One of the most common sleep disorders (SS) is insomnia (IS). According to the International Classification of Sleep Disorders, it is characterized as “recurrent disturbances in the initiation, duration, consolidation or quality of sleep, occurring despite the availability of sufficient time and conditions for sleep” [1–3].
The incidence of ID in children is significant and ranges from 30 to 56%. As a rule, the largest percentage occurs at an early age of up to 3 years, which is associated with the immaturity of multiple hypnogenic centers in the central nervous system and their frequent suffering in the perinatal period [2]. Lack of sleep manifests itself in rapid fatigue during the day, decreased activity and performance, impaired growth and development of children and deterioration in the quality of life of their parents. In rare cases, prolonged and severe sleep disorders can lead to more serious consequences [4–6].
The search for medications that level the nervous system is relevant, especially in young children. Drugs registered in Russia (phenibut, nervohel, persen) are not indicated for use in children under 2 years of age [2, 7, 8]. Therefore, the drug Dormikind, which is approved for use in children from the neonatal period, attracts a lot of attention. Dormikind has established itself as the first choice drug for the correction of ID in young children [9].
Dormikind contains a complex of active components of plant and mineral origin in low pharmacological concentrations: Cypripedium pubescens
(Slipper fluffy, Orchidaceae family),
Magnesium carbonicum
(magnesium carbonate),
Zincum valerianicum
(zinc valerianate). Each of the active components has clear indications for use1 and provides complementary effects.
The purpose of this study is to obtain additional information about the effectiveness of Dominide NS therapy in young children.
pharmachologic effect
Kindinorm N is a homeopathic medicine whose action is aimed at eliminating behavioral and cognitive disorders in children and adults.
The active ingredients of the drug effectively relieve typical symptoms of a nervous disorder , such as impaired concentration, increased excitability, anxiety , problems with assimilation of information, sleep disorders and a general state of weakness.
In addition, the drug has a sedative and stabilizing effect on the patient’s mental state.
Hamomilla pharmaceutical D12 - eliminates anxiety and dissatisfaction, calms and promotes good sleep.
Potassium phosphate D6 - eliminates nervous exhaustion , helps treat sleep disorders (night terrors, sleepwalking ), helps normalize brain activity, stimulating the ability to absorb information.
Delphinium staphysagria D12 - helps treat physical diseases caused by nervous disorders, has a calming effect.
Valerian D6 - has a calming effect (cumulative effect), eliminates symptoms of psychological hypersensitivity, normalizes mood and psychological perception of the surrounding reality.
results
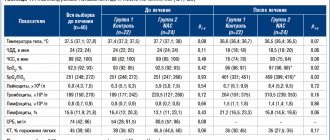
NA and difficulty falling asleep were the only complaints of participants upon inclusion in the study. In group 1, 28 (43.7%) children were diagnosed with acute insomnia, and 36 (56.3%) - chronic. In group 2 - in 46 and 54% of children, respectively.
Before the start of therapy, the assessment of deviations in the emotional-behavioral sphere in the 1st group was 3.8±0.7 points, in children of the 2nd group - 3.5±0.9 points, without identifying significant intergroup differences. Minor neurological changes in the form of increased muscle tone were noted in 15 (23.4%) children in group 1 and in 13 (26%) in group 2.
The duration of night sleep in children of the 1st group was 9.3±0.5 hours, in the 2nd group - 10.13±0.5, awakenings during sleep were observed 3.7±0.8 times versus 3.3± 0.9 times 2nd group. The duration of falling asleep in group 1 was 44.2±3.1 minutes, and night wakefulness was 2.2±0.6 hours. In group 2, the corresponding indicators were 38.2±4.6 minutes and 1.9 ±0.6 h.
In group 1, 8 (13%) children fell asleep on their own in bed, 26 (41%) were rocked in their arms, and 16 (25%) fell asleep and slept in bed with their parents. In group 2 the picture was similar (Table 2).
Table 2. Characteristics of falling asleep before treatment according to the BISQ questionnaire, n (%) Note. * - several answers were possible.
