- Types and causes of placental insufficiency
- Diagnosis of placental insufficiency
- Treatment of placental insufficiency
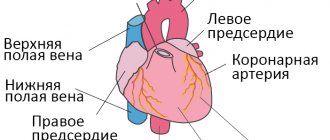
Most women know that the placenta connects mother and baby during pregnancy and through it, nutrients and oxygen are supplied to the baby.
Are there situations when the placenta stops performing its function correctly and fully? Is it possible to somehow prevent this?
Fetoplacental insufficiency: definition
During conception, together with the fetus, the placenta or baby's place, as it is popularly called, simultaneously forms and develops. This is a unique temporary organ; it appears only during pregnancy, and when the child is born, the placenta follows. It performs the most important functions:
- filters toxins and harmful substances and prevents them from reaching the baby;
- provides the fetus with nutrition and oxygen and removes carbon dioxide;
- produces the synthesis of hormones responsible for the course of pregnancy;
- responsible for the safety of the fetus.
Sometimes it happens that the placenta stops performing some function or is completely switched off, that is, it ages earlier than expected, nutritional metabolism and blood flow are disrupted - this is called fetoplacental insufficiency.
What is the function of the placenta?
So, the placenta is an important organ that is formed only during pregnancy. The placenta is formed from the chorion - the embryonic membranes of the fetus. At the very beginning of pregnancy, chorionic villi - outgrowths of the membrane - evenly cover the entire surface of the fetal egg; starting from the second month of pregnancy, on one side of the fetal egg, the villi begin to lengthen, increase in size and form the placenta.
The baby's blood flows inside the villi, and outside they are washed with the mother's blood. Between the bloodstream of mother and baby there is only one layer of cells, which acts as a barrier between the body of mother and child. Thanks to this membrane, the blood of the mother and fetus does not mix.
However, in recent years it has become known that fetal blood cells do penetrate the placental barrier into the mother’s bloodstream, and thanks to this, it has become possible to conduct genetic tests and determine chromosomal abnormalities, Rh factor and fetal sex using the blood of a pregnant woman (non-invasive prenatal test).
In the placenta there is a constant exchange of substances between mother and child. Oxygen and nutrients flow from the mother's blood to the fetus, and carbon dioxide and metabolic products that must be excreted from the body return to the mother from the fetus.
The placental barrier performs an immunological function, since it allows the passage of some protective antibodies - blood cells that ensure the fight against infectious agents; in addition, it is impermeable to some harmful substances, viruses and bacteria. Unfortunately, the placental barrier is easily overcome by drugs, alcohol, nicotine, components of many medications and some viruses.
An important function of the placenta is the production of hormones and biologically active substances. First of all, these are hormones that are important for successful pregnancy, for example, human chorionic gonadotropin, placental lactogen, estrogens, etc.
Unfortunately, things don’t always work out quite well. For a variety of reasons, at different stages of pregnancy, deviations in the development and functioning of the placenta can occur. These changes never go unnoticed for mother and baby, and often have dire consequences.
If the placenta ceases to perform its functions fully, so-called placental insufficiency develops. In essence, it consists in deterioration of blood circulation in the mother-placenta-fetus system.
Causes
Numerous factors influence the development of FPN:
- diseases of the thyroid gland, hypothalamus (diabetes mellitus, asthma, heart problems and others);
- Iron deficiency anemia provokes the development of FPN during pregnancy. The body at this time needs double the amount of iron, often a deficiency occurs, which leads to dysfunction of the blood flow, coagulation, and this can disrupt blood circulation in the placenta;
- infectious diseases and infections. In the early stages they can lead to miscarriage, and in the second and third trimester to pathology of the child’s place;
- abnormal structure of the uterus, surgical operations and diseases of the female organs (fibroids, endometriosis and others);
- multiple pregnancy;
- late gestosis;
- Rhesus conflict;
- placenta previa;
- poor nutrition of women;
- abuse of alcohol, cigarettes, drugs and medications.
The list of reasons is extensive and therefore, in order to monitor the situation and the development of pregnancy, you need to visit a doctor in a timely manner and undergo all recommended examinations.
For several years now, prenatal screening has been actively used; it is a set of procedures (ultrasound, biochemical blood test, examination of the patient), which makes it possible to identify pathologies in the early stages.
Treatment of placental insufficiency
There are currently no specific treatments for placental insufficiency, since there are no drugs that selectively improve uteroplacental blood flow. That is why all measures to combat placental insufficiency are aimed at prevention. If the patient is at high risk for the development of placental insufficiency, from early pregnancy she is prescribed medications whose effectiveness is well proven and which prevent the early development of severe placental dysfunction.
If, during additional methods of assessing the condition of the fetus, initial disturbances in the supply of oxygen to the baby are detected, drug treatment is carried out aimed at increasing the flow of blood and oxygen through the placenta and mandatory control examinations during the therapy. If the changes are serious and the baby experiences a severe deficiency of oxygen and nutrients, his condition suffers, then in such cases an emergency delivery is performed.
Classification of FPN
FPN by period of education:
- Primary – develops before the 16th gestational week, the main reasons being problems in implantation and placentation.
- Secondary - occurs after 16 weeks due to external unfavorable factors.
FPN by stage of development:
- Acute form - develops quickly and at any time during pregnancy, even the formation of pathology during childbirth is possible. The organ stops supplying oxygen, resulting in hypoxia and the threat of death for the child. More often this occurs due to thrombosis, abruption, or placental infarction.
- Chronic form - develops mainly due to problems in the blood circulation of the uterus-placenta. The pathology forms in the second trimester and actively manifests itself in the last weeks of gestation.
