Pharmacological properties of the drug Methylprednisolone aceponate
GCS for external use. Has anti-inflammatory and anti-allergic effects. It has virtually no systemic GCS or mineralocorticoid effect. Suppresses the skin inflammatory reaction, reduces vascular permeability. Systemic absorption does not exceed 2.5%. Possessing high lipophilicity, it quickly penetrates through the stratum corneum of the skin into the dermis, where it undergoes hydrolysis with the formation of active metabolites - 17-propionate and free methylprednisolone. Both derivatives have greater affinity for steroid receptors than the parent compound. After entering the systemic circulation, it is quickly conjugated with glucuronic acid and inactivated. It is excreted mainly by the kidneys in the form of metabolites. The half-life is about 16 hours. It does not accumulate in the body.
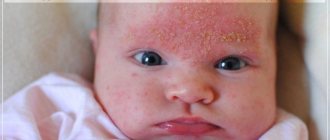
Currently, atopic dermatitis (AD) is considered a chronic recurrent allergic skin disease that occurs mainly in early childhood in individuals with a hereditary predisposition. The disease has age-related features of the localization and morphology of inflammation foci, is characterized by skin itching and is caused by hypersensitivity to both allergens and nonspecific irritants [1–3].
In clinical practice, the term “atopy” has been used since the 1920s, and later the term “atopic dermatitis” was used, characterized by an unusual response of the skin to common irritants [4]. In 1980, JM Hanifin and G. Rajka [5] proposed the currently generally accepted criteria for diagnosing AD; over 37 years of use, the criteria have been modified.
According to modern concepts, the most effective approach to the treatment of AD is complex therapy. In children, a necessary component is a hypoallergenic diet and the elimination of risk factors in everyday life. In clinical practice, antihistamines can be used for the treatment of AD; according to indications, sodium thiosulfate, magnesium sulfate, pro- and prebiotics, vitamins A, E [1, 6].
The leading role in the complex treatment of AD is played by external therapy, the purpose of which is to suppress skin inflammation, hydrate the skin, prevent and eliminate secondary infection, and improve the barrier functions of the skin. The choice of external agents for treatment is determined by the age of the child, the phase of the disease, and the presence of complications [1, 6, 7]. The use of such traditional means as tar, sulfur, naphthalan, ichthyol, antiseptic - Dorogov stimulant (ASD) (3rd fraction), menthol, anesthesin, is limited by their sharp specific odor, which can lead to exacerbation of both the skin and respiratory processes syndrome that can be observed in AD. In modern medical practice, the main role in the treatment of exacerbations of AD is given to topical glucocorticosteroids (GCS) due to their high efficiency. The drugs are recognized as first-line treatment for AD, while topical calcineurin inhibitors, recommended in some publications, are classified [8] as second-line due to unproven safety. In addition, a pilot study demonstrated the benefits of using methylprednisolone aceponate compared to pimecrolimus [9]. The benefits of methylprednisolone aceponate compared to tacrolimus were also shown [10]. Topical corticosteroids are the optimal means for relieving exacerbation of AD from the standpoint of evidence-based medicine [11].
The first use of the topical corticosteroid hydrocortisone in 1950 revolutionized dermatology. In the 1960s–1970s. more effective agents were synthesized, the potential of which increased due to either halogenation or methylation of the main molecule of the steroid framework [11].
For use in pediatric practice, topical corticosteroids are subject to particularly strict criteria: such a drug must have a strong anti-inflammatory effect, low systemic bioavailability, rapid onset of action and minimal local side effects [6, 7, 11].
Halogenated topicals are widely used in dermatological practice.
In the Russian Federation, a lot of halogenated GCS is used [12]. When prescribing them, it is necessary to take into account that such drugs are genotoxic in vitro, and chromosomal aberrations of peripheral blood are indicated [13]. The use of halogenated steroid drugs leads to a decrease in the synthesis of fibroblasts, collagen, glycosaminoglycans, a slowdown in the rate of cell division of the epidermis and dermis, which predisposes to the development of skin atrophy, as well as side effects such as perioral dermatitis, stretch marks, hypertrichosis, acneiform folliculitis, and pigmentation disorders. Long-term circulation of halogenated drugs in the blood determines the suppression of the production and disruption of the circadian rhythm of endogenous cortisol [14].
