Rengalin oral solution 100 ml fl/pack card x1
RENGALIN
Features and Benefits
INSTRUCTIONS for medical use of the drug
Trade name Rengalin Dosage form: solution for oral administration Composition (per 100 ml) of solution for oral administration Active substances: Antibodies to bradykinin, affinity purified 0.12 g* Antibodies to histamine, affinity purified 0.12 g* Antibodies to morphine, affinity purified 0. 12 g*
Excipients: maltitol 6.0 g, glycerol 3.0 g, potassium sorbate 0.165 g, anhydrous citric acid 0.02 g, purified water up to 100 ml.
* are administered as a mixture of three active aqueous dilutions of the substance, diluted 10012, 10030, 10050 times, respectively. Description Colorless or almost colorless transparent liquid. Pharmacotherapeutic group: Other antitussive drugs. ATC code: R05DB Pharmacological properties Pharmacodynamics It has been experimentally shown that the components of the drug modify the activity of the ligand-receptor interaction of endogenous regulators with the corresponding receptors: antibodies to morphine (a component of the drug) modify the activity of the ligand-receptor interaction of endogenous regulators with opiate receptors, antibodies to histamine - with H1 histamine receptors, antibodies to bradykinin - with bradykinin receptors, while the combined use of the components leads to an increased antitussive effect.
In addition to the antitussive effect, the complex drug, due to its constituent components, has anti-inflammatory, anti-edema, anti-allergic, antispasmodic (antibodies to histamine, antibodies to bradykinin) and analgesic effects (antibodies to morphine).
The complex drug Rengalin, due to the modification of histamine-dependent activation of H1 receptors and bradykinin-dependent activation of B1 and B2 receptors, selectively reduces the excitability of the cough center of the medulla oblongata and inhibits the central parts of the cough reflex. By inhibiting pain sensitivity centers in the thalamus, it blocks the transmission of pain impulses to the cerebral cortex. Inhibits the flow of pain impulses from the periphery due to a decrease in the release of tissue and plasma algogens (histamine, bradykinin, prostaglandins, etc.). Unlike narcotic analgesics, it does not cause respiratory depression, drug dependence, and does not have a narcotic or hypnotic effect.
Alleviates the manifestations of acute pharyngitis, laryngitis and bronchitis, reducing bronchospasm. Relieves systemic and local symptoms of allergic reactions by influencing the synthesis and release of histamine and bradykinin from mast cells.
Pharmacokinetics The sensitivity of modern physicochemical methods of analysis (gas-liquid chromatography, high-performance liquid chromatography, gas chromatography-mass spectrometry) does not allow assessing the content of ultra-low doses of antibodies in biological fluids, organs and tissues, which makes it technically impossible to study the pharmacokinetics of the drug Rengalin.