The most common is chronic placental insufficiency; it is divided into several forms:
- compensated – not dangerous to the child’s life and is stopped by the body’s defense mechanism. With such a diagnosis, the doctor manages the pregnancy more carefully and, if necessary, prescribes therapy;
- decompensated - poses a threat to the development of the fetus, easily bypasses protection and requires treatment. Can cause hypoxia, developmental delays and cause pathologies of the heart and blood vessels;
- subcompensated - this form does not respond to protective mechanisms and causes complications in the fetus (pathologies and diseases), disrupts the normal course of pregnancy;
- critical – the most dangerous. The functioning of the placenta is almost completely disrupted and the death of the fetus is inevitable.
FPN degrees:
- 1 – distinguish 1a – pathology due to impaired blood flow in the uterus-placenta and 1b – impaired in the form of fetus-placenta;
- 2 – dysfunction of blood flow in both forms: uterus-placenta and fetus-placenta;
- 3 – critical degree. The blood flow in the fetus-placenta form is disrupted, and the uterus-placenta can either function or work, but not fully.
Therapy, the life of the child and his development depend on the type, form and degree of pathology.
Types and causes of placental insufficiency
Doctors distinguish between acute and chronic placental insufficiency:
Acute placental insufficiency
This is a condition that requires emergency medical intervention. It is characterized by a rapid deterioration of placental blood flow. Acute placental insufficiency occurs mainly as a result of placental abruption or death of individual areas of placental tissue, for example, due to the formation of blood clots in blood vessels. The cause of detachment may be abdominal trauma or antiphospholipid syndrome.
Phospholipids are complex fats that are part of the membranes of all cells in the body. In some cases, the body's immune system produces large amounts of antibodies to some of its own phospholipids and proteins that bind these lipids. They are called antiphospholipid antibodies and when interacting with the cells of the body, they cause cell damage and activation of the blood coagulation system, which leads to blood clots.
Antiphospholipid syndrome is the most common cause of thrombotic complications during pregnancy, including placental abruption and acute placental insufficiency. Placental abruption can also be caused by severe gestosis, a dangerous complication of the second half of pregnancy, manifested by edema, increased pressure and the appearance of protein in the urine.
Acute placental insufficiency develops when more than 2/3 of the surface of the placenta is detached.
If acute placental insufficiency develops, it is necessary to perform a cesarean section as quickly as possible to save the life of the baby and mother.
Chronic placental insufficiency
Chronic placental insufficiency occurs much more often in pregnant women. In this case, the formation and maturation of the placenta is disrupted, uteroplacental and fetal-placental blood flows are reduced, gas exchange and metabolism in the placenta is limited, and the synthesis of placental hormones is reduced. All these changes determine the insufficient supply of oxygen and nutrients to the baby and cause retarded growth and development of the fetus.
The causes of placental insufficiency most often are previous abortions, especially surgical abortion during the first pregnancy, smoking, while the number and strength of cigarettes smoked do not matter, since tobacco smoke, not nicotine, has a negative effect on the formation of defective blood vessels of the placenta.
The risk group for the development of placental insufficiency also includes women with chronic diseases, such as arterial hypertension, iron deficiency anemia, pyelonephritis, diabetes mellitus, and thyroid diseases. In recent years, there has been a significant increase in placental insufficiency caused by bacteria, viruses, and fungi. The reason for this may be either an acute infection suffered by the expectant mother during pregnancy or the activation of a chronic infectious process in the body of a pregnant woman.
Uterine pathology plays an important role in the formation of chronic placental insufficiency: endometriosis, uterine malformations (saddle-shaped, bicornuate). Doctors also consider uterine fibroids to be a risk factor. Of course, a number of medications have an adverse effect on the formation of the placenta and fetal development. Currently, a list of drugs that are not approved for use during pregnancy has been determined.
Also of great importance in the development of placental insufficiency is thrombophilia - an increased tendency of the body to form blood clots - thrombi in the vessels.
In some cases, placental insufficiency may be due to the presence of chromosomal abnormalities in the fetus, in particular with Down syndrome (the presence of an additional 21st chromosome in the fetus) or Edwards syndrome (an additional 18th chromosome in the fetus), a dysfunction of the placenta is diagnosed already in the early stages of pregnancy.
It should be noted that among the pregnancy complications that most often lead to the development of chronic placental insufficiency, a significant factor is preeclampsia (or late gestosis) - these are complications of the second half of pregnancy, manifested by edema, increased pressure and the appearance of protein in the urine. Regardless of the factors contributing to the development of placental insufficiency, it is based on circulatory disorders in the uteroplacental complex, leading to disruption of all functions of the placenta. Consequently, symptoms of chronic placental insufficiency will be caused by a lack of oxygen and nutrients to the fetus.
This is, first of all, intrauterine growth retardation - a lag in the size of the fetus and a slowdown in its growth rate. There are often changes in fetal motor activity. At first there may be some increase in movements, and then a decrease. Violation of the protective function of the placenta leads to intrauterine infection of the fetus under the influence of pathogenic (disease-causing) microorganisms penetrating the placenta. The fetus, whose development occurs under conditions of placental insufficiency, is at a much greater risk of trauma during childbirth; they have impaired adaptation to extrauterine life and increased morbidity in the first year of life.
Based on the time of occurrence, doctors divide placental insufficiency into early and late.
Early (or primary) placental insufficiency
Develops before 16 weeks of pregnancy. It occurs already at the stage of placenta formation and is associated with diseases of a pregnant woman that existed before pregnancy, for example, pathology of the uterus, chronic arterial hypertension, and endocrinological diseases. In this case, the formation of defective vessels in the placenta occurs.
Late (or secondary) placental insufficiency
Occurs after 16 weeks of pregnancy and is most often associated with diseases that arose during pregnancy. Most often, these are iron deficiency anemia (that is, a decrease in the concentration of hemoglobin and iron in the blood), gestational diabetes mellitus (that is, a violation of the body's absorption of glucose that occurs during pregnancy), and previous viral and bacterial infections.