In pediatric practice, it is preferable to use non-halogenated topical agents. These include methylprednisolone aceponate, which has high anti-inflammatory activity [11], lipophilicity, and improved pharmacokinetics compared to previously created drugs [4].
The purpose of this work was to evaluate data on the effectiveness and safety of the use of methylprednisolone aceponate (Advantan, BAYER) in the complex therapy of children suffering from AD.
Material and methods
The work used data presented in MEDLINE/PubMed for the query “methylprednisolone aceponate atopic dermatitis”. Inclusion criteria: compliance with the search query, publication in English. The exclusion criterion was inconsistency with the publication of the drug and/or nosological form. 26 publications were found, 17 of them are considered in the review. In addition, 9 publications containing the necessary definitions are presented [1–6, 12–14].
results
Methylprednisolone aceponate belongs to the second class of topical corticosteroids, i.e. 100–150 times more effective than hydrocortisone. Advantan's active metabolite, 6α-methylprednisolone-17-propionate, binds to intracellular glucocorticoid receptors. The steroid receptor complex interacts with specific sections of DNA, thereby causing a series of biological effects. In particular, this leads to the induction of the synthesis of the lipocortin-1 protein, which suppresses the activity of the key enzyme of the arachidonic acid cascade, phospholipase A2, as a result of which the synthesis of pro-inflammatory prostaglandins and leukotrienes is disrupted. Other mechanisms of the anti-inflammatory effect of GCS have been established: inhibition of the formation and all functions of macrophages, inhibition of the synthesis of proinflammatory cytokines, enhancement of the vasoconstrictor effect of adrenaline, etc. [7, 10]. Topical use of methylprednisolone aceponate leads to the suppression of inflammatory and allergic skin reactions, as well as reactions associated with increased proliferation, which leads to a reduction in objective symptoms (such as erythema, swelling, lichenification) and subjective sensations (itching, irritation, pain). When methylprednisolone aceponate is used externally in an effective dosage (0.1%), systemic exposure is minimal. After repeated application of the drug to large surfaces (40–60% of the skin surface), as well as use under an occlusive dressing, no dysfunction of the adrenal glands is observed, the level of cortisol in plasma and its circadian rhythm remain within normal limits, and there is no decrease in the level of cortisol in daily urine [ 6, 11].
When it enters the systemic circulation, 6α-methylprednisolone-17-propionate quickly and completely binds to the transport protein transcortin, and then undergoes conjugation with glucuronic acid in the liver and is excreted in a conjugated form by the kidneys [7, 11].
The effectiveness of therapy largely depends on the correct choice of dosage form. When choosing it, it is necessary to take into account such morphofunctional features of the skin in children as constant anatomical and physiological development, rapid change of layers of the epidermis, loose arrangement of keratinizing cells, intense mitosis not only in the basal, but also in the spinous and granular layers, increased sensitivity of the skin to external influences, abundant vascularization of the skin, one layer of endothelial cells near the vessels [6].
The choice of external agents for treatment is determined by the age of the child, the phase of the disease, and the presence of complications [15–17]. Methylprednisolone aceponate is approved for use in children starting from 4 months of age [12]. The undoubted advantages of the drug include the possibility of its use on vulnerable areas of the skin, in particular on the face. The use of 0.1% methylprednisolone aceponate allows you to choose the optimal dosage form for the appropriate age group. For children of the first year of life, an emulsion based on 67.5% water is preferred; for children under 3 years of age, an emulsion and cream based on 60% water is preferred. From the age of three, ointment can be used on foci of chronic inflammation, and fatty ointment can be used on foci of persistent chronic inflammation, especially in areas with a pronounced stratum corneum [6, 7, 11].
Studies on the properties of methylprednisolone aceponate have been conducted in different countries. Thus, domestic practice has accumulated experience in the effective use of methylprednisolone aceponate in pediatric practice [6]. Reliable clinical evidence of the high effectiveness of methylprednisolone aceponate when used in children of the first year of life has been obtained [16, 17]. The drug has a pronounced antipruritic effect in chronic itching, the leading symptom of AD [17].