Indications for use: Acute and chronic diseases of the respiratory tract, accompanied by cough and bronchospasm. Productive and non-productive cough with influenza and ARVI, acute pharyngitis, laryngotracheitis, acute obstructive laryngitis, chronic bronchitis and other infectious-inflammatory and allergic diseases of the upper and lower respiratory tract. Contraindications: Children under 3 years of age. Increased individual sensitivity to the components of the drug. Hereditary fructose intolerance (due to the presence of maltitol). With caution: diabetes mellitus. Use during pregnancy and breastfeeding The safety of Rengalin in pregnant women and during breastfeeding has not been studied. During pregnancy and breastfeeding, the drug is used only if the expected benefit to the mother outweighs the potential risk to the fetus and child. The benefit/risk ratio is determined by the attending physician. Directions for use and dosage: Inside. For one dose – 1-2 teaspoons (5-10 ml) – outside meals. It is advisable to keep the solution in the mouth before swallowing for maximum effect of the drug. Use 1-2 teaspoons 3 times a day. Depending on the severity of the condition in the first three days, the frequency of administration can be increased to 4-6 times a day. The duration of therapy depends on the severity of the disease and is determined by the attending physician. Side effects Possible reactions of increased individual sensitivity to the components of the drug. If these side effects get worse, or you notice any other side effects not listed in the instructions, tell your doctor. Overdose In case of accidental overdose, dyspeptic symptoms (nausea, vomiting, diarrhea) are possible due to the fillers included in the drug (maltitol, glycerol). Treatment is symptomatic. Interaction with other drugs During clinical studies, no data were obtained on the interaction of the drug Rengalin with drugs used as concomitant therapy. Special instructions Patients with diabetes should remember that each teaspoon (5 ml) of the drug contains 0.3 g of maltitol, which corresponds to 0.02 bread units (XU). Insulin is required for the metabolism of maltitol, although due to slow hydrolysis and absorption in the gastrointestinal tract, the need for insulin is low. The energy value of maltitol is 10 kJ or 2.4 kcal/g, which is significantly less than that of sucrose. The energy value of one teaspoon of the drug is approximately 5.7 kJ (1.37 kcal). The effect on the ability to drive vehicles and machinery has not been studied. Release form: Oral solution. 100 ml in bottles made of OS brand glass or painted glass, sealed with tamper evident caps, with a polyethylene dropper. Each bottle, along with instructions for medical use, is placed in a cardboard pack. Storage conditions: At a temperature not exceeding 25 °C. Keep out of the reach of children. Do not freeze. Shelf life: 3 years. Do not use after the expiration date. Conditions for dispensing from pharmacies: Without a prescription. Name, address of the manufacturer of the medicinal product / organization accepting claims LLC NPF MATERIA MEDICA HOLDING, Russia, 127473, Moscow, 3rd Samotechny lane, 9. Address of the place of production of the medicinal product Russia, 454139, Chelyabinsk, st. Buguruslanskaya, 54.
Rengalin tablets d/rassas No. 20
Compound
Active ingredients (per 1 tablet):
- Antibodies to bradykinin, affinity purified – 0.006 g*
- Antibodies to histamine, affinity purified – 0.006 g*
- Antibodies to morphine, affinity purified – 0.006 g*
* applied to isomalt in the form of a mixture of three active aqueous-alcoholic dilutions of the substance, diluted 10012, 10030, 10050 times, respectively.
Excipients: isomalt - 0.506 g, microcrystalline cellulose - 0.0275 g, magnesium stearate - 0.0055 g, anhydrous citric acid - 0.005225 g, colloidal silicon dioxide - 0.00275 g, sodium cyclamate - 0.00275 g, sodium saccharin - 0.000275 g.
Pharmacokinetics
The sensitivity of modern physicochemical methods of analysis (gas-liquid chromatography, high-performance liquid chromatography, gas chromatography-mass spectrometry) does not allow assessing the content of the active ingredients of the drug Rengalin in biological fluids, organs and tissues, which makes it technically impossible to study pharmacokinetics.
Indications for use
Acute and chronic diseases of the respiratory tract, accompanied by cough and bronchospasm. Productive and non-productive cough with influenza and ARVI, acute pharyngitis, laryngotracheitis, acute obstructive laryngitis, chronic bronchitis and other infectious-inflammatory and allergic diseases of the upper and lower respiratory tract.
Contraindications
- Increased individual sensitivity to the components of the drug.
- Rengalin is not recommended for use in children under 3 years of age due to insufficient data on efficacy and safety.
Directions for use and doses
Inside. For one dose - 1 tablet (keep in mouth until completely dissolved). Take 1-2 tablets 3 times a day without meals. Depending on the severity of the condition in the first three days, the frequency of administration can be increased to 4-6 times a day.
Storage conditions
The drug should be stored out of the reach of children, protected from light at a temperature not exceeding 25°C.
Best before date
3 years. Do not use after the expiration date.
special instructions
Before using the drug Rengalin, you should consult your doctor.
Description
Other antitussive drugs.
Use in children
The use of the drug in children under 3 years of age is contraindicated (due to insufficient data on effectiveness and safety).
