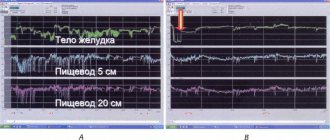
AMOXYCLAVE por. d/prig. susp. 400+57 mg/5 ml vial. 140 ml
Directions for use and doses
Amoxiclav powder for the preparation of a suspension for oral administration is prescribed orally.
The daily dose of suspensions is 125+31.25 mg/5 ml and 250+62.5 mg/5 ml (to facilitate correct dosing, a dosage pipette is inserted into each package of suspensions 125+31.25 mg/5 ml and 250+62.5 mg/5 ml, graduated at 5 ml, with a scale of division of 0.1 ml or a dosing spoon with a capacity of 5 ml, with ring marks in the cavity at 2.5 and 5 ml). Newborns and children up to 3 months - 30 mg/kg/day (for amoxicillin), divided into 2 doses (every 12 hours).
Children over 3 months - from 20 mg/kg for mild and moderate infections to 40 mg/kg for severe infections and lower respiratory tract infections, otitis media, sinusitis (amoxicillin) per day, divided into 3 doses (every 8 h).
The daily dose of Amoxiclav suspension 400 mg + 57 mg/5 ml is calculated per kg of body weight, depending on the severity of the infection. From 25 mg/kg - for mild and moderate infections to 45 mg/kg - for severe infections and lower respiratory tract infections, otitis media, sinusitis (in terms of amoxicillin) per day, divided into 2 doses. To facilitate correct dosing, a dosage pipette is inserted into each package of the 400 mg + 57 mg/5 ml suspension, graduated simultaneously into 1, 2, 3, 4, 5 ml and into 4 equal parts. Suspension 400 mg+57 mg/5 ml is used in children over 3 months.
Exact daily doses are calculated based on the child's body weight.
The maximum daily dose of amoxicillin is 6 g for adults and 45 mg/kg for children.
The maximum daily dose of clavulanic acid (in the form of potassium salt) is 600 mg for adults and 10 mg/kg for children.
In patients with impaired renal function, the dose should be adjusted based on the maximum recommended dose of amoxicillin.
Patients with creatinine Cl >30 ml/min do not require any dose adjustment.
Adults and children weighing more than 40 kg (the indicated dosage regimen is used for moderate and severe infections)
For patients with creatinine Cl 10-30 ml/min - 500/125 mg 2 times a day.
With Cl creatinine
For patients on hemodialysis, the recommended dose is 500/125 mg every 24 hours, plus 500/125 mg during dialysis and another dose at the end of dialysis (since serum concentrations of amoxicillin and clavulanic acid are reduced).
Children weighing less than 40 kg
With creatinine Cl 10-30 ml/min, the recommended dose is 15/3.75 mg/kg 2 times a day (maximum 500/125 mg 2 times a day).
With Cl creatinine
For hemodialysis, the recommended dose is 15/3.75 mg/kg once a day. Before hemodialysis - 15/3.75 mg/kg. To restore appropriate concentrations of the drug in the blood, it is necessary to take another dose of 15/3.75 mg/kg after hemodialysis.
The course of treatment is Amoxiclav for 5-14 days. The duration of treatment is determined by the attending physician. Treatment should not continue for more than 14 days without repeated medical examination.
Amoxiclav powder for the preparation of solution for intravenous administration
Children: with body weight less than 40 kg - the dose is calculated depending on body weight.
Under 3 months with body weight less than 4 kg - 30 mg/kg (in terms of the entire drug AmoxiclavR) every 12 hours.
Under 3 months with a body weight of more than 4 kg - 30 mg/kg (in terms of the entire drug AmoxiclavR) every 8 hours.
In children under 3 months of age, AmoxiclavR should be administered only by slow infusion over 30-40 minutes.
Children from 3 months to 12 years - 30 mg/kg (in terms of the entire drug AmoxiclavR) with an interval of 8 hours, in case of severe infection - with an interval of 6 hours.
Children with impaired renal function
Dose adjustments are based on the maximum recommended dose of amoxicillin. For patients with creatinine Cl levels above 30 ml/min, dose adjustment is not necessary.