It is important to divide placental insufficiency into compensated and decompensated forms.
Compensated placental insufficiency
It develops, for example, when there is a threat of miscarriage and mild forms of late gestosis, if these complications can be successfully corrected with medication.
Decompensated placental insufficiency
Causes development of delayed fetal development, chronic intrauterine hypoxia, up to fetal death.
Symptoms
There are no identical and exact signs of FPN. It all depends on the form of the pathology. Naturally, in case of acute insufficiency, the symptoms cannot be ignored: the woman’s well-being changes, bleeding begins, and the child suddenly calms down and stops moving.
The chronic form proceeds calmly, only slight bleeding is possible periodically, and the size of the abdomen does not correspond to the norm for the duration of pregnancy.
That is why a woman must attend all prescribed ultrasounds and screenings. It is with the help of these procedures that the doctor can assess the functionality and condition of the placenta.
Fetoplacental insufficiency and perinatal complications in pregnant women with iron deficiency anemia
According to modern data, iron deficiency at the end of the gestational process develops in all pregnant women without exception, either in a latent or overt form. This is due to the fact that pregnancy is accompanied by additional loss of iron: 320–500 mg of iron is spent on an increase in hemoglobin and increased cellular metabolism, 100 mg on the construction of the placenta, 50 mg on an increase in the size of the uterus, 400–500 mg on the needs of the fetus. As a result, taking into account the reserve fund, the fetus is provided with iron in sufficient quantities, but at the same time, pregnant women often develop iron deficiency conditions of varying severity [9,14]. The negative impact of IDA on the course of pregnancy is explained by the fact that developing hypoxia can cause stress in the body of the mother and fetus, stimulating the synthesis of corticotropin-releasing hormone (CRH). Elevated CRH concentrations are a major risk factor for preterm labor, preeclampsia, and premature rupture of amniotic fluid. CRH increases fetal cortisol release, which may inhibit fetal growth. The result of these complications of IDA can be oxidative stress of erythrocytes and the fetoplacental complex [7,11,17]. With a long course of anemia, the function of the placenta is disrupted, its trophic, metabolic, hormone-producing and gas exchange functions change, and placental insufficiency develops. Preeclampsia often occurs (in 40–50%); premature birth occurs in 11–42%; weakness of labor is observed in 10–15% of women in labor; hypotonic bleeding during childbirth – 10%; the postpartum period is complicated by purulent-septic diseases in 12% and hypogalactia in 38% of postpartum women [7,9,10,13]. Fetoplacental insufficiency (FPI) in IDA is caused by a sharp decrease in the level of iron in the placenta, changes in the activity of respiratory enzymes and metalloproteinases [12]. A.P. Milovanov believes that one of the significant mechanisms in the development of hypoxic, circulatory, tissue and hemic hypoxia in the placenta is the pathology of the spiral arteries of the uterus [6]. According to G.M. Savelyeva et al. (1986), FPI of any etiology is based on disorders of the placental circulation, including microcirculation, and metabolic processes, which are closely related and often interdependent. They are accompanied by changes in blood flow not only in the placenta, but also in the body of the mother and fetus. This fully applies to FPN that develops during pregnancy complicated by IDA [19]. The main criteria for IDA are low color index, hypochromia of erythrocytes, decreased serum iron content, increased total iron-binding capacity of blood serum and clinical signs of hyposiderosis. The most important indicator of anemia is the hemoglobin level, at which anemia should be diagnosed. This value has repeatedly changed towards increasing the minimum value: 100, 110 g/l (WHO, 1971). Mild (I) degree of anemia is characterized by a decrease in hemoglobin levels to 110–90 g/l; average (II) degree – from 89 to 70 g/l; severe (III) – 70 g/l or less [2, 4]. Treatment of IDA involves, in addition to eliminating the main cause of this pathological condition, the use of iron supplements. An ideal antianemic drug should contain the optimal amount of iron, have minimal side effects, have a simple regimen of use, and the best effectiveness/price ratio. However, many iron-containing preparations have a number of disadvantages that create problems when using them: unpleasant organoleptic properties, low bioavailability, the ability to irritate the mucous membrane of the gastrointestinal tract, which often causes dyspeptic symptoms. From this point of view, there is justification for interest in the problem of finding new methods for treating IDA that can influence not only the condition of the pregnant woman, but also prevent adverse complications in the fetus associated with impaired functioning of the FPC [1,7,13,14]. Treatment of IDA in pregnant women should be comprehensive. First of all, you need to pay attention to your diet. However, the main type of therapy for IDA in pregnant women is iron supplements [1,9,18]. Of great clinical interest is Sorbifer Durules with a high content of Fe2+ (100 mg) and ascorbic acid (60 mg), which creates more favorable conditions for the absorption of iron in the intestine and ensures its higher bioavailability. The purpose of the study was to evaluate the effectiveness of the use of iron-containing drugs in the prevention of FPN and perinatal complications in pregnant women with anemia. Materials and methods of research We observed 115 pregnant women with IDA in the second and third trimesters of gestation. Pregnant women were divided into two groups. Group 1 included 75 pregnant women in whom anemia was diagnosed in the second trimester of pregnancy; Group 2 (comparison group) consisted of 40 patients who were admitted to the institute before delivery at 35–40 weeks of gestation. All pregnant women received IDA therapy with the iron-containing drug Sorbifer Durules in a continuous mode from the second trimester (1 tablet per day), and in pregnant women of the 2nd group this drug was used at 36–38 weeks (1 tablet 2 times a day). The age of the patients ranged from 22 to 37 years. 37 (49.6%) patients of the 1st group and 21 (52.5%) of the 2nd group had their first birth, 38 (50.4%) and 19 (47.5%) had a second birth. Among the features of the obstetric and gynecological anamnesis in pregnant women of both groups, menstrual irregularities should be noted in 17 (22%) and 16 (40%), respectively, spontaneous miscarriages - in 18 (24%) and 10 (25%). 5 (7%) patients of group 1 and 6 (15%) patients of group 2 had a history of perinatal losses. 