Pharmacodynamics
It has been experimentally shown that the components of the drug modify the activity of the ligand-receptor interaction of endogenous regulators with the corresponding receptors: antibodies to morphine - with opiate receptors; antibodies to histamine – with histamine H1 receptors; antibodies to bradykinin – with B1 bradykinin receptors; in this case, the combined use of the components leads to an increase in the antitussive effect.
In addition to the antitussive effect, the complex drug, due to its constituent components, has anti-inflammatory, decongestant, antiallergic, antispasmodic (antibodies to histamine, antibodies to bradykinin) and analgesic effects (antibodies to morphine).
The complex drug Rengalin, due to modification of histamine-dependent activation of H1 receptors and bradykinin-dependent activation of B2 receptors, selectively reduces the excitability of the cough center of the medulla oblongata and inhibits the central parts of the cough reflex. By inhibiting pain sensitivity centers in the thalamus, it blocks the transmission of pain impulses to the cerebral cortex. Inhibits the flow of pain impulses from the periphery due to a decrease in the release of tissue and plasma algogens (histamine, bradykinin, prostaglandins and others). Unlike narcotic analgesics, it does not cause respiratory depression, drug dependence, and does not have a narcotic or hypnotic effect.
Alleviates the manifestations of acute pharyngitis, laryngitis and bronchitis, reducing bronchospasm. Relieves systemic and local symptoms of allergic reactions by influencing the synthesis and release of histamine and bradykinin from mast cells.
Side effects
Reactions of increased individual sensitivity to the components of the drug are possible.
Use during pregnancy and breastfeeding
The safety of using the drug Rengalin in pregnant women and during breastfeeding has not been studied. If it is necessary to take the drug, the risk/benefit ratio should be taken into account.
Interaction
To date, no cases of incompatibility with other drugs have been reported.
Overdose
In case of accidental overdose, dyspeptic symptoms are possible due to the fillers included in the drug.
Impact on the ability to drive vehicles and operate machinery
Rengalin does not affect the ability to drive vehicles and other potentially dangerous mechanisms.
Cough treatment drug "Rengalin"
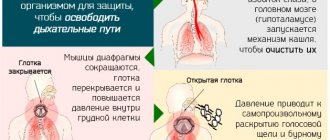
Release-active antibodies to bradykinin, histamine and morphine in Rengalin modify the activity of ligand-receptor interaction with B1 and B2 bradykinin receptors, with H1 histamine receptors and with opiate receptors, exerting a regulatory effect on the central and peripheral parts of the cough reflex: release-active antibodies to bradykinin contribute to the rapid resolution or reduction or reduction of inflammation in the respiratory tract and influence the formation of the cough reflex by suppressing the synthesis and release of bradykinin and relaxing the smooth muscles of the respiratory organs; release-active antibodies to histamine affect the histamine-dependent activation of histamine receptors, reduce vascular permeability, hyperproduction of mucus and reduce swelling of the mucous membrane of the respiratory tract - release-active antibodies to morphine regulate the activity of the cough center through opioid receptors (in particular, µ-receptors), have an inhibitory effect on the pain center and peripheral pain impulses, providing analgesic action (Figure 1).
Figure 1. Active components and pharmacological targets of Rengalin.
The regulatory effect of Rengalin components on specific receptors leads to an increase in the antitussive effect when they are combined in the drug. Since the complex effect of Rengalin is realized due to the combined influence of its components on the main pathogenetic mechanisms of the occurrence and maintenance of cough - the central and peripheral parts of the cough reflex and inflammation in the respiratory tract - the use of the drug is effective in the treatment of any type of cough: dry, wet, residual. Thanks to its regulatory action at the receptor level, Rengalin is effective at any stage of the infectious-inflammatory process during acute respiratory infection (ARI): in the prodrome, acute period and recovery stage. Rengalin has the ability to suppress dry cough associated with receptor hyperreactivity, and in the presence of an inflammatory process, it effectively converts a non-productive cough into a productive one. This feature of the action of Rengalin allows you to solve various therapeutic problems in the treatment of patients with dry/non-productive and wet/productive cough throughout the entire period of ARI (Figure 2).