Adults and children over 12 years of age or weighing more than 40 kg - 1.2 g of the drug (1000+200 mg) with an interval of 8 hours, in case of severe infection - with an interval of 6 hours.
Prophylactic doses for surgical interventions: 1.2 g during induction anesthesia (if the operation lasts less than 2 hours). For longer operations - 1.2 g up to 4 times a day.
For patients with renal insufficiency, the dose and/or interval between doses of the drug should be adjusted depending on the degree of insufficiency
The course of treatment is 5-14 days. The duration of treatment is determined by the attending physician. When the severity of symptoms decreases, it is recommended to switch to oral forms of Amoxiclav to continue therapy.
pharmachologic effect
Amoxicillin is the active substance included in Amoxiclav 400 mg + 57 mg, a semi-synthetic broad-spectrum antibiotic with activity against many gram-positive/gram-negative microorganisms. At the same time, amoxicillin is susceptible to destruction by beta-lactamases, so the spectrum of activity of amoxicillin does not extend to microorganisms that produce this enzyme.
Clavulanic acid is an active substance included in Amoxiclav 400 mg + 57 mg, a beta-lactamase inhibitor, structurally related to penicillins, has the ability to inactivate a wide range of beta-lactamases found in microorganisms resistant to penicillins and cephalosporins. Clavulanic acid is sufficiently effective against plasmid beta-lactamases, which most often cause bacterial resistance, but is not effective against type I chromosomal beta-lactamases, which are not inhibited by clavulanic acid.
The presence of clavulanic acid in the Amoxiclav 400 mg + 57 mg suspension protects amoxicillin from destruction by enzymes - beta-lactamases, which allows expanding the antibacterial spectrum of amoxicillin.
The following microorganisms are sensitive to Amoxiclav 400 mg + 57 mg:
- Gram-positive aerobes: Streptococcus agalactiae (1, 2), Listeria monocytogenes, Bacillus anthracis, Nocardia asteroids, Enterococcus faecalis, Streptococcus pyogenes (1, 2), methicillin-sensitive Staphylococcus aureus, methicillin-sensitive coagulase-negative Staphylococcus spp., Staphylococcus saprophyticus, other beta -hemolytic streptococci Streptococcus spp. (12);
- Gram-positive anaerobes: Clostridium spp., Peptostreptococcus magnus, Peptostreptococcus spp., Peptococcus niger, Peptostreptococcus micros;
- Gram-negative aerobes: Neisseria gonorrhoeae, Haemophilus influenzael, Vibrio cholerae, Pasteurella multocida, Moraxella catarrhalisl (Branhamella catarrhalis), Helicobacter pylori, Bordetella pertussis;
- Gram-negative anaerobes: Porphyromonas spp., Capnocytophaga spp., Prevotella spp., Eikenella corrodens, Bacteroides spp. (including Bacteroides fragilis), Fusobacterium spp., Fusobacterium nucleatum;
- Other: Leptospira icterohaemorrhagiae, Treponema pallidum, Borrelia burgdorferi.
For the following microorganisms, acquired resistance to the drug Amoxiclav 400 mg + 57 mg is likely:
- Gram-negative aerobes: Proteus spp. (including Proteus vulgaris and Proteus mirabilis), Escherichia coli (1), Salmonella spp., Klebsiella spp. (including Klebsiella pneumoniae (1) and Klebsiella oxytoca), Shigella spp.;
- Gram-positive aerobes: Enterococcus faecium, Streptococcus pneumonia (1, 2), Corynebacterium spp., Streptococcus spp. groups
The following microorganisms are naturally resistant to the action of amoxicillin in combination with clavulanic acid:
- Gram-negative aerobes: Stenotrophomonas maltophilia, Pseudomonas spp., Enterobacter spp., Yersinia enterocolitica, Legionella pneumophila, Hafnia alvei, Citrobacter freundii, Serratia spp., Providencia spp., Morganella morganii, Acinetobacter spp.;
- Other: Mycoplasma spp., Chlamydia psittaci, Chlamydia spp., Coxiella burnetii, Chlamydia pneumoniae.