According to neurosonography, mild ventriculomegaly was detected in 15.6% of patients in group 1 and in 14% in group 2. Also, according to EEG data, there was an increase in the duration of the process of falling asleep (up to 30-50 minutes or more), an increase in the number of fragmentary and complete awakenings during the process of falling asleep and staying asleep, a violation of the depth of sleep with a predominance of superficial stages (1st and 2nd stages of the slow-wave sleep phase - FMS); reduction in sleep duration (due to prolonged falling asleep and frequent awakenings) with the absence, in most examinations, of the paradoxical sleep phase and rapid awakening with motor and emotional restlessness of the child; frequent detection of benign sleep myoclonus, including subsequent fragmentary and complete awakenings and restlessness of the child.
After 14 days of treatment (2nd visit), the average assessment of deviations in the emotional-behavioral sphere decreased and amounted to 2.4±0.7 points for group 1 (hereinafter - compared with the initial results; t
-Wilcoxon test,
p
<0.001), for group 2 - 3.3±0.8 points,
p
=0.062.
The noted decrease in the indicator in patients of the 1st group significantly exceeded the dynamics in the 2nd group: 1.4 ± 0.9 points and 0.2 ± 0.7 points, respectively (hereinafter - for intergroup comparisons; U
- test,
p
<0.0001).
The average score on the IMOS scale in group 1 was recorded at 3.1±0.8 points versus 2.1±0.8 points in group 2 ( p
<0.001). In group 1, 22 (34%) patients showed a significant improvement in sleep quality and condition, 25 (39%) children showed improvement, and 17 (27%) children showed no improvement. In the 2nd period, no recovery or significant improvement was observed in any child, in 18 (36%) there was a slight positive change, in 20 (40%) there were no changes, in 12 (24%) children falling asleep worsened, the frequency of awakenings increased , sleep became more superficial.
Tolerability of therapy in group 1 was rated as very good in 12 (19%) cases, as good in 48 (75%) cases, however, parents were dissatisfied with the drug regimen; mild symptoms were reported in 4 (6%) patients. excitability in the first 3 days of use, which did not require discontinuation of the drug. Parents of children in group 2 reported good tolerance of behavioral therapy in 7 (14%) cases, which was significantly less than in group 1 (χ2 criterion, p <
0.001), satisfactory tolerability - in 26 (52%) children, 17 (34%) children had a deterioration in sleep quality, which required additional instructions on compliance with all requirements of behavioral therapy.
After 14 days, 12.5% of parents were completely satisfied with the results of treatment in group 1, while in group 2 there were no excellent ratings. In group 1, treatment satisfaction of 4 points was recorded in 48.4% of cases and in group 2 - in 18% (χ2 criterion, p
<0.001). A neutral assessment was given by 20.3% of parents of patients in group 1 and 36% in group 2; in 19 and 32% of cases (groups 1 and 2, respectively) parents were dissatisfied with the treatment (in addition, 14% parents of children in group 2 expressed an extremely negative assessment of behavioral therapy, which may have been due to their personal and characterological characteristics).
At the 3rd visit, positive dynamics were revealed in both groups. The average values for assessing deviations in the emotional-behavioral sphere compared to the initial data decreased to 1.3±0.7 points in group 1 ( p
<0.001) and up to 2.8±0.7 points in group 2 (
p
<0.001).
Differences between groups were statistically significant ( p
< 0.001). No significant changes in neurological and somatic status were recorded.
The average sleep duration in group 1 increased to 10.8±0.8 hours ( p
<0.001), the number of awakenings decreased to 1.8±0.7 times (
p
<0.001), and the duration of night wakefulness decreased to 0.9±0.7 hours (
p
<0.001).
The same indicators in children of the 2nd group were 10.6±0.6 hours, 2.8±0.7 times, 1.4±0.5 hours. The duration of night wakefulness was significantly different from the initial level ( p
<0.001) .
