Prevalence, etiology and general treatment approaches
Infections of the upper respiratory tract (URT) and ENT organs are among the most common diseases. In the structure of overall morbidity in Moscow, they account for approximately 19% and are in second place among all pathologies [1].
Approximately 5–15% of adults and 5% of children suffer from some form of sinusitis [1]. In Russia, about 10 million people suffer from acute sinusitis annually [2], and at least 80% of them are people of working age [3]. It is also one of the most widespread diseases in children.
The share of acute otitis media in the structure of diseases of the ENT organs accounts for 20–30% [4, 5]. In Europe, its incidence in the adult population is 1–5% per year [6]. In the United States, acute otitis media occurs annually in 10 million people [5], affecting about 14% of the population [7]. During the first year of life, 62% of children experience at least one episode of the disease; by the age of two to three years – 80–94% [8, 9]. Acute otitis media occupies a leading position in the structure of seeking medical care in pediatrics, accounting for 33% of all doctor visits [8,9].
The main clinical manifestation of tonsillopharyngitis is a sore throat, which is observed in an adult on average two to three times a year [10]. Pharyngitis caused by group A beta-hemolytic streptococcus is one of the most common bacterial infections in children under two years of age. Chronic tonsillitis occurs in 2–3% of children aged 3 years, 6.5% – 5–6 years old, 12–13% – 10–12 years old, and 25–35% of people aged 18–20 years [11]. Its prevalence among frequently ill children aged 2–6 years reaches 43% [12].
Infections of the upper respiratory tract and ENT organs are potentially dangerous in terms of the development of serious complications and deaths, the risk of which increases significantly in the case of irrational treatment of the primary disease. Thus, tonsillitis and pharyngitis can be complicated by infections of neighboring organs (otitis media, sinusitis, bronchitis), and also cause severe regional complications (peritonsillar, lateral pharyngeal and retropharyngeal abscesses, etc.), requiring emergency surgical interventions, and systemic complications - rheumatism, glomerulonephritis, vasculitis, etc. Acute otitis media is one of the leading causes of meningitis, brain abscess and thrombosis of the asigmoid sinus [4]. Sinusitis can lead to the development of severe orbital and intracranial complications.
Infections of the upper respiratory tract and ENT organs are also associated with significant financial losses. For example, in the USA, direct and indirect costs associated with acute otitis media exceed $3.5 billion per year [13], and $3.5 billion is spent annually on the treatment of acute bacterial sinusitis [14], including in children up to 12 years – 1.8 billion [15]. In Russia, the average duration of temporary disability for acute sinusitis is 11.6 days [16]. Economic losses caused only by temporary disability are estimated at 232 million US dollars, or 0.15% of the gross domestic product of the Russian Federation [16]. Thus, the correct choice of treatment for infections of the upper respiratory tract and ENT organs is, along with clinical, of great economic importance. Adequate treatment of these infections is also an important factor in the prevention of bacterial resistance.
Unfortunately, the widespread increase in the resistance of the main causative agents of infections of the upper respiratory tract and ENT organs to many antibacterial agents, including first-line drugs, significantly limits the choice of antibiotics for empirical therapy. For example, the spread of beta-lactamase-producing strains of H. influenzae and M. catarrhalis and the emergence of penicillin-resistant pneumococci are already calling into question the legality of using amoxicillin as a first-line drug for acute otitis media and other community-acquired infections [17].
In addition, in recent years there have been changes in the etiological structure of infections of the upper respiratory tract and ENT organs, which necessitates a revision of approaches to the management of patients with infections of this localization. First of all, this is due to the increasing role of “atypical” bacterial pathogens (M. pneumoniae, C. pneumoniae) in the etiology of acute respiratory diseases (ARI), laryngotracheitis and other respiratory tract infections, especially in young people and children [18]. For example, in a randomized study whose participants included 353 children aged 1 to 14 years with recurrent acute respiratory infections and 208 healthy children (control group), “atypical” pathogens were identified in 54% of patients in the main group compared to 3.8% children from the control group (p < 0.0001) [18]. This study also showed that short-term (within a month) and long-term (within six months) clinical benefit was observed significantly more often in patients receiving azithromycin along with symptomatic therapy than in children receiving symptomatic therapy alone. Moreover, the long-term clinical effect of azithromycin (reduction in the frequency of relapses) was expressed in infections caused not only by “atypical” pathogens, but also by other pathogens. The results of the study led to the conclusion that “atypical” bacteria play a fairly large role in the occurrence of recurrent respiratory tract infections in children and that long-term therapy with azithromycin can not only significantly improve the course of an acute episode of the disease, but also reduce the risk of relapses.