Notes:
1) For these bacteria, clinical studies have established the effectiveness of using Amoxiclav 400 mg + 57 mg.
2) Strains of these types of microorganisms do not produce beta-lactamases and are sensitive to amoxicillin, and therefore, presumably, to the combination of amoxicillin + clavulanic acid.
Drug interactions
The simultaneous use of probenecid and Amoxiclav 400 mg + 57 mg is not recommended. Probenecid reduces renal tubular secretion of amoxicillin.
The use of allopurinol during treatment with amoxicillin may increase the likelihood of allergic reactions.
An antibiotic can affect intestinal flora.
The drug Amoxiclav 400 mg + 57 mgm should not be used together with bacteriostatic chemotherapeutic agents, antibiotics (chloramphenicol, macrolides, tetracyclines or sulfonamides).
The simultaneous use of Amoxiclav with methotrexate may increase the toxicity of the latter (leukopenia, thrombocytopenia, formation of skin ulcers).
Analogs
Analogs of the Amoxiclav 400 mg + 57 mg suspension contain a combination of amoxicillin/clavulanic acid. Preparations with one of the components do not have a wide spectrum of action of the drug. An absolute analogue is Augmentin.
Cheap analogues with the same active ingredient:
- Amoxicillin;
- Amosin;
Substitutes with a similar effect, but a different active ingredient:
- Azithromycin;
- Flemoxin Solutab;
- Sumamed;
- Suprax;
Use in children
The use of Amoxiclav 400 mg + 57 mg is possible according to indications in compliance with the dosage regimen.
The bottle with the powder must be shaken vigorously, water must be added in two additions (up to the mark) to achieve complete dissolution of the powder.
| Volume of finished suspension | Required amount of water |
| 35 ml | 29.5 ml |
| 50 ml | 42 ml |
| 70 ml | 59 ml |
| 140 ml | 118 ml |
The suspension should be shaken well before use.
To prepare the suspension, the powder is diluted with boiled water at room temperature.
The prepared suspension of Amoxiclav 400 mg + 57 mg is stored in the refrigerator.
It is not recommended to heat the drug before use; room temperature is sufficient.
After administration, the dosing pipette is washed with boiled water.
Possible side effects
In children, the described side effects are extremely rare. Liquid Amoxiclav 400 mg + 57 mg is well tolerated by children, sometimes children experience stomach upset or skin reactions that go away after stopping the antibiotic.
From the digestive system:
- loss of appetite, nausea, vomiting, diarrhea;
- rarely - stomach pain, liver dysfunction, increased activity of liver enzymes (ALT or AST);
- cholestatic jaundice, hepatitis, pseudomembranous colitis.
Allergic reactions:
- itching, urticaria, erythematous rash;
- rarely - exudative erythema multiforme, angioedema, anaphylactic shock, allergic vasculitis;
- rarely - exfoliative dermatitis, Stevens-Johnson syndrome, acute generalized exanthematous pustulosis.
From the hematopoietic system, lymphatic system:
- rarely - reversible leukopenia (including neutropenia), thrombocytopenia;
- very rarely - hemolytic anemia, reversible increase in prothrombin time (when used together with anticoagulants), eosinophilia, pancytopenia.
From the nervous system:
- dizziness, headache;
- very rarely - convulsions (may occur in patients with impaired renal function when taking high doses of Amoxiclav 400 mg + 57 mg), hyperactivity, anxiety, insomnia.
From the urinary system:
- very rarely - interstitial nephritis, crystalluria.
Other:
- rarely - development of superinfection, candidiasis.
Use during pregnancy and breastfeeding
The use of Amoxiclav 400 mg + 57 mg may be associated with an increased risk of developing necrotizing enterocolitis in newborns. The use of Amoxiclav should be avoided during pregnancy, especially in the first trimester, unless the benefits of use outweigh the potential risks.
The active components of Amoxiclav 400 mg + 57 mg pass into breast milk, the baby may experience diarrhea, as well as fungal infection of the mucous membranes. Use during breastfeeding is only possible after a thorough risk assessment.