case reports of rhabdomyolysis have been received in patients receiving concomitant azithromycin and statins.
Carbamazepine
Pharmacokinetic studies involving healthy volunteers did not reveal a significant effect on the plasma concentrations of carbamazepine and its active metabolite in patients receiving concomitant azithromycin.
Cimetidine
In pharmacokinetic studies of the effect of a single dose of cimetidine on the pharmacokinetics of azithromycin, no changes in the pharmacokinetics of azithromycin were detected when cimetidine was used 2 hours before azithromycin.
Indirect anticoagulants (coumarin derivatives)
In pharmacokinetic studies, azithromycin did not affect the anticoagulant effect of a single 15 mg dose of warfarin administered to healthy volunteers. Potentiation of the anticoagulant effect has been reported after simultaneous use of azithromycin and indirect anticoagulants (coumarin derivatives). Although a causal relationship has not been established, the need for frequent monitoring of prothrombin time should be considered when using azithromycin in patients receiving indirect oral anticoagulants (coumarin derivatives).
Cicposporin
In a pharmacokinetic study involving healthy volunteers who took azithromycin (500 mg/day once) orally for 3 days and then cyclosporine (10 mg/kg/day once), a significant increase in maximum plasma concentration (Cmax) and area under the concentration-time curve (AUC0-5) of cyclosporine. Caution is advised when using these drugs together. If simultaneous use of these drugs is necessary, it is necessary to monitor the concentration of cyclosporine in the blood plasma and adjust the dose accordingly.
Efavirenz
Concomitant use of azithromycin (600 mg/day once) and efavirenz (400 mg/day) daily for 7 days did not cause any clinically significant pharmacokinetic interaction.
Fluconazole
Concomitant use of azithromycin (1200 mg once) did not change the pharmacokinetics of fluconazole (800 mg once). The total exposure and half-life of azithromycin did not change with simultaneous use of fluconazole, however, a decrease in Cmax of azithromycin was observed (by 18%), which had no clinical significance.
Indinavir
The simultaneous use of azithromycin (1200 mg once) did not cause a statistically significant effect on the pharmacokinetics of indinavir (800 mg 3 times a day for 5 days).
Methylprednisolone
Azithromycin does not have a significant effect on the pharmacokinetics of methylprednisolone.
Nelfinavir
The simultaneous use of azithromycin (1200 mg) and nelfinavir (750 mg 3 times a day) causes an increase in the equilibrium concentrations of azithromycin in the blood serum. No clinically significant side effects were observed and no dose adjustment of azithromycin was required when used concomitantly with nelfinavir.
Rifabutin
The simultaneous use of azithromycin and rifabutin does not affect the concentration of each drug in the blood serum. Neutropenia has sometimes been observed with simultaneous use of azithromycin and rifabutin. Although neutropenia has been associated with the use of rifabutin, a causal relationship between the use of the combination of azithromycin and rifabutin and neutropenia has not been established.
Sildenafil
When used in healthy volunteers, there was no evidence of the effect of azithromycin (500 mg/day daily for 3 days) on the AUC and Cmax of sildenafil and its main circulating metabolite.
Terfenadine
In pharmacokinetic studies, there was no evidence of interaction between azithromycin and terfenadine. There have been isolated cases reported where the possibility of such an interaction could not be completely excluded, but there was no concrete evidence that such an interaction occurred.
It has been found that the simultaneous use of terfenadine and macrolides can cause arrhythmia and prolongation of the QT interval.
Theophylline
There was no interaction between azithromycin and theophylline.
Triazolam/midazolam
No significant changes in pharmacokinetic parameters were detected with simultaneous use of azithromycin with triazolam or midazolam in therapeutic doses.
Trimethoprim/sulfamethoxazole
Concomitant use of trimethoprim/sulfamethoxazole with azithromycin did not show a significant effect on Cmax, total exposure or renal excretion of trimethoprim or sulfamethoxazole. Azithromycin plasma concentrations were consistent with those found in other studies.
Review of clinical guidelines for the treatment of acute uncomplicated lower urinary tract infection
A review of the recommendations of the European Association of Urology and the Infectious Diseases Society of America on the treatment of acute uncomplicated lower urinary tract infection is presented. It is noted that the basis of therapy is the use of antibacterial drugs, the choice of which should be made taking into account the clinical picture, the sensitivity of the most likely pathogen, the effectiveness of the drug confirmed in clinical studies, as well as its cost and availability.