Another recent study showed that, if not adequately treated, acute tonsillopharyngitis in children associated with atypical bacteria can lead to unfavorable outcomes and is associated with a high risk of relapse [19]. These data make us more critical of modern recommendations, which do not support the use of antibiotics in most patients with acute infections of this localization, and contribute to increasing the role of macrolides in their therapy.
Currently, azithromycin has actually already acquired the status of a first-line antibiotic for the treatment of infections of the respiratory tract and ENT organs, despite the fact that in most recommendations it is given the position of a drug alternative to beta-lactams [20]. This is due to the favorable pharmacological properties of azithromycin, proven effectiveness and safety, and the increasing etiological role of atypical pathogens.
Rationale for the feasibility of using azithromycin for infections of the respiratory tract and ENT organs from a pharmacological point of view
Like other macrolides, azithromycin is active against the main causative agents of infections of the respiratory tract and ENT organs (S. pneumoniae, S. pyogenes, M. catarrhalis), including atypical pathogens (mycoplasma, chlamydia). Its advantage over other macrolides is its clinically significant activity against the common causative agent of acute otitis media and acute bacterial sinusitis, H. influenzae. Like other macrolides, azithromycin has a bacteriostatic effect, but due to its unique pharmacokinetic properties it is capable of creating very high interstitial and intracellular concentrations and, accordingly, has a bactericidal effect, including on pathogens located inside cells [21].
Azithromycin is characterized by post-antibiotic effects against S. pyogenes, S. pneumoniae, H. influenzae, L. pneumophila. In terms of the duration of the post-antibiotic effect against a number of microorganisms, for example, H. influenzae and Legionella pneumophila, it is superior to clarithromycin [22, 23]. The effect of azithromycin is enhanced by its anti-inflammatory and immunomodulatory properties [24].
Azithromycin differs from erythromycin and other macrolides in improved pharmacokinetic properties: it is superior to other antibiotics of this group in acid resistance (300 times higher than erythromycin), is more consistently absorbed from the gastrointestinal tract and has greater bioavailability. The high lipophilicity of azithromycin determines its wide distribution in the body (volume of distribution - 31 l/kg) and good penetration into tissues and biological fluids. Its concentrations in various tissues after a single administration are 20–1000 times higher than those in blood plasma [25, 26]. Very high concentrations of the drug are created in the respiratory tract and ENT organs: in bronchial secretions, lungs, alveolar fluid, tonsils, adenoids, middle ear and paranasal sinuses [27, 28]. They far exceed the minimum inhibitory concentrations of the antibiotic for the most common pathogens that cause infections in the corresponding localization.
Azithromycin is significantly superior to erythromycin in its ability to penetrate into the paranasal sinuses [29]. Its maximum concentrations in the mucous membrane of the maxillary sinus are created within two hours after administration and significantly exceed the level of the antibiotic in the blood serum. Research conducted at the L.S. Strachunsky showed that two hours after taking 500 mg of azithromycin, its concentrations in the mucous membrane of the maxillary sinus were eight times higher than those in the blood serum, while the maximum concentrations of erythromycin were approximately three times lower than serum concentrations [30]. In a placebo-controlled study in animals with experimental rhinosinusitis, azithromycin, creating high concentrations at the site of infection, ensured rapid clearance of bacteria and disappearance of inflammation [31]. In contrast, the new fluoroquinolone with bactericidal action, moxifloxacin, which creates low concentrations in the lesion and has a short post-antibiotic effect, had little effect on bacterial clearance and the inflammatory process [31].
Azithromycin very quickly reaches high concentrations in the tonsils and adenoids. 2–24 hours after administration, its concentrations in the tonsils exceed the MIC for S. pyogenes by 30 times [30]. The half-life of azithromycin from tonsil tissue reaches 3.2 days [32]. In children aged two to eight years who received a three-day course of treatment with an azithromycin suspension at a dose of 10 mg/kg/day, the level of the antibiotic in the tonsils and adenoids four days after completion of therapy was more than 900 times higher than its serum concentrations [26]. In patients with recurrent or chronic tonsillitis, after oral administration of azithromycin at a dose of 250 mg twice daily, high concentrations of the drug in the tonsils persisted for seven days [26].