88.6% of pregnant women in both groups had various extragenital diseases: pathology of the cardiovascular system in 12 (16%) pregnant women in group 1, in 6 (15%) in group 2; chronic tonsillitis – in 12 (16%) and 7 (17.5%) patients, respectively; chronic bronchopulmonary diseases – in 5 (6.6%) and 3 (7.5%); type 1 diabetes mellitus – in 8 (11%) and 9 (22.5%); thyroid pathology – in 5 (6.6%) and 4 (10.0%), respectively. The listed somatic diseases and complications of obstetric and gynecological history created an unfavorable background for the development of pregnancy, causing deviations during gestation. Laboratory diagnosis of anemia was based on determination of hemoglobin content, red blood cell count, serum iron and blood color index. The study of utero-placental-fetal blood flow was carried out on a Voluson-730 ultrasound device equipped with a specialized sensor (RAB 4-8p), using color Doppler mapping and pulsed Doppler imaging of the umbilical cord artery, fetal thoracic aorta, fetal middle cerebral artery and placental vessels. Qualitative analysis of blood flow velocity curves included determination of the systole-diastolic ratio (S/D) in the listed vessels (normative S/D indicators in the aorta are up to 5.6, in the umbilical artery up to 2.8, in spiral arteries 1.60 - 1.80 , middle cerebral artery 3.5–5.0) [10,11,15]. An increase in cerebral blood flow serves as a manifestation of compensatory centralization of fetal circulation during intrauterine hypoxia in conditions of reduced placental perfusion. According to D. Arduini et al. [16], Doppler studies indicate that fetuses with intrauterine growth restriction (IUGR) and anemia have a significant decrease in the pulsatility index in the middle cerebral artery. Researchers find that middle cerebral artery pulsatility readings are the best test for identifying this pathology. With fetal hypoxia, resistance to blood flow in the common carotid artery and middle cerebral artery decreases, and resistance in the aorta and umbilical artery increases (sensitivity of the method 89%, specificity 94%). When visually assessing the results of a 3D study of the selected area of the placenta, attention was paid to the nature of the distribution of the vascular component and the organization of blood vessels in the area under study. During computer processing of placentograms, the following parameters were calculated: VI – vascularization index, FI – blood flow index. Standard indicators of uteroplacental blood flow, developed in the perinatal diagnostics department of MONIIAG: central zone – VI 4.0–8.1; FI 42.0–45.0; paracentral – VI 3.8–7.6; FI 40.5–43.7; peripheral – VI 2.8–5.9; FI 37.5–42.1 [12]. To verify the ultrasound signs of FPN, the morphological state of the placentas after birth was studied. Study results and discussion Various clinical manifestations of anemia (pallor of the skin and visible mucous membranes, tachycardia, weakness, decreased performance, dizziness, paresthesia of the lower extremities) were present in 12 (16.0%) patients of group 1 and in 20 (50% ) – 2nd group. The course of this pregnancy was aggravated by early toxicosis in 36 (48%) and 27 (67.5%) patients of groups 1 and 2, respectively, and the threat of miscarriage in the first trimester - in 18 (24.0%) and 26 ( 65.0%). The second trimester of pregnancy was complicated by the threat of miscarriage in 8 (10.6%) women of the 1st group and in 18 (45.0%) of the 2nd group, hydrops of pregnancy - in 5 (6.6%) and 11 (27 .5%) respectively. In the third trimester, the main complications of gestation were gestosis of mild and moderate severity - in 6 (8.0%) and 9 (22.5%) pregnant women of the 1st and 2nd observation groups, the threat of premature birth - in 5 (6, 6%) and 8 (20%), and in 3 pregnant women of group 1 and 7 in group 2, despite the therapy, premature birth occurred at 35–36 weeks of pregnancy. Diffuse thickening of the placenta was diagnosed in 4 (5.3%) pregnant women of group 1 and 5 (12.5%) in group 2, FPN – in 16 (21.3%) and 23 (57.5%), IUGR – in 15 (20.6%) patients of group 1 and in 26 (65.0%) of the comparison group, oligohydramnios – in 12 (16.0%) and 7 (17.5%), polyhydramnios – in 4 (5.3%) and 5 (12.5%), respectively. It is noteworthy that the most severe gestational complications - FPN and IUGR were observed in patients with anemia of II and III degrees (Table 1). These same women also had the most serious extragenital diseases (diabetes mellitus, arterial hypertension, bronchopulmonary diseases). Childbirth in patients in the comparison group was significantly more often complicated by untimely rupture of water and labor anomalies; the afterbirth and early postpartum periods – bleeding. The course of the postpartum period was much more often pathological. The presented data indicate significantly more frequent complications during pregnancy, childbirth and the postpartum period in patients of group 2 (p<0.05). Gestational complications were observed significantly less frequently in patients with mild anemia. In particular, they showed no signs of intrauterine fetal suffering. This suggests that the incidence and severity of gestational complications correlate with the severity of anemia. All pregnant women received complex therapy for gestational complications, including prevention or treatment of FPN (antiplatelet, antioxidant therapy, hepatoprotectors). Indicators of red blood in pregnant women with IDA before and during treatment are presented in Figure 1. The increase in the average hemoglobin level in group 1 after treatment relative to the baseline was 23.2 g/l, serum iron - 11.6 µmol/l, whereas in group 2, no significant positive dynamics in red blood parameters were noted and the increase in hemoglobin level was 5 g/l, and the level of serum iron remained almost at the original level. Indicators of volumetric uteroplacental blood flow in pregnant women of both groups are presented in Table 2. Our studies indicated a decrease in vascularization of the placenta (hypovascularization) in patients of the 2nd group, but in the 1st group slightly reduced indicators were recorded in the peripheral zones, while in the 2nd group group they were low in all zones, which was caused by vascular spasm and beginning rheological disturbances in the intervillous space. In patients of both the 1st and 2nd groups, placental circulatory disorders correlated with changes in maternal and fetal hemodynamics, which was expressed in a significant increase in resistance in the spiral arteries, in the umbilical cord vessels and aorta, and C/D indicators in the spiral arteries approached linear form (Table 3). In group 2, there was a tendency towards a greater increase in S/D. At the same time, S/D in the middle cerebral artery of the fetus was increased only in pregnant women of group 2. Only one patient of group 2 with severe anemia (Hb 68 g/l) with increased resistance to blood flow in the umbilical cord artery and fetal aorta showed a decrease in it in the middle cerebral artery. The child was born with severe anemia (Hb 112 g/l). When drugs aimed at improving