Table 1. Risk factors for developing acute UTI in women
Table 2. EAU recommended antimicrobial therapy for acute uncomplicated cystitis
Table 3. IDSA recommendations for the treatment of acute uncomplicated UTI
Introduction
Lower urinary tract infections (LUTIs) are among the most common. In the United States, about 15% of all antibiotics prescribed in the population are used in connection with urinary tract infections [1]. A similar situation is observed in some European countries [2]. Every year, more than 7 million visits to outpatient specialists in America are caused by cystitis, about 100 thousand hospitalizations are caused by acute pyelonephritis. In Russia, 26–36 million cases of cystitis are registered annually. During their lifetime, 20–25% of women experience acute cystitis, every third of them experiences a relapse within a year, and in 10% the disease becomes a chronic recurrent form [3].
Numerous clinical guidelines are available today regarding the diagnosis and treatment of urinary tract infections. The most widespread among them are the recommendations of the European Association of Urology (EAU) and the Infectious Diseases Society of America (IDSA). This article provides an overview of the recommendations of these professional societies, particularly those related to the treatment of acute uncomplicated lower urinary tract infection (AUTI).
EAU recommendations
Epidemiology, etiology and pathophysiology
Acute uncomplicated UTI involves sporadic episodes or recurrences of acute cystitis in otherwise healthy people. As a rule, acute uncomplicated UTI affects women without any significant structural and functional disorders of the urinary tract, kidney disease and concomitant diseases that can have a negative impact on the outcome of the inflammatory process. In men, this condition is observed extremely rarely.
Almost half of women experience at least one episode of UTI during their lifetime. Approximately every third woman under the age of 24 experiences an episode of acute cystitis [4]. The most well-known predisposing factor in the development of acute cystitis is diabetes mellitus [5]. Other risk factors for developing an episode of UTI are listed in Table. 1.
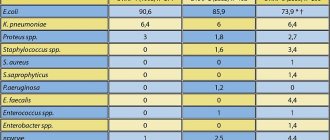
The spectrum of pathogens causing acute UTI is mainly represented by Escherichia
coli
(70–95%) and
Staphylococcus saprophyticus
(5–10%).
of Enterobacteriaceae
are much less common -
Proteus mirabilis
and
Klebsiella
spp. [6].
Diagnostics
The diagnosis of acute uncomplicated cystitis can most likely be established on the basis of the clinical picture (dysuria, frequent urination and urgency) in the absence of risk factors for complicated infection, as well as vaginal discharge and irritative symptoms [7, 8]. It should be borne in mind that in elderly patients, these symptoms may be due to reasons other than UTI [9].
In patients with a compensated form of diabetes mellitus, an episode of acute cystitis can be classified as uncomplicated. However, patients with long-term diabetes mellitus may develop neurogenic urinary disorders, which should be regarded as complicated UTI.
It is necessary to differentiate between UTI and asymptomatic bacteriuria, which should not be classified as an infectious disease. Asymptomatic bacteriuria does not require specific therapy, unless it is detected during screening of bacterial colonization in pregnant women.
Laboratory diagnosis of UTI is limited to the use of test strips for semi-quantitative determination of the presence of bacteria [10, 11]. Routine bacteriological examination of urine is not required. Conducting a urine culture is indicated only:
- if acute pyelonephritis is suspected;
- lack of effect from therapy or relapse of the disease within two to four weeks after completion of therapy;
- atypical symptoms in women [12, 13];
- the presence of acute UTI in pregnant women;
- suspected presence of UTI in men.
A quantitative concentration of uropathogens ≥ 103 CFU/ml is microbiological confirmation of acute UTI in patients with symptoms of uncomplicated cystitis [14]. Invasive research methods, such as cystoscopy, are not indicated for acute uncomplicated UTI in women.
Treatment
The prescription of antibacterial drugs is the basis for the treatment of acute uncomplicated UTI, which is confirmed by a number of studies on the comparative use of antibacterial drugs and placebo [15]. The antibacterial drug is selected taking into account factors such as the sensitivity of the most likely pathogen, the frequency of side effects, the effectiveness of the drug according to clinical studies, its cost and availability.
According to the above criteria, in most European countries, the drugs of choice are fosfomycin trometamol (3 g once), pivmecillinam (400 mg three times daily for three days), and macrocrystalline nitrofurantoin (100 mg twice daily for five days). [16–18]. These regimens are indicated for the treatment of acute UTTI in women and should not be used in men. It should be noted that of the drugs listed above, only fosfomycin trometamol is available in Russia today.