High levels of azithromycin are detected in the mucous membranes of the ethmoid sinus and in the tympanic cavity [26]. In children with otitis media, within 24 hours after the start of therapy, azithromycin concentrations in the middle ear reach 8–9 mg/kg [33].
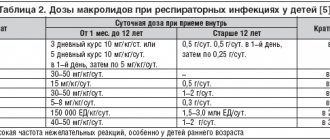
Data on antibiotic concentration levels in the respiratory tract and ENT organs obtained in pharmacokinetic studies are summarized in the table.
Azithromycin is metabolized to a small extent with the formation of inactive metabolites [36]. The drug is excreted primarily in bile. Elimination of azithromycin has a biphasic nature, the half-life from the body is 35–79 hours [37, 38] and does not change with renal and liver failure and cirrhosis of the liver [39, 40].
Favorable pharmacokinetic properties make it possible to take azithromycin once a day in short courses of three to five days, and for some infections even as a single dose. No significant differences in the pharmacokinetics of the antibiotic (accumulation in macrophages, tissue and cellular concentrations) were found when using three- and five-day courses of treatment [41].
The unique regimen of azithromycin provides it with advantages over other antibiotics in terms of patient adherence to therapy. This is facilitated by the possibility of using it regardless of food intake. Although azithromycin is recommended to be taken before meals, several targeted studies have demonstrated that food does not significantly affect the bioavailability of this antibiotic in various dosage forms [42]. The advantage of azithromycin in pediatrics is the favorable organoleptic properties of the pediatric suspension of the drug [43, 44].
Azithromycin is well tolerated. Adverse reactions with its use rarely develop. The incidence of adverse drug reactions in adults and children is about 9% [45], while with erythromycin treatment it reaches 30–40% [46]. Serious adverse reactions with the use of azithromycin in clinical studies were recorded extremely rarely, and their cause-and-effect relationship with the drug has not been definitively established. According to the results of meta-analyses, the rate of azithromycin discontinuation due to adverse reactions is 0.7% for lower respiratory tract infections and 0.8% for upper respiratory tract infections [47, 48].
Azithromycin has low allergenic potential. Allergic reactions are observed in less than 1% of patients, while when treated with penicillins they develop in 10% of cases, and with cephalosporins in 4% [46]. The most common side effects are azithromycin dyspeptic disorders, the incidence of which, according to several controlled clinical studies, ranges from 6 to 9% [49]. Thus, in pediatric clinical studies, diarrhea was observed in 1–6% of participants, abdominal pain in 1–4%, nausea in 0.5–2%, and vomiting in 1–6%. Elevated liver enzyme levels occurred in 0–1% of children receiving azithromycin versus 2–4% of children receiving erythromycin [49].
The absence of a pronounced effect on the enzymes of the cytochrome P450 system in the liver provides azithromycin with a low potential for clinically significant drug interactions, which determines its fairly high safety when used in combination with other drugs. In this indicator, azithromycin is superior to most macrolides, which, in terms of their effect on cytochrome P450, are arranged in the following order: clarithromycin > erythromycin > roxithromycin > azithromycin > spiramycin [50].
The use of azithromycin is quite safe during pregnancy. It is the only semi-synthetic macrolide classified by the FDA as category B. This category includes drugs that do not have an adverse effect on the fetus and the course of pregnancy in animal experiments. There are also clinical data confirming the safety of azithromycin in pregnant women [29].
The use of macrolides for empirical treatment of infections of the respiratory tract and ENT organs is also justified from the point of view of the low level of resistance to them in common pathogens of this localization in Russia. According to the multicenter study PeGaS-1 (2000), the frequency of pneumococcal resistance to azithromycin is 5%, to erythromycin – 6%, to clarithromycin – ranges from 2 to 13% [24]. Moreover, according to Japanese authors, azithromycin remains active against pneumococci resistant to other macrolides [51]. “Overcoming” the low level of resistance of microorganisms is apparently associated with the pharmacokinetic properties of azithromycin.