The period of falling asleep decreased in both groups. In group 1, the mean values decreased to 26.2±4.8 min ( p
<0.001), in group 2 - up to 33.4±3.0 min (
p
<0.001).
Moreover, the intergroup difference was significant ( p
<0.001). The percentage change in the average values of the discussed indicators compared to the base ones is shown in Fig. 1.
Rice.
1. Changes (in%) in the average values of the assessed sleep indicators before and after treatment (full course 28 days). 1 — increase in the duration of night sleep; 2 — reduction in the number of night awakenings; 3 — reduction in the duration of night wakefulness; 4 - reduction in the duration of the period of falling asleep. In addition, other characteristics of falling asleep also changed (Table 3).
Table 3. Dynamics of falling asleep in children during treatment, n (%) Note. * — intragroup differences (before and after treatment) are significant (Wilcoxon t-test, p<0.001); ** — intergroup differences are significant (U-test, p<0.001); # — intergroup differences in frequencies are significant (F-test, p=0.004); ## — intergroup differences in frequencies are significant (χ2 test, p=0.009). Thus, in group 1, the proportion of children who fell asleep while feeding reached 14%, the proportion of children who fell asleep on their own (alone in the room or alone in bed) increased to 19%; The share of those who were rocked in bed by their parents before bed also increased to 50%. At the same time, the frequency of cases of children rocking in their arms, as well as falling asleep in bed with their parents (from 25 to 13%) decreased significantly and statistically (from 41 to 5%). In group 2, positive dynamics were also noted, but no statistically significant changes were observed.
Thus, in children of group 1, during therapy, the duration of falling asleep decreased by 1.7 times (versus 1.1 times in group 2), the proportion of children falling asleep in their arms decreased by 8.7 times (by 1.3 times in the 2nd group) and 2 times in the parents’ bed (1.5 times in the 2nd group). Against this background, the number of children who successfully fell asleep while rocking in their beds increased by 3.2 times (compared to 1.2 times in group 2). The duration of night sleep increased on average by 1.5 hours (0.5 hours in group 2). Significant dynamics were noted in the emotional and behavioral state of children after therapy: irritability, excitability, fatigue, anxiety decreased, the total assessment score significantly improved by almost 3 times (1.3 times in group 2; p
<0,01).
Average IMOS score (Fig. 2)
Rice.
2. Distribution of various options for assessments on the IMOS scale after completion of treatment (full course 28 days). 1 - complete recovery; 2 - significant improvement; 3 - improvement; 4 - no dynamics; 5 - deterioration of condition. *—differences are significant (p<0.01). after the end of treatment was 4.2±0.7 points in group 1 versus 2.6±0.7 points in group 2 ( p
<0.001).
According to frequency analysis data (see Fig. 2), in group 1, 20 (31%) children, according to doctors, achieved complete recovery, 37 (58%) had a significant improvement, condition 4 (6%) patients were characterized as improvement, only 3 (5%) patients showed no dynamics. In the 2nd group, complete recovery, according to medical assessment, was not achieved: in 5 (10%) children a significant improvement was noted, in 23 (46%) there was an improvement, in 19 (38%) patients no dynamics were observed. In addition, in 3 (6%) patients of group 2, doctors noted a slight deterioration during behavioral therapy. By the time of completion of treatment in group 1, the proportion of children with recovery or significant improvement was 89% versus 10% in group 2 ( F
-test,
p
<0.001).
According to EEG data, the following changes were noted in patients: a decrease in the duration of the process of falling asleep (less than 15-20 minutes) with a faster achievement of the stage of superficial sleep (in 84 and 40% of children in groups 1 and 2, respectively); reduction in the number of fragmentary and complete awakenings (in some cases 2 times or more) and the child’s restlessness with the achievement, in some cases, of deep stages of sleep (usually the 3rd stage of FMS) - 45.3% in children of the 1st group and 18 % in group 2.
According to neurosonography with Doppler sonography, in 28.1% of children, a reduction in changes in the anterior cerebral artery and normalization of blood flow velocity in the vein of Galen were detected.