the function of the FPC, as well as an iron-containing drug, were included in therapy, positive dynamics were noted in volumetric blood flow indices in patients of both groups compared to the initial data, however, in pregnant women of group 2 they remained slightly lower than in patients 1 -th group and normative (Table 4). Indicators of C/D during Doppler measurements of the vessels of the mother and fetus during treatment in group 1 approached the normative values. In group 2, S/D in the umbilical cord artery and fetal aorta tended to normalize, while increased resistance remained in the spiral arteries and middle cerebral artery of the fetus, which is apparently due to the inclusion in this group of pregnant women with moderate and moderate anemia. severe severity and insufficient effect of short-term therapy. When studying the hormonal function of the placenta in pregnant women with anemia, it was found that only 38% of women in the 1st group and 25% of the 2nd group had normal function. In 22.0% and 25.0% of pregnant women, respectively, it was tense, and in 12% and 20% of patients in groups 1 and 2, depletion of the hormonal function of the placenta was noted (Table 5). It is known that it is very difficult to achieve compensation for FPN when FPC function is depleted. As our studies have shown, the positive effect of the therapy in the majority of patients with depleted FPC function is associated with early initiation of treatment for anemia and FPN in patients of group 1. In group 1, 63 (84%) pregnant women were delivered through the vaginal canal, and 10 (13.3%) patients had a planned caesarean section. Indications for a planned cesarean section were placenta previa - in one case, severe diabetes mellitus - in 3 cases, absolute unpreparedness of the body for childbirth and old age - in 4 patients; in one case each there was a scar on the uterus after a cesarean section and after a myomectomy. Emergency caesarean section was performed in 2 (3.0%) pregnant women due to severe gestosis and progressive FPN at 31–32 weeks. FGR I degree was observed in 13.3% and degree II – in 6.7% of newborns. In this group there were 2 children born in a state of asphyxia. In 12 (16%) newborns, the Apgar score at the 1st minute was 7 points, at the 5th minute in all children - 8 and 9 points. The average body weight of newborn mothers of group 1 reached 3215.0 g (2650.0–3390.0 g). Indicators of cerebral blood flow were within the normative values (S/D=3.3–3.4; IR=0.70–0.71). Thus, the birth of more than 75% of healthy newborns in pregnant women with IDA diagnosed in the second trimester of gestation is, of course, a consequence of adequately conducted and pathogenetically substantiated therapy. All newborns of group 1 were discharged home in satisfactory condition, but 18 (24.0%) of them were not on the 4th–5th, but on the 6th–8th day after birth. 15 (37%) pregnant women of group 2 were delivered through the natural birth canal, 18 (45%) were delivered through the planned abdominal route. In this group, indications for a planned cesarean section were decompensation of FPN in 8 pregnant women, a scar on the uterus after cesarean section in one observation, acute fetal hypoxia in 4, in one case - severe gestosis, in 4 cases - old age of the primigravida in combination with pathology cardiovascular system and FPN. 7 (17.5%) patients underwent emergency abdominal delivery due to progressive FPN. FGR I degree was observed in 10 (25%) and degree II – in 9 (22.5%) newborns. 7 (17.5%) newborns were born in a state of asphyxia (with an Apgar score of 5–6 points at 1 minute). In 15 (37.5%) children, at the 1st minute the Apgar score was 7 points; at the 5th minute, these newborns had an Apgar score of 8 points. The average body weight of newborns in mothers of group 1 reached 2800.0 g (2600.0–3060.0 g). Indicators of cerebral blood flow were also within the normative values (S/D = 3.3–3.4; IR = 0.70–0.71; PI = 1.3–1.4). Only one child born with severe anemia had reduced cerebral blood flow. In 27 (67.5%) newborns, the period of early neonatal adaptation proceeded satisfactorily; these children were discharged home in a timely manner. All newborns born to mothers with moderate and severe IDA had malnutrition, their weight and body length corresponded to the 3–10th percentile level; 6 (15%) newborns were transferred to the second stage of treatment and 7 (17.5%) were transferred to the intensive care unit. When analyzing the reasons that complicated the period of early adaptation of children, it was revealed that a high percentage of complications was observed in group 2 (33%). In the 1st group, the number of newborns with complications was slightly less, although this indicator is also quite high (24%). Most often, both groups noted respiratory disorders and infectious complications, convulsive syndrome. A characteristic feature of a group of newborn mothers with the UDA was a delay in the healing of the umbilical wound, which indicates a decrease in regenerative processes due to the presence of medium -sized severity in mothers. There are single messages about the influence of the expectation on the morphological features of the structure of the placenta [5]. According to the author, there are characteristic morphological changes in the placenta depending on the time of detection, the degree of anemia and the therapy. According to our observations, when studying women, with a characteristic morphofunctional feature, the dissociated type of maturation of cotyledons, the presence of pseudoinfarcts, aphunctional zones, focal necrosis of villi, sclerosis of the stroma of villi and their thrombosis. An increase in sclerosed villi is directly dependent on the severity of anemia. With anemia of mild and moderate severity, the safety of the synthiotrophoblast is 80–70%, while with anemia of severe degree, safety does not exceed 60%. Patients of the 1st and 2nd groups significantly vary the morphological characteristics of the reservoir: in pregnant women who received anti -enemic therapy from the II trimestra of pregnancy, there were a large mass and size of the placenta, the plethary of the villi, the preservation of syncytiotropoblast, compensatory changes in mitochondria, which is aimed at improving metabolism In the placenta and preservation of its ability to synthesize. In the study, the reservoir in women of the 2nd group revealed that they are characterized by an increase in sclerosed and fibrinoid -replaced villi and their pathological rapprochement, blood vessels, accumulation of red blood cells in the inter -pervide space, microinfarcts. Conclusions 1. The development of anemia during pregnancy negatively affects the course of the gestational process and is a serious factor in the inhibition of postnatal adaptation. 2. Timely and adequate treatment, the prophylactic use of antienemic drugs from the early stages of gestation - the key to the successful completion of pregnancy for both the mother and the newborn.