Evidence of the effectiveness of azithromycin for infections of the upper respiratory tract and ENT organs
The high effectiveness of azithromycin for infections of the upper respiratory tract and ENT organs in adults and children has been proven in controlled clinical studies. Between 1991 and 2001, the effectiveness of azithromycin in infections of the upper respiratory tract and ENT organs was studied in at least 29 randomized clinical trials, which included 7240 patients, including 4263 children [52]. Along with five-day courses of treatment, shorter treatment regimens have also been studied to improve adherence to treatment and reduce its cost. Three-day courses of azithromycin therapy were compared with 7–14-day courses of clarithromycin, roxithromycin, phenoxymethylpenicillin, amoxicillin/clavulanate, and cefaclor. In most studies, a short course of azithromycin was not inferior in clinical and bacteriological effectiveness to standard courses of comparator drugs, and in some it was even superior to them.
Currently, azithromycin is the only antibiotic approved by the FDA for short-term use in acute bacterial sinusitis. The basis for approval was the results of a multicenter, randomized, double-blind trial involving 936 patients [53]. It compared the effectiveness and safety of two treatment regimens: azithromycin 500 mg once daily for 3 or 6 days and amoxicillin/clavulanate 625 mg 3 times daily for 10 days. The effectiveness of all treatment regimens was the same, however, when treating with amoxicillin/clavulanate, side effects were observed more often (51.1%) than when using 3-day (31.1%; p < 0.001) or 6-day courses of azithromycin (37.6 %; p < 0.001). There were also more patients in the amoxicillin/clavulanate group who dropped out of the study (n = 28) due to side effects than in the azithromycin groups (n = 7 and 11, respectively, for the 3- and 6-day courses).
The comparable effectiveness of three-day courses of azithromycin with standard courses of treatment with amoxicillin/clavulanate was confirmed in three more clinical studies, and in two of them the effect of azithromycin developed faster than the effect of the comparison drug. The results of a domestic randomized clinical and pharmacoeconomic study showed that a three-day course of azithromycin provides cure for acute sinusitis in a shorter period of time than a ten-day course of amoxicillin/clavulanate, more effectively prevents relapses of the disease, is better tolerated, is characterized by fewer adverse reactions than the comparison drug, and is more profitable from an economic point of view [54].
Controlled studies have demonstrated the high clinical and bacteriological effectiveness of azithromycin for tonsillopharyngitis in adults and children. It has been shown that azithromycin is not inferior in clinical effectiveness to phenoxymethylpenicillin and erythromycin. There is evidence of faster relief of symptoms of the disease in patients receiving three- and five-day courses of azithromycin, compared with patients receiving a standard course of phenoxymethylpenicillin and roxithromycin [55, 56]. At the same time, some studies have revealed a lower bacteriological effectiveness of azithromycin compared to phenoxymethylpenicillin [57–59] and a higher rate of disease relapses in the long term in patients treated with azithromycin [60, 61]. This may be due to the insufficient dose of azithromycin used in these studies.
A meta-analysis of controlled clinical trials showed that the effectiveness of azithromycin for tonsillopharyngitis largely depends on the dose and treatment regimen [58]. In children, the course dose of the drug should be 60 mg/kg (3 days at 20 mg/kg or 5 days at 12 mg/kg). According to the results of the meta-analysis, at this course dose, azithromycin was significantly (p < 0.00001) superior in effectiveness to 10-day courses of treatment with comparison antibiotics. Moreover, bacteriological failure in children receiving azithromycin at a course dose of 60 mg/kg was observed five times less often than when using ten-day courses of comparison antibiotics. On the contrary, at a course dose of 30 mg/kg, azithromycin was inferior (p = 0.02) in effectiveness in pediatrics to standard courses of comparison antibiotics. In children, three-day treatment regimens were less effective than five-day ones. In contrast, in adult patients, three-day treatment regimens were superior to five-day regimens. When using three-day treatment regimens (500 mg/day) in adults, there was a trend towards higher efficacy of azithromycin compared with ten-day courses of comparison antibiotics (p = 0.14).
The only clinical study used a single dose of azithromycin (30 mg/kg) in patients with tonsillitis, which was not inferior in effectiveness to a ten-day course of treatment with a comparator antibiotic [62], but the question of the possibility of using a single dose of azithromycin for the treatment of acute tonsillopharyngitis requires further study.