At the final assessment on the IMPSS integral scale (Fig. 3)
Rice.
3. Distribution of scores on the IMPSS scale after completion of treatment (full course 28 days). 1 - extreme dissatisfaction; 2 - dissatisfaction; 3 - neutral; 4 - satisfaction; 5 - complete satisfaction. *—differences are significant (p<0.01). 70% of parents of patients in group 1 expressed complete satisfaction with the therapy (versus 12% of parents in group 2); 40% of parents of children in group 1 (16% in group 2) were satisfied with the treatment; A neutral assessment was given by 6% of parents from group 1 and approximately 4% from group 2. A significant proportion of parents of children in group 2 noted that they were dissatisfied with the treatment, with 8% being very satisfied. In group 1, the proportion of parents who rated the treatment unsatisfactorily was 8%. The average value on the IMPSS scale in group 1 was statistically significantly higher than in group 2: 4.5±0.9 points and 3.1±1.3 points, respectively ( p
<0.001).
The average treatment tolerability in group 1 was 3.3±0.5 points versus 1.9±0.7 points in group 2 ( p
<0.001).
In group 1, 38% of parents rated the tolerability of therapy as excellent (in group 2, the maximum rating was not recorded), the rating “good” was received from 59 and 20% of parents in groups 1 and 2, respectively; Tolerability of therapy was assessed as 3 points (satisfactory) by 3% of parents of patients in group 1 and ½ of parents from group 2. 30% of parents of patients in group 2 were dissatisfied with the tolerability of behavioral therapy, which should be considered not in terms of the development of adverse events, but due to difficulties in its implementation. In general, excellent and good tolerability of IN treatment in group 1 was significantly more common: in 62 (97%) children compared to group 2 - in 10 (20%) children ( p
<0.001).
Instructions for use of Kindinorm (Method and dosage)
The instructions for Kindinorm for children indicate that the drug is prescribed for the treatment of patients aged one to twelve years and older.
For children aged from one to five years, the drug is prescribed two granules one to three times a day.
For children aged six to eleven years, Kindinorm is prescribed three granules one to three times a day.
For adults and children twelve years of age and older, the medicine is prescribed five granules one to three times a day.
After the patient's condition improves, the frequency of taking the medication can be reduced. It is better to take the granules half an hour before or after a meal. It is recommended to dissolve the granules rather than write them down with water.
To treat children under three years of age, the granules must be dissolved in a small amount of water (a teaspoon of liquid is enough).
The course of treatment is determined by the doctor individually, but in general treatment lasts no more than eight weeks. If there is no improvement or worsening of the patient’s condition, after two months of treatment, it is necessary to consult a doctor.
Special instructions for the use of the drug Kindinorm
When using homeopathic medicines, a temporary initial deterioration is possible. In this case, you should reduce the frequency and dosage of the drug and, after regression of undesirable symptoms, resume taking the drug according to the instructions. In case of severe primary deterioration, accompanied by poor tolerability of the drug, its use should be discontinued. Do not use the drug in children under 1 year of age due to the lack of clinical studies. In accordance with generally accepted principles, the drug can be used during pregnancy and lactation only after assessing the ratio of the expected benefit to the mother and the possible risk to the fetus/child.
Kindinorm's analogs
The drug Kindinorm has a fairly large number of analogues that have a similar effect:
- Notta;
- Engystol;
- Job the Kid;
- Viburcol;
- Stodal;
- Calm down;
- Cinnabsin;
- Gastrokind;
- Immunokind;
- Enterokind;
- Angin-Heel S;
- Vertigohel;
- Thyroidea Compositum;
- Tsinabsin;
- Euphorbium;
- Homeovox;
- Salvia;
- Dentokind;
- Dormikind;
- Dantinorm Baby;
- Mulimen;
- Rhinital;
- Mastodinon;
- Cyclodion;
- Atma;
- Nervochel;
- Voqara.