The article was published in the journal “Russian Bulletin of Obstetrician-Gynecologist” 2009, No. 5: 72–77
Literature 1. Beloshevsky E.A. Iron deficiency in adults, children and pregnant women. Voronezh 2000; 121. 2. Vorobyov P.A. Anemic syndrome in clinical practice. M 2001; 168. 3. Grishchenko O.V., Lakhno I.V., Pak S.A. and others. Modern approach to the treatment of fetoplacental insufficiency. Women's Reproductive Health 2003; 1:13:18–22. 4. Dvoretsky L.I. Algorithms for diagnosis and treatment of iron deficiency anemia. RMJ 2002; 2:6:22–27. 5. Zhilyaeva O.D. Clinical and anatomical features of the mother-placenta-fetus system during pregnancy against the background of iron deficiency anemia: Abstract of thesis. dis…. Ph.D. honey. Sci. M 2005; 24. 6. Milovanov A.P. Pathology of the mother-placenta-fetus system. Guide for doctors. M: Medicine 1999: 351–368. 7. Murashko L.E. Placental insufficiency: Current issues in the pathology of childbirth, fetus and newborn: A manual for doctors. M 2003; 38–45. 9. Serov V.N., Prilepskaya V.N., Zharov E.V. and others. Iron deficiency conditions at different periods of a woman’s life: Information manual for obstetricians and gynecologists. M 2002; 15. 10. Strizhakov A.N., Baev O.R., Timokhina T.F. Fetoplacental insufficiency: pathogenesis, diagnosis, treatment. Vopr gin akush i perinatol 2003; 2:2:53–63. 11. Titchenko L.I., Krasnopolsky V.I., Tumanova V.A. and others. The role of 3-D Doppler examination of the placenta in a comprehensive assessment of the fetoplacental system in pregnant women at high risk of perinatal pathology. Akush and Gin 2003; 5: 16–20. 12. Shekhtman M.M. Guide to extragenital pathology in pregnant women. M 1999; 815. 13. Shakutina M.K. Modern views on the pathogenesis of anemia during pregnancy: Abstract. dis…. Ph.D. honey. Sci. N Novgorod 1995; 21. 14. Alen LH Biological mechanisms that might underlie iron's effects on fetal growth and preterm birth. J Nutr 2001; 131:28–2:581–589. 15. Aranyosi J., Zatick J., Jakab A.Jr. et al. Practical aspects of Doppler sonography in obstetrics. Orv Hetil 2003; 144:34:1683–1686. 16. Arduini D., Capponi M., Rizzo G. Sonographic automated volume count (SonoAVC) in volume measurement of fetal fluid–filled structures: comparison with virtual organ computeraided anaLysis (VOCAL). Ultrasound Obstet Gynec 2008; 32:1:111–112. 17. Casparis D., Del Carlo P., Branconi F. et al. Effectiveness and tolerability of oral liquid ferrous gluconate in iron–deficient anemia in pregnancy and in the immediate post–partum period: comparison with other liquid or solid formulations containing bivalent or tnvalent iron. Minerva Ginecol 1996; 48:11:511–518. 18. Hercberg S., Preziosi P., Galan P. Iron deficiency in Europe. Public Health Nutr 2001; 4:2:(B):537–545. 19. Ponka P. Cellular iron metabolism. Kidney Int 1999; 55: Suppl. 69:2–11.
Diagnostics
To make an accurate diagnosis of FPN, a comprehensive examination is carried out, including the following steps:
- the doctor examines the patient’s medical history, collects all data and complaints;
- external examination and measurement of the abdomen and weight to determine how the fetus is growing;
- smear analysis;
- Fetal ultrasound;
- CHT and phonocardiography;
- Dopplerography is the study of blood flow.
There is a risk group that includes certain categories of women who are more likely to develop FNP during pregnancy:
- age from 35;
- work in heavy production;
- poor and meager nutrition;
- multiple pregnancy;
- history of abortion, surgery, diseases of the female organs;
- having chronic diseases;
- abusing bad habits.
Such patients require increased attention.
The essence of Doppler measurements of fetoplacental blood flow
During pregnancy, a new structure is formed in the female body, within which the relationship “future mother - placenta - baby” takes place.