Figure 2. Features of the action of Rengalin for different types of cough.
Rengalin has virtually no contraindications and is approved for use in children over 3 years of age for acute and chronic respiratory diseases accompanied by cough, including bronchospasm - productive and non-productive cough in acute respiratory viral infections (ARVI), acute pharyngitis, laryngitis, tracheitis, bronchitis, chronic bronchitis and other infectious, inflammatory and allergic diseases of the upper and lower respiratory tract. Rengalin is produced both in tablet form - in the form of lozenges, and in a liquid dosage form (oral solution), specially designed for the treatment of cough in children. It is recommended to use the drug 1-2 tablets sublingually or 1-2 teaspoons (5-10 ml) 3 times a day without meals until recovery with the possibility of increasing the frequency of administration to 4-6 times a day depending on the severity of the patient’s condition (Figure 3).
Figure 3. Rengalin dosage regimen.
When using Rengalin, depending on the severity of cough and the severity of the patient's condition, a differentiated, individualized approach to therapy is recommended. So, in the case of short-term episodes of cough that bother the patient, but do not disturb his sleep - 1 tablet (1 teaspoon, 5 ml) 3 times a day. In case of frequent cough that disrupts the patient’s usual daily activity and sleep - 2 tablets (2 teaspoons, 10 ml) 3 times a day. In the case of a severe, debilitating cough that bothers the patient day and night, for the first 3 days you can increase the number of doses to 6, prescribing 2 tablets (2 teaspoons, 10 ml) per dose, and then switch to a regimen of 2 tablets (2 teaspoons). spoons, 10 ml) 3 times a day. The minimum duration of the course of treatment is 3 days and if the cough has stopped, then the treatment can be completed. If the cough persists, then Rengalin is continued until the cough stops bothering the patient. Rengalin makes cough therapy during ARI comfortable both for the doctor, eliminating the need to select and replace the drug depending on the type of cough and phase of the disease, and for the patient, accelerating recovery, improving quality of life and increasing adherence to therapy. The use of Rengalin allows one to avoid polypharmacy in the treatment of cough in ARI, minimizing the drug load on the body and reducing the cost of treatment. In multicenter randomized studies in children and adults, it has been clinically proven that Rengalin has an antitussive and “cough-optimizing” effect - it reduces its severity and duration, accelerates the transition of a dry cough to a wet one, facilitating its rapid relief, and reduces the risk of bacterial complications (Figure 4).
Figure 4. Clinically proven effects of Rengalin in ARI.
In the article “ Rengalin is a new effective and safe drug in the treatment of cough. Results of a multicenter comparative randomized clinical trial in patients with acute respiratory infections " (A.L. Akopov et al., 2015) presents data from a clinical trial conducted in 9 centers of the Russian Federation. The study conducted a comparative assessment of the effectiveness and safety of Rengalin and the codeine-containing drug Codelac® in the treatment of cough in ARI in adults. All study participants had a dry/nonproductive cough due to ARI (pharyngitis, laryngitis, laryngotracheitis, tracheitis, tracheobronchitis, bronchitis) lasting from 12 hours to 7 days. The results presented in the article prove that a progressive decrease in the intensity of daytime and nighttime cough begins from the first day of using Rengalin, and its antitussive activity is comparable to Codelac® (p <0.025). At the end of the 7-day course of taking Rengalin, the severity of the cough decreased by almost 100%. Nonproductive/dry frequent cough was completely cured in 76% of patients; in the remaining patients of the Rengalin group it persisted in the form of “residual cough” (single short-term episodes of cough during the day). At the same time, Rengalin contributed to the recovery of patients from cough during ARI without the development of secondary bacterial complications (bronchitis, pneumonia). Also, while taking Rengalin, there was an improvement in the quality of life and night sleep with 100% adherence of patients to treatment. Rengalin did not cause respiratory depression, narcotic or hypnotic effects, was well tolerated by patients and was combined with other drugs for the treatment of ARI and underlying pathology. Rengalin is effective throughout the entire period of the infectious-inflammatory process in the respiratory tract and allows you to obtain an antitussive (antitussive) effect in the initial period of acute respiratory infection and a protussive (“cough optimizing”) effect in the subsequent period of the disease. According to doctors, Rengalin has a high efficiency index. It was concluded that the combined composition of Rengalin and the complementary action of its components allows one to achieve the main therapeutic goal for cough against the background of ARI - cure for cough.