Azithromycin has proven to be an effective treatment for acute otitis media. It has been shown to be effective in three- and five-day courses in clinical studies, and in 2003 it was approved by the FDA for the treatment of acute otitis media in children in a single dose.
The effectiveness and safety of a single dose of azithromycin (30 mg/kg) have been studied in a fairly large number of clinical studies in children with uncomplicated acute otitis media. The summarized results of four studies indicate that, in general, the clinical effectiveness of a single dose of azithromycin in children with acute otitis is 84%, including for infections caused by Streptococcus pneumoniae - 91%, Haemophilus influenzae - 77%, Moraxella catarrhalis - 100 %, Streptococcus pyogenes – 64%, mixed infection of S. pneumoniae and H. influenzae – 25% [63]. The drug was very well tolerated: side effects were rare and mainly manifested as mild transient gastrointestinal disorders. In the two largest studies, the incidence of side effects with azithromycin was lower than that of the comparator antibiotics amoxicillin and amoxicillin/clavulanate.
According to the results of a meta-analysis, the frequency of failure when using short (less than five days) courses of azithromycin and 7-10-day courses of amoxicillin does not differ [64]. At the same time, azithromycin is significantly less likely (19%; p < 0.05) to cause side effects than amoxicillin.
Thus, azithromycin meets almost all the requirements for “ideal” drugs for the treatment of infections of the upper respiratory tract and ENT organs:
· exhibits high activity against the main pathogens of infections in a given localization, including “atypical” pathogens, the etiological role of which has recently been increasing;
· has favorable pharmacokinetic properties, allowing one to achieve high concentrations of the antibiotic at the site of infection and apply it once a day in short courses;
· has proven efficacy and safety in adequate clinical studies and is well tolerated by patients;
· thanks to the convenient treatment regimen and good tolerability, it allows for high patient adherence to treatment;
· is associated with low levels of resistance in major pathogens and may “overcome” low levels of resistance due to unique pharmacokinetic properties.
Chemical properties[edit | edit code]
Macrolides get their name from the macrocyclic lactone ring (14-membered in erythromycin and clarithromycin and 15-membered in azithromycin), to which at least one deoxysugar residue is connected. Clarithromycin differs from erythromycin in the methyl group that replaces the hydrogen of the hydroxyl group at position 6, and azithromycin has an additional nitrogen atom with a methyl group attached to its lactone ring. Due to these structural differences, azithromycin and clarithromycin are more stable in an acidic environment, penetrate tissue better and have a wider spectrum of action. The chemical formulas of macrolides are as follows:
Structural formula of erythromycin Structural formulas of clarithromycin and azithromycin
Antimicrobial activity[edit | edit code]
Erythromycin usually has a bacteriostatic effect, but in high concentrations it can act bactericidal on highly sensitive microorganisms. In vitro, erythromycin is most active against aerobic gram-positive cocci and rods (Steigbiegel, 2000). The MIC for sensitive strains of Streptococcus pyogenes and Streptococcus pneumoniae ranges from 0.015 to 1 μg/ml. However, the number of erythromycin-resistant streptococcal strains is increasing. The mechanism of resistance is the same for all macrolides, therefore such strains are cross-resistant to other drugs in this group. Due to the widespread use of macrolides, the proportion of Streptococcus pyogenes strains resistant to them can reach 40% (Seppala et al., 1997; Esposito et al., 1998). In Streptococcus pneumoniae, the prevalence of macrolide resistance is particularly high among penicillin-resistant strains, being 60% compared with 5% among penicillin-susceptible strains (Thomsberry et al., 1997; Thomsberry et al., 1999). The MIC of erythromycin for viridans streptococci is 0.06–3.1 μg/ml.
Some staphylococci are sensitive to erythromycin, but the MIC for them varies widely (for Staphylococcus epidermidis - from 8 to more than 32 μg/ml, for Staphylococcus aureus - from 0.12 to more than 128 μg/ml). Hospital strains of Staphylococcus aureus are often resistant to macrolides; in addition, Staphylococcus aureus may become resistant during treatment. Macrolide-resistant strains of Staphylococcus aureus exhibit cross-resistance to clindamycin (Fass, 1993). Many gram-positive bacilli are sensitive to erythromycin: the MIC for Clostridium perfringens is 1 μg/ml, for Corynebacterium diphtheriae - 0.2-3 μg/ml, for Listeria monocytogenes - 0.25-4 μg/ml.