Its blood flow system is also separate. Any violation of it can have a negative impact on the fetus. To diagnose such disorders, Doppler measurements of fetoplacental blood flow are used. It allows you to assess the movement of blood through the vessels within this blood flow system, thanks to which the doctor is able to identify any violations that pose a threat to the health and life of the mother and child.
Dopplermetry (abbreviated as “DPM”) is an ultrasound examination technique that allows you to determine the indicators of blood movement in the vessel being examined. It is based on the Doppler effect, which causes a frequency shift when acoustic ultrasonic waves are reflected from objects in motion. In this case, these objects are blood cells that move through the vessels, forming blood flow.
During the procedure, blood flow in the uterus and umbilical cord arteries is assessed in pregnant women.
Therapy
There is no universal treatment regimen. This is due to the fact that pathology has many forms and types and can develop due to a variety of factors. Therefore, doctors in most cases send the expectant mother to the hospital to monitor the intensity of FPN and take correct measures.
Under no circumstances should you prescribe treatment on your own! At the first signs of malaise, a decrease in the number of movements, or bleeding, you should immediately consult a doctor.
Features of DPM of uteroplacental blood flow
The pattern of ultrasound examination and Doppler testing for the patient is no different. The procedure is performed transabdominally, with the pregnant woman lying on her back or side. The diagnostician applies a medical gel to the area being examined, which allows for better visualization. It eliminates the presence of air between the ultrasound scanner sensor and the surface of the skin and ensures their tight contact.
Sometimes the study is performed transvaginally. It is prescribed if you need to obtain a detailed image. The process uses sensors whose power is sufficient to provide visualization of vessels located at a distance of no more than 10 cm. The sensor is inserted directly into the vagina and thus a scan is carried out.
Complications
Placental insufficiency may not have a detrimental effect on the child if it occurs in a certain form. In acute cases and in severe cases, it can provoke the development of serious diseases:
- intrauterine developmental delay;
- hypoxia;
- pathologies of the heart, blood vessels, nervous system;
- death.
The gynecologist considers each case individually, and possible complications depend on many factors.
Diagnosis of placental insufficiency
It is almost impossible to treat already developed placental insufficiency, so doctors are actively seeking to identify pregnant women at risk of developing placental dysfunction. If placental insufficiency is detected in the 3rd trimester of pregnancy, unfortunately, there is no effective treatment. Therefore, all methods of identifying in the early stages of pregnancy those women whose placenta formation has undergone disturbances are being very actively used.
First of all, when registering for pregnancy, the most significant risk factors are identified - smoking, previous abortions, family history (low birth weight, tendency to thrombosis), the presence of chronic heart disease, vascular disease, diabetes mellitus.
Preventive measures against the development of placental insufficiency are especially relevant and necessary until 16-17 weeks of pregnancy, when the formation of placental structures occurs.
Prenatal screening, which is carried out at 11-14 weeks of pregnancy, provides significant assistance in assessing the risk of developing placental insufficiency. It is carried out to identify Down syndrome, Edwards syndrome and other chromosomal diseases in the fetus. Currently, the most urgent thing is to conduct comprehensive early screening of pregnant women to predict the risk of developing placental insufficiency, preeclampsia and intrauterine growth retardation. Since this type of diagnostics is one of the most modern and advanced, unfortunately, it is not yet included in the list of services provided in the antenatal clinic as part of compulsory medical insurance, but is available to everyone at prenatal diagnostic centers.
Determination of proteins produced by the placenta
First of all, the PAPP-A protein is determined; it is also a marker of fetal chromosomal abnormalities. A decrease in the concentration of PAPP-A in the blood at 11-14 weeks of pregnancy occurs in pregnant women who have a high risk of placental insufficiency and fetal growth retardation.
The second placental hormone that helps in assessing the risk of placental insufficiency is PIGF (placental growth factor). Its concentration in the blood decreases long before the first manifestations of placental insufficiency. Its definition is not used as widely as PAPP-A, but nevertheless, many laboratories have already included this protein in prenatal screening of the 1st trimester. Measuring blood flow in the vessels of the uterus is extremely important when conducting 1st trimester screening. It has been unequivocally proven that the narrowing of the vessels of the uterus, determined during the study, indicates the inferiority of the formation of the placenta, which will worsen with increasing gestational age and will lead to a decrease in the baby’s nutrition and oxygen supply, that is, to the development of placental insufficiency and delayed fetal development. With normal sizes of the uterine vessels at 11-14 weeks of pregnancy, the risk of severe placental insufficiency is negligible.
The next mandatory screening ultrasound examination is carried out at 20-21 weeks of pregnancy. In this case, it is necessary to take measurements of the fetus to assess whether there is growth retardation. Indeed, with oxygen starvation, the growth rate of the fetus slows down and its size begins to lag behind the norm for each stage of pregnancy. In addition, the doctor must evaluate the condition and maturity of the placenta. During ultrasound, Doppler measurements of the uterine vessels are also performed to identify early changes that precede the clinical manifestations of placental insufficiency.
In patients belonging to the high-risk group, in addition to ultrasound and Doppler measurements, daily monitoring of blood pressure fluctuations is also carried out, determination of the amount of protein in a urine test collected per day, and evaluation of blood coagulation system parameters.
The third ultrasound is performed for all expectant mothers at 30–34 weeks of pregnancy. The doctor measures the circumference of the baby's head and abdomen, the length of the bones of his arms and legs, and calculates the estimated weight of the fetus. These measurements allow the doctor to make sure that the baby is developing normally. Also important is the structure of the placenta, the presence of signs of aging in it, as a result of which it usually ceases to fully supply the baby with blood, which means that he ceases to have enough oxygen and nutrients and the child’s development is disrupted. During an ultrasound, the amount and type of amniotic fluid is assessed, which can also change with intrauterine fetal suffering.