The effectiveness and safety of the use of Rengalin in the treatment of cough in ARI in children has been proven. ON THE. Geppe, E.G. Kondurina et al. in the article “ Rengalin is a new drug for the treatment of cough in children. Interim results of a multicenter comparative randomized clinical trial (2014) present the results of the use of the liquid dosage form of Rengalin in the treatment of cough in ARI (rhinitis, pharyngitis, laryngitis, laryngotracheitis, tracheitis) in pediatric practice. In 14 research centers, a randomized clinical study of the safety and effectiveness of Rengalin in the treatment of cough in children aged 3 to 17 years with clinically confirmed ARI (rhinitis, pharyngitis, laryngitis, laryngotracheitis, tracheitis) and dry/non-productive cough lasting from 12 hours to 3 days. This study proved that Rengalin quickly reduces cough, which helps normalize sleep - after 3 days, the proportion of patients in the Rengalin group with significant improvement/recovery according to the results of daytime assessments was 90%, at night - 88% (versus 81 and 88% in the Sinecoda group , respectively). A complete absence of night cough was observed within 3 days from the start of treatment with Rengalin in 52% of patients versus 34% in the Sinecod group (p = 0.0003). It has been proven that Rengalin reduces the need for additional mucolytic therapy - after 3 days of therapy with Rengalin, Ambroxol was prescribed to 66% of patients versus 95% in the Sinecod group (p<0.0001). It has been established that, in addition to cough, Rengalin affects the severity of other clinical manifestations of ARI and prevents its generalization. The severity of the antitussive activity of Rengalin in ARI with cough syndrome was comparable to Sinecode. Along with a high level of safety, according to doctors, Rengalin has a high efficiency index.
In the article by Yu.L. Mizernitsky et al. “ The effectiveness of a combination drug of ultra-low doses of antibodies to inflammatory mediators for dry cough in children with respiratory infections ” (collection “Childhood Pulmonology: Problems and Solutions”, 2014), presents the results of a comparative randomized study of the effectiveness and safety of using the tablet dosage form of Rengalin in children from 3 to 10 years old. Compared with Sinecode®, Rengalin contributed to a faster transition from a non-productive dry cough to a productive wet cough, starting from the 2nd day of therapy (p<0.05). A positive anti-inflammatory effect of Rengalin was also noted, which was confirmed by a more rapid decrease in elevated body temperature, starting from the 2nd day of treatment (p <0.05). During therapy with Rengalin, no additional administration of expectorants or mucolytics was required. With its use, no cases of allergic reactions, bacterial complications, or exacerbations of concomitant chronic pathology have been registered. The combined composition of the drug Rengalin allows you to effectively control cough, despite the clinical differences in its variants - it is effective in the treatment of both dry and productive wet cough in acute respiratory infections, which is due to the effect of its components on various pathogenetic mechanisms of cough syndrome.