Erythromycin has no effect on most enterobacteria, but is active against other gram-negative microorganisms. In vitro, it is moderately active against Haemophilus influenzae (MIC 1-32 μg/ml) and Neisseria meningitidis (MIC 0.4-1.6 μg/ml), highly active against most strains of Neisseria gonorrhoeae (MIC 0.12-2 μg/ml). ml; Steigbigel, 2000). In addition, it acts on Pasteurella mul-tocida, Borrelia spp. and Bordetella pertussis. Strains of Bacteroides fragilis are often resistant to erythromycin (MIC 2–32 μg/ml), and Campylobacter jejuni are sensitive (MIC 0.5–4 μg/ml). Erythromycin is effective against infections caused by Mycoplasma pneumoniae (MIC 0.004–0.02 μg/ml) and Legionella pneumophila (MIC 0.01–2 μg/ml). For most strains of Chlamydia trachomatis, the MIC is 0.06–2 μg/ml. In vitro, some atypical mycobacteria, including Mycobacterium scrofulaceum, are also sensitive to erythromycin. The susceptibility of Mycobacterium kansasii and Mycobacterium avium-intracellulare varies (Molavi and Weinstein, 1971). Mycobacterium fortuitum is resistant to erythromycin.
Clarithromycin is slightly more active than erythromycin against strains of streptococci and staphylococci that are sensitive to the latter, and is moderately active against Haemophilus influenzae and Neisseria gonorrhoeae. In addition, clarithromycin has a good effect on Moraxella catarrhalis, Chlamydia spp., Legionella pneumophila, Borrelia burgdorferi, Mycoplasma pneumoniae, Mycobacterium leprae (Chan et al., 1994).
Azithromycin is generally less active than erythromycin against gram-positive bacteria (streptococci, enterococci), but slightly stronger than erythromycin and clarithromycin against Haemophilus influenzae and Campylobacter spp. (Peters et al., 1992). Azithromycin is highly active against Moraxella catarrhalis, Pasteurella multocida. Chlamydia spp., Mycoplasma pneumoniae. Legionella pneumophila, Borrelia burgdorferi, Fusobacterium spp. and Neisseria gonorrhoeae.
A microorganism is considered sensitive to new macrolides (clarithromycin and azithromycin) if its MIC does not exceed 2 μg/ml. An exception is Haemophilus influenzae: the MIC for strains sensitive to clarithromycin does not exceed 8 μg/ml, and the MIC for strains sensitive to azithromycin is 4 μg/ml.
Azithromycin and clarithromycin are more active than erythromycin against Mycobacterium avium-intracellulare. New macrolides also act on some protozoa (Toxoplasma gondii, Cryptosporidium spp., Plasmodium spp.).
Read also[edit | edit code]
- Antibiotics (antimicrobial agents) Choice of antibiotic
- Combination antibiotic therapy
- Prophylactic antibiotic therapy
- Mechanisms of action of antibiotics
- Sulfonamides, trimethoprim/sulfamethoxazole
- Quinolones and urinary antiseptics
- Beta-lactam antibiotics Penicillins
- Cephalosporins
- Carbapenems
- Beta-lactamase inhibitors
- Tetracyclines
- Antituberculosis drugs (antimycobacterial) Isoniazid
- Rifampicin
- Ethambutol
- Streptomycin
- Pyrazinamide
- Other anti-tuberculosis drugs
Side effects[edit | edit code]
Erythromycin rarely causes severe side effects. Allergic reactions include fever, eosinophilia, and rash, which may occur alone or in combination. After discontinuation of the drug, the symptoms quickly disappear. The most severe side effect is cholestatic hepatitis. It is mainly caused by erythromycin estolate, very rarely by erythromycin ethylsuccinate or erythromycin stearate (Ginsburg and Eichenwald, 1976). The disease begins approximately 10-20 days after the start of treatment with nausea, vomiting and cramping abdominal pain. The pain is often the same as with | acute cholecystitis, which may lead to unnecessary surgery. Jaundice soon appears, sometimes accompanied by fever, leukocytosis, eosinophilia and increased aminotransferase activity. Liver biopsy reveals cholestasis, periportal infiltration of neutrophils, lymphocytes and eosinophils, and sometimes necrosis of hepatocytes. Manifestations of hepatitis rarely persist for more than a few days after discontinuation of the drug. It is possible that cholestatic hepatitis results from an allergic reaction to erythromycin estolate (Tolman et al., 1974). There may be a slight increase in serum liver enzyme activity (McCormack et al., 1977).