Doppler
Doppler testing of the vessels of the placenta and umbilical cord (a method for studying the speed of blood flow in these vessels) also allows you to assess the baby’s well-being. The doctor examines the blood flow in the arteries of the uterus, umbilical cord, heart and brain of the child. This study allows you to determine whether the placenta is working well, whether there are signs of a lack of oxygen in the baby, or the development of gestosis in the mother. When the speed of blood flow in any vessel decreases, we can talk about fetal nutritional disorders of varying degrees of severity.
A timely examination allows us to identify the initial stages of blood supply deficiency. In such cases, treatment can prevent serious complications, such as hypoxia and intrauterine growth retardation. Dopperometry is carried out at 20–21 weeks and at 30–32 weeks of pregnancy; if there are changes, monitoring is carried out at least every two weeks.
Cardiotocography
This is an important method for assessing the condition of the fetus. CTG is performed during pregnancy of 33 weeks or more, since only at this stage of the baby’s intrauterine development is full regulation of the activity of the fetal cardiovascular system established by the centers of the spinal cord and brain. The fetal heartbeat is recorded for 20–40 minutes, and if necessary, the study can be extended to 1.5 hours.
The device detects and records the baby's heart rate. The obstetrician-gynecologist evaluates the heartbeat recording curve, episodes of decrease and sharp increase in the fetal heart rate and, based on these data, makes a conclusion about how comfortable the baby feels in the mother’s stomach. For example, when the concentration of oxygen in the blood of the fetus decreases, its supply to the cells of the nervous system also decreases, which in turn affects the heart rate. In the normal course of pregnancy, CTG is performed after 33 weeks once every 10–14 days, sometimes more often. Some clinics currently offer the service of continuous CTG monitoring, which becomes relevant if there are signs of placental insufficiency. A pregnant woman is given a monitor that records changes in the baby’s cardiac activity and this data is transmitted via the Internet to the attending physician.
Prevention
Prevention methods are universal and available to everyone:
- healthy lifestyle;
- high-quality and vitamin-rich nutrition;
- good dream;
- physical activity, in the absence of contraindications;
- it is necessary to monitor the state of the reproductive system constantly, avoid abortions and diseases whenever possible;
- absence of stress and neuroses;
- Visit your doctor promptly during pregnancy and all medical procedures.
Of course, it is impossible to completely predict the development of some pathologies. But a healthy lifestyle, monitoring your own well-being and planning a pregnancy all increase your chances of safely carrying your baby to term.
Quite often, absolutely healthy women have problems during gestation; this may be hereditary, or some illnesses have worsened due to increased workload, so it is important to regularly see a doctor and notice even small changes in the child’s well-being and activity. Attentiveness will help to identify the pathology of FPN in a timely manner.
How does FPN occur?
Fetoplacental insufficiency is a pregnancy pathology in which placental blood flow is disrupted. The presence of FPN can be suspected by changes in the frequency and strength of the baby's movements. A sharp increase or decrease in fetal movements, and even more so their disappearance, often indicates an unfavorable condition of the baby, hypoxia and placental insufficiency.
The consequences of this pathology are immediate and very dangerous for the fetus, because it is through the placental blood flow that nutrients and oxygen are delivered to the baby. When the speed and level of blood flow decreases in any part of the mother-placenta-fetus system, the baby begins to lack the substances necessary for normal growth and development. With long-term or rapidly developing FPN, not only the development, but also the life of the baby is threatened.
What reasons can lead to disruption of the functioning of an organ that is so important for mother and baby? Most often, FPN develops against the background of acute and chronic diseases of the mother, pregnancy pathologies, or diseases of the placenta itself. Here are the most common risk factors for developing FPN.
- Extragenital (not related to gynecology) diseases of the mother. This includes cardiovascular failure, arterial hypertension (increased blood pressure), kidney pathologies (acute and chronic pyelonephritis, glomerulonephritis, urolithiasis, renal failure), endocrine diseases (diabetes mellitus, pathology of the adrenal glands, thyroid gland, pituitary gland), etc. All These diseases affect the speed and level of blood flow in the mother's body, which, in turn, causes a decrease in placental blood flow.
- Diseases of the genital area of a pregnant woman - endometritis (inflammation of the lining of the uterus), inflammation of the appendages, sexually transmitted diseases (ureaplasmosis, mycoplasmosis, chlamydia, herpes, cytomegalovirus, etc.). Infectious agents and their toxins that penetrate the placental barrier are retained by the placental tissue. In places where pathogens invade, pockets of inflammation form, in which blood circulation normal to the placenta is disrupted.
- Pathologies of pregnancy. The most common cause of placental insufficiency in this group is gestosis. One of the manifestations of this pathology is a persistent increase in blood pressure in the blood vessels, which, of course, very quickly affects the level of placental blood flow. Another reason is a violation of hemostasis (the ratio of factors of the coagulation and anticoagulation system of the blood, providing the necessary viscosity and speed of movement through the vessels), often detected in the second and third trimester. Less commonly, placental insufficiency develops against the background of prolonged severe anemia of pregnant women, a disease characterized by a decrease in the level of hemoglobin in the blood.
- Pathologies of the placenta. Like any other human organ, the placenta itself can also become diseased. This disease is called placentitis and is expressed in significant swelling of the placenta and disruption of placental blood flow. The cause of placentitis is mainly viruses that can penetrate the placental barrier. Sometimes foci of inflammation are limited to certain areas of the placenta; in this case, as a result of the disease, petrificates are formed at the site of inflammation - foci of calcification. A large amount of petrificates also impedes placental blood flow.
- Anomalies in the development of the placenta. This includes hypotrophy (“skinny” placenta), additional placental lobules, partial detachment and improper attachment of the placenta.