Numerous evidence has been presented regarding the advisability of using methylprednisolone aceponate in preschool children [6, 18–20]. The use of the drug in adolescents is justified [7, 10, 21].
Experience with the use of Advantan has shown that the elimination of itching and inflammation under the influence of the drug leads to an improvement in the general condition, in particular, eliminates sleep disorders in various age groups, which plays a very significant role in the treatment of children suffering from AD [16, 17, 22].
Methylprednisolone aceponate in the form of one of four dosage forms (emulsion, cream, ointment, fatty ointment) is recommended for use once a day [23]. A single use of a drug with a high level of effectiveness and safety significantly increases adherence to therapy [23].
Currently, methods for the treatment of AD have been developed that involve the use of methylprednisolone aceponate in alternation with moisturizers [24, 25].
Significant advantages of using methylprednisolone aceponate compared with mometasone furoate have been shown [26], completely refuting an earlier report of their comparable anti-inflammatory activity [27].
Methylprednisolone aceponate is well tolerated by patients of various age groups [6, 7, 11, 15–25]. We found the only description of the development of contact sensitization to the drug in a patient previously sensitized with 17-hydrocortisone butyrate [28].
Thus, the vast majority of studies conducted indicate the high effectiveness and safety of external use of methylprednisolone aceponate (Advantan, BAYER) in various age groups of children. Choosing the optimal dosage form of the drug depending on age and clinical characteristics will increase the therapeutic effect.
Advantan cream external 0.1% 15g tube
Pharmacological group:
Glucocorticosteroid for topical use ATC code: D07AC14
Pharmacodynamics:
The active component of Advantan, methylprednisolone aceponate, is a non-halogenated steroid.
When applied externally, Advantan suppresses inflammatory and allergic skin reactions, as well as reactions associated with increased proliferation, which leads to a reduction in objective symptoms of inflammation (erythema, swelling, weeping, etc.) and subjective sensations (itching, irritation, pain etc.).
When methylprednisolone aceponate is used topically at the recommended dose, the systemic effect is minimal in both humans and animals. After repeated application of Advantan to large surfaces (40-60% of the skin surface), as well as application under an occlusive dressing, no dysfunction of the adrenal glands is observed: the level of cortisol in plasma and its circadian rhythm remain within normal limits, and there is no decrease in the level of cortisol in daily urine.
During clinical studies, the use of Advantan for up to 12 weeks in adults and up to 4 weeks in children (including young children) did not reveal the development of skin atrophy, telangiectasia, stretch marks and acne-like rashes. Methylprednisolone aceponate (especially its main metabolite, 6 alpha-methylprednisolone 17-propionate) binds to intracellular glucocorticosteroid receptors. The steroid receptor complex binds to specific regions of the DNA of immune response cells, thereby causing a series of biological effects. In particular, the binding of the steroid receptor complex to the DNA of immune response cells leads to the induction of macrocortin synthesis. Macrocortin inhibits the release of arachidonic acid and, thereby, the formation of inflammatory mediators such as prostaglandins and leukotrienes. Inhibition of the synthesis of vasodilating prostaglandins by glucocorticosteroids and potentiation of the vasoconstrictor effect of adrenaline lead to a vasoconstrictor effect
Pharmacokinetics:
Methylprednisolone aceponate is hydrolyzed in the epidermis and dermis. The main and most active metabolite is 6alpha-methylprednisolone-17-propionate, which has a significantly higher affinity for glucocorticosteroid receptors in the skin, which indicates the presence of its “bioactivation” in the skin.
The degree of percutaneous absorption depends on the condition of the skin, the dosage form and the route of administration (with or without an occlusive dressing). Percutaneous absorption in children and adults with atopic dermatitis (neurodermatitis) and psoriasis was no more than 2.5%, which is only slightly higher compared to healthy volunteers (0.5-1.5%).
After entering the systemic circulation, 6alpha-methylprednisolone-17-propionate quickly conjugates with glucuronic acid and is thus inactivated. Metabolites of methylprednisolone aceponate are eliminated mainly by the kidneys with a half-life of about 16 hours.
Methylprednisolone aceponate and its metabolites do not accumulate in the body.