Escherichia
coli
strains currently maintain sensitivity to fosfomycin . However, Spanish researchers have discovered an increase in resistance to fosfomycin in ESBL-producing Escherichia coli. It should be noted that in this study, more than 60% of fosfomycin-resistant strains were isolated from nursing home patients. Consequently, resistant strains were predominantly hospital-acquired pathogens of complicated urinary infections, and the results of the study cannot be directly extrapolated to patients with acute uncomplicated cystitis [19]. In general, compared to cephalosporins and fluoroquinolones, the formation of resistance of uropathogens to fosfomycin occurs much more slowly and with minimal collateral damage, which became one of the key factors in recommending its inclusion in a number of first-line drugs for the treatment of acute cystitis.
Alternative drugs, according to the EAU, are trimethoprim (both in monotherapy and in combination with sulfonamide), as well as fluoroquinolones. Trimethoprim/sulfamethoxazole (160/800 mg twice daily for three days) or trimethoprim (200 mg for five days) should only be used in areas where Escherichia coli
to them exceeds 80%, which excludes their use in Russia [20, 21].
Although Escherichia coli
to fluoroquinolones is still acceptable in some countries, this group of antibiotics is no longer the drug of choice for the treatment of acute UTI due to the significant incidence of side effects and high levels of resistance.
Aminopenicillins are not used for empirical treatment of acute UTI due to the widespread high level of resistance of Escherichia coli to them. Aminopenicillins in combination with beta-lactamase inhibitors (ampicillin/sulbactam or amoxicillin/clavulanate), as well as first- and second-generation oral cephalosporins in general, can be used in selected cases, but for short-term therapy they are inferior in effectiveness to the drugs of choice and are not recommended for empirical therapy [22, 23].
In table Table 2 presents the EAU-recommended drugs for the treatment of acute uncomplicated UTI.
IDSA Recommendations
Macrocrystalline nitrofurantoin (100 mg twice daily for five days)
The results of four randomized comparative studies on the use of macrocrystalline nitrofurantoin in patients with acute uncomplicated UTI allowed the following conclusions to be drawn [16, 24–26]:
1) macrocrystalline nitrofurantoin at a dose of 100 mg twice daily for seven days has similar clinical efficacy:
- with ciprofloxacin (100 mg twice daily for three days) – 93 compared to 95%;
- trimethoprim/sulfamethoxazole (160/800 mg twice daily for seven days) – 93 compared with 95%;
- fosfomycin (3 g once) – 89 compared to 90%.
2) macrocrystalline nitrofurantoin at a dose of 100 mg twice daily for five days is equivalent in clinical and microbiological effectiveness to trimethoprim/sulfamethoxazole (160/800 mg twice daily for three days);
3) macrocrystalline nitrofurantoin at a dose of 100 mg four times a day for three days is superior to placebo in the treatment of acute uncomplicated UTI.
We can talk about the high clinical (88–93%) and bacteriological (81–92%) effectiveness of the study drug.
The analyzed randomized studies indicate the high effectiveness of macrocrystalline nitrofurantoin against acute uncomplicated UTI. The course of therapy can be reduced to five days while maintaining clinical and bacteriological effectiveness. Macrocrystalline nitrofurantoin is not registered in Russia.
Trimethoprim/sulfamethoxazole (160/800 mg twice daily for three days)
Trimethoprim/sulfamethoxazole has traditionally been considered the drug of choice for the treatment of acute uncomplicated UTI in the United States. However, due to the sharp increase in Escherichia coli
For this drug (mainly outside America), the indications for its use have been revised.
These recommendations are based on four comparative randomized trials of the effectiveness of trimethoprim/sulfamethoxazole in patients with acute uncomplicated cystitis [25, 27–29].
Two studies compared the effectiveness of trimethoprim/sulfamethoxazole and fluoroquinolones [25, 27]. In both cases, a longer – seven-day – course of trimethoprim/sulfamethoxazole was administered. Trimethoprim/sulfamethoxazole demonstrated comparable early clinical and bacteriological effectiveness, but was inferior to ciprofloxacin in terms of late bacteriological effectiveness [25].