In the article by A.B. Malakhov “ Rengalin: a new solution to the problem of cough in acute respiratory infections ” provides data on the features of the mechanism of action of Rengalin. It is known that in ARI, in the first stages of development of the infectious-inflammatory process, the so-called “dry” inflammation with high excitability of cough receptors predominates, which is clinically manifested by an irritating, frequent cough. During this period of the disease, the effect of Rengalin is largely mediated by its effect on opiate receptors. It is worth noting that in the presence of codeine-like action, Rengalin does not have the risk of developing side effects characteristic of centrally acting antitussives - it does not cause respiratory depression, drug dependence, and does not have narcotic or hypnotic effects. Subsequently, when exudative-catarrhal inflammation predominates in the clinical picture of ARI, the regulating effect of Rengalin components on bradykine and histamine receptors becomes more significant. This, in addition to the antitussive, provides an anti-inflammatory, bronchodilator effect, and, accordingly, a reduction in the severity of edema, normalization of sputum discharge, which contributes to the rapid relief of cough.
In a review of the literature on current ideas about the pathogenesis of cough in ARI, “A new solution to the problem of choosing a drug for pathogenetically based treatment of cough in children” (Karpova E.P., 2015), modern approaches to the treatment of cough are considered using the example of a new and innovative technology production of the drug Rengalin. Data are presented on the results of experimental and clinical studies confirming that Rengalin regulates the reflex and inflammatory mechanisms of cough in ARI. Thanks to the combined composition and the modulating effect of its components on bradykinin, histamine and opiate receptors, Rengalin has an antitussive, anti-inflammatory and bronchodilator effect and is effective for any cough - dry, wet, residual - at all stages of the infectious-inflammatory process. The article concludes that Rengalin has the optimal set of properties necessary to successfully combat cough in children. And the proven high effectiveness and safety of Rengalin allow it to be recommended for widespread use in ARI with cough syndrome in children.
The mechanism of action of Rengalin has been deciphered and proven in preclinical studies in Russia and abroad. The pronounced antitussive effect of Rengalin has been proven in various standard models of cough, including capsaicin-induced - significant from the point of view of the drug’s effect on chemoreceptors (C-fibers) that respond to inflammatory stimuli (for example, bradykinin, histamine), and induced by citric acid, to a greater extent reflecting the effect of the drug on mechanoreceptors (A-fibers) that respond to irritants (for example, sputum). Published scientific data, including the results of studies by Dugina JL et al. (2011), Churina A.A. et al. (2013) ( “Assessment of the efficacy of a novel antitussive drug in capsaicin-induced cough model in guinea pigs” and “Study of the antitussive properties of the drug rengalin and its components in a model of cough caused by aerosol administration of capsaicin in guinea pigs” ), indicate proven in the course of a comparative blind experimental study, the pronounced antitussive effect of Rengalin. Moreover, the severity of the effect of Rengalin was not inferior and even exceeded the comparison drug butamirate citrate.
In publications of the results of research carried out at the Research Institute of Immunology (Moscow) under the leadership of Shilovsky I.P. and Khaitova M.R., “Therapeutic effectiveness of rengalin in an experimental model of allergic bronchial asthma in mice” (Babakhin A.A. et al., 2013) , “New opportunities in the treatment of bronchial asthma using drugs containing release-active antibodies" (Sekirina M.A. et al., 2013) and "Management of allergen-induced bronchial asthma and virus-induced asthma exacerbation using release-active rabbit polyclonal antibodies" (Myslivetc M. et al., 2014 ) provides evidence of the anti-inflammatory and bronchodilator effects of Rengalin. With its use, the severity of inflammation in the bronchi decreased, the number of alveolar macrophages and neutrophils in bronchial lavages decreased, relative eosinophilia and lymphocytosis in the peripheral blood decreased, and the results of a histomorphological assessment of general inflammation in the lungs indicated its decrease by 50% compared to the control group. Rengalin also reduces the amount of IgG in the blood serum and does not cause an increase in IgE levels. The bronchodilator effect of the drug was confirmed in experimental conditions using a model of ovalbumin-induced bronchial asthma - the use of Rengalin promotes a statistically significant reduction in bronchial hyperreactivity comparable to the effects of dexamethasone and desloratadine compared to the group without treatment.