Reviews about Kindinorm
Parents' reviews of Kindinorm are unanimous. According to mothers and fathers who gave their children this homeopathic medicine, persuading the baby to take it is a matter of a couple of seconds. Children associate the pleasant sweet taste of the medicine with a treat, so even a long course of treatment goes without problems.
Reviews about Kindinorm for children from the children themselves, who are already able to express their thoughts coherently, again come down to a pleasant taste sensation.
Many children over 6-7 years of age noted that they did not like taking conventional medications because of the unpleasant bitter taste and the inconvenient form of release (children generally have a negative attitude toward pills). And Kindinorm granules taste pleasant and do not cause any inconvenience when absorbed.
Reviews from doctors about Kindinorm, although in most cases are positive, are not always so colorful. Experts warn parents against treating children with the drug “on the advice of friends,” since Kindinorm is a medicine and the fact that it is homeopathic does not eliminate the possibility of developing unwanted reactions.
Material and methods
An open, prospective, randomized, comparative, controlled clinical trial was conducted at the Consultative and Diagnostic Center of Sechenov University and the Moscow Children's State Clinic No. 120 from November 2016 to October 2022.
Inclusion criteria
Patients included in the study were: age (from 6 months to 2 years 6 months inclusive), diagnosed with NA or difficulty falling asleep, written consent of the child’s representatives to participate.
Non-inclusion criteria:
severe neurological and (or) somatic disorders, inability of the child’s representatives to comply with the conditions of the Study Protocol.
We examined 114 children (58% boys and 42% girls), who were divided into two groups using the simple randomization method. Group 1 included 64 children (average age 13.4±6.9 months), group 2 included 50 children (average age 13.1±6.6 months). All children had practically normal physical development and indicators of somatic health (Quetelet II index in the 1st group was 16.2±1.3 kg/m2; in the 2nd group - 15.8±1.4 kg /m2).
Patients of the 1st group were prescribed Dormikind (DHU - Deutsche Homoeopathie-Union, Germany) 1 tablet 4 times a day with meals for 28 days (the tablet was first dissolved in 20 ml of water). Children of the 2nd group received behavioral therapy for NS (method of gradual “extinction” [10])2.
All patients were examined 3 times: before the start of treatment (1st visit), on the 14th day (2nd visit) and on the 28th day (3rd visit) of treatment.
The effectiveness of therapy was assessed taking into account the characteristics of the emotional-behavioral sphere (Table 1),
Table 1. Scale for assessing the emotional-behavioral sphere of quality characteristics of sleep/falling asleep according to the BISQ questionnaire 4 and the patient’s diary. The change in the average values of the assessed parameters after treatment was determined as a percentage relative to the initial values. In addition, the effectiveness of therapy was taken into account on the IMOS5 scale, and the degree of satisfaction with therapy - on the IMPSS6 scale. Tolerability of treatment was assessed using a 4-point scale (very good tolerability - 4 points, unsatisfactory - 1 point). These indicators were compared at the 2nd and 3rd visits.
At the 1st and 3rd visits, all participants underwent electroencephalography (EEG) in a state of wakefulness and sleep (Encephalan-131−01 device); in the presence of an open fontanel, neurosonography was performed.
Statistical analysis and processing of results were performed using Statistica 10.0 (Statsoft Inc., USA). Differences were considered significant at p
≤0.05.
The significance of the differences was assessed using
the Mann-Whitney U
Kindinorm price, where to buy
The price of the drug Kindinorm, unlike many analogues of homeopathic remedies, is accessible to all social strata of the population.
The average cost of pellets varies from 309 to 465 rubles and depends on the individual sales conditions established by the seller.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Kindinorm granules homeopath.
10gDeutsche Homoopathie Union RUB 615 order
Pharmacy Dialogue
- Kindinorm (gran. 10g)DHU
RUR 638 order
show more
Pharmacy24
- Kindinorm N 10 g granules Deutsche Homeopathy-Union DKHU-Arznaimittel GmbH & Co.KG, Nimechchina
147 UAH.order
PaniPharmacy
- Kindinorm H Kindinorm N gran.10g Germany, DHU
168 UAH order
show more