In a study by D. Kavatha et al. The effectiveness of a three-day course of trimethoprim/sulfamethoxazole 160/800 mg twice daily and cefpodoxime proxetil 100 mg twice daily was compared [28]. Clinical efficacy was observed in 100% of cases in the trimethoprim/sulfamethoxazole group and in 92% in the cefpodoxime group. Similar results were obtained for bacteriological efficiency.
M. A. Minassian et al. [29] compared trimethoprim/sulfamethoxazole (160/800 mg twice daily for three days) and macrocrystalline nitrofurantoin (100 mg twice daily for five days). Complete clinical cure after 30 days of therapy was observed in 79% of patients in the trimethoprim/sulfamethoxazole group and in 84% of patients in the nitrofurantoin group. Short-term clinical effectiveness after five to nine days of therapy was equivalent in both groups.
Thus, we can talk about a pronounced clinical effect of this drug, provided Escherichia coli
no higher than 20%. Consequently, its use in Russia is inappropriate due to the excess of the specified resistance value.
Fosfomycin trometamol (3 g once)
Two studies were analyzed that compared the effectiveness of fosfomycin trometamol (3 g once) and macrocrystalline nitrofurantoin (100 mg twice daily for seven days) [26] and trimethoprim (100 mg twice daily for five days) [29 ]. In a study by G. E. Stein et al. [26] confirmed comparable short-term clinical efficacy of fosfomycin and nitrofurantoin (91 and 95%, respectively). Late clinical efficacy was also high and not significantly different (93–94%). Late microbiological efficacy was higher in the fosfomycin group (96%) compared with nitrofurantoin (91%). In a study by M.A. Minassian et al. [29] showed identical short-term bacteriological efficacy (83%) of fosfomycin and trimethoprim.
in vitro studies are available
, demonstrating the activity of fosfomycin against vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and ESBL-producing gram-negative microorganisms [30]. Due to its convenient dosage regimen, as well as high clinical and bacteriological efficacy, fosfomycin is the first choice drug for the treatment of acute uncomplicated UTI in a number of countries, which is confirmed by EAU recommendations.
Pivmecillinam (400 mg twice daily for three to seven days)
This drug is an oral form of mecillinam. Pivmecillinam is active exclusively against gram-negative uropathogens.
Two studies were analyzed [31]. One of them compared the effectiveness of different doses, regimens and durations of taking the drug. Pivmecillinam 200 mg three times daily for seven days, 200 mg twice daily for seven days, and 400 mg twice daily for three days resulted in short-term clinical efficacy in 62, 64, and 55% of cases, respectively, and also to bacteriological effectiveness in 93, 94 and 84% of cases, respectively.
Another randomized trial compared the effectiveness of pivmecillinam and norfloxacin, which were taken according to the same regimen: 400 mg twice daily for three days. In the pivmecillinam group, lower clinical (82 and 88%) and bacteriological efficacy (75 and 91%, respectively) were found compared to the norfloxacin group.
This drug is not available in many countries, including it is not registered in Russia. However, in those regions where it is available, its use against pathogens of acute uncomplicated UTI is justified due to the significant level of clinical and bacteriological effectiveness, as well as the low level of resistance of Escherichia coli
.
Fluoroquinolones
An analysis of 12 randomized studies was conducted that studied the effectiveness of various fluoroquinolone drugs against the causative agent of acute uncomplicated UTTI. The authors of the recommendations concluded that fluoroquinolones remain effective against UTI pathogens, but increasing resistance to these drugs around the world sharply limits their use. That is why fluoroquinolones in Europe today are considered alternative drugs. In Russia, the prescription of this class of antibiotics as first-choice therapy for the treatment of acute uncomplicated UTI is also considered inappropriate.
IDSA recommendations for the treatment of acute uncomplicated UTI are presented in Table. 3.
Conclusion
The choice of antibacterial drug should be based on the severity of clinical symptoms, data on antibiotic resistance in a particular region, and also take into account the patient’s allergy history, availability and cost of drugs. If acute pyelonephritis is suspected (fever, pain in the lumbar region, prolonged presence of symptoms of acute cystitis), it is not recommended to use drugs that accumulate poorly in the kidneys.
Most of the antibiotics recommended by the EAU and IDSA for use as first-choice drugs for the treatment of acute uncomplicated UTI are either not registered in Russia, or their use is impractical due to the high level of resistance. The only available drug that has the required level of sensitivity against the causative agent of UTI is currently fosfomycin trometamol, which leads to its widespread use in our country.