When taken orally, especially in large doses, erythromycin often causes epigastric pain, sometimes quite severe. With intravenous administration, gastrointestinal disorders are also possible - cramping abdominal pain, nausea, vomiting, diarrhea. Erythromycin has been shown to enhance gastrointestinal motility by binding to motilin receptors (Smith et al., 2000). Gastrointestinal disturbances are dose-dependent and are more common in children and young adults (Seifert et al., 1989). Longer infusion (over 1 hour) and pre-administration of glycopyrronium bromide alleviate these symptoms (Bowler et al., 1992). IV administration of the drug at a dose of 1 g, even when diluted in a large volume of liquid, is often accompanied by thrombophlebitis. Slow administration reduces the risk of this complication.
There are reports that erythromycin causes cardiac arrhythmias, including prolongation of the QT interval and, against its background, ventricular tachycardia. In most cases, these disorders occurred in patients with heart disease or were observed when erythromycin was prescribed concomitantly with drugs such as cisapride and terfenadine (Brandriss et al., 1994).
The use of erythromycin in large doses (erythromycin glucoheptonate or erythromycin lactobionate, 4 g/day IV, or large doses of erythromycin estolate orally) may be accompanied by transient hearing loss (Karmody and Weinstein, 1977).
Mechanism of action[edit | edit code]
Macrolides are bacteriostatic antibiotics that inhibit protein synthesis by reversibly binding to the 505 ribosomal subunit (Fig. 47.3; Brisson-Noel et al., 1988). Macrolides act on the same target as chloramphenicol, competitively inhibiting its binding to ribosomes (Fig. 47.2). A change in the 50S ribosomal subunit due to mutation, which disrupts the binding of macrolides to the target, leads to the development of drug resistance. Unlike chloramphenicol, which prevents the formation of a peptide bond, macrolides act at the stage of translocation - the transfer of a newly synthesized peptidyl-tRNA molecule from the aminoacyl site of the ribosome to the peptidyl site.
Gram-positive bacteria accumulate almost 100 times more erythromycin than gram-negative bacteria.
In an alkaline environment, the antimicrobial activity of the drug is much higher, probably because in its non-ionized form, which predominates at high pH, it penetrates bacterial cells much better (Sabath et al., 1968; Vogel et al., 1971).
Acquired resistance to macrolides is due to three main mechanisms:
- active removal of the drug from the cell (in staphylococci the transporter is encoded by the mrsA gene, in Streptococcus pyogenes - by the mefA gene, in Streptococcus pneumoniae - by the mefE gene),
- a decrease in the affinity of ribosomes for the drug, due to their methylation under the influence of the inducible or constitutive enzyme methyltransferase (this enzyme is encoded by the egtA, egtB and egtC genes)
- hydrolysis of macrolides by enterobacterial esterases (Barth61dmy et al., 1984).
The second mechanism, mediated by the egt genes, determines resistance not only to macrolides, but also to lincosamides and streptogramins (MLSB phenotype). All of these drugs act on the same target, the methylation of which leads to the formation of resistance. There is another mechanism of macrolide resistance found in Bacillus subtilis, Campylobacter spp. and gram-positive cocci. It is caused by chromosomal mutations that change the structure of the protein 508-ribosomal subunit.
Drug interactions[edit | edit code]
Erythromycin and clarithromycin interact with other drugs (Periti et al., 1992). Erythromycin enhances the effects of astemizole, carbamazepine, glucocorticoids, cyclosporine, digoxin, ergot alkaloids, terfenadine, theophylline, triazolam, valproic acid and warfarin, probably by inhibiting liver microsomal enzymes involved in the metabolism of these drugs (Ludden, 1985; Martell et al. , 1986; Honig et al., 1992). Clarithromycin, which is similar in structure to erythromycin, interacts with the same drugs. Azithromycin, apparently, does not enter into drug interactions, since, unlike erythromycin and clarithromycin, it contains a 15-membered lactone ring. However, azithromycin should be prescribed concomitantly with the drugs listed above with caution.