From the end of 2022 until today, the attention of the entire healthcare and world community as a whole has been focused on one big problem - the spread of a new coronavirus infection, which WHO has characterized as an emergency of international concern. One of the most important questions that has arisen for doctors is how to treat covid-19 in adults and children. We talked about this with the head of the Department of Children's Infectious Diseases of the Krasnoyarsk State Medical University, the chief specialist in infectious diseases in children of the Ministry of Health of the Russian Federation for the Siberian Federal District and Krasnoyarsk Territory, Doctor of Medical Sciences, Professor Galina Petrovna Martynova.
-Galina Petrovna, tell us what difficulties practitioners have encountered during the pandemic.
-Coronaviruses are a large family of viruses that can infect humans and some animals; they have been known to us since the 60s of the last century. Until 2002, coronaviruses were considered to be agents that caused mild upper respiratory tract disease. However, in 2002, a highly pathogenic strain of SARS-CoV was discovered, which caused an epidemic of SARS, and 10 years later - MERS-CoV. Therefore, the appearance of another representative of the alphacoronavirus SARS-CoV-2 in December 2022 in the city of Wuhan (PRC) did not initially cause much concern. But today it is already absolutely clear that COVID-19 is a completely new infection that has swept the whole world and has posed a number of diagnostic and treatment challenges for medical workers.
Particularly great difficulties in providing medical care to patients occurred at the beginning of the pandemic, when the scale of the spread of the new coronavirus infection COVID-19 became obvious. The therapy was prescribed, essentially, off-label due to the lack of reliable evidence of the effectiveness and safety of certain medications prescribed to patients. But today, a sufficient amount of information has been accumulated about the epidemiology, clinical features, prevention and treatment of this disease, a number of clinical studies have been conducted that have confirmed the effectiveness and safety of a number of drugs that can be used in the treatment of COVID-19 in adults and children.
-There is an opinion that children practically do not get sick from COVID-19. How susceptible are they to infection?
-Children are susceptible to SARS-CoV-2: according to statistics, they make up 5 to 7% of those infected with the new coronavirus infection. However, compared to adults, children have a milder form of COVID-19. Only 10% of infected children require hospitalization, and only 1% develop severe disease.
In most cases, the new coronavirus infection COVID-19 in children occurs as a mild form of acute respiratory infection, but at the same time, despite the absence of pronounced symptoms of intoxication, physical and auscultatory changes in the lungs, 20–50% of children develop pneumonia (according to computed tomography of the chest).
It is noteworthy that, along with the new coronavirus infection, today there is a traditional seasonal increase in morbidity caused by other respiratory viruses: rhinoviruses, respiratory syncytial virus, adenovirus, influenza virus and parainfluenza. But the seasonal rise of ARVI in modern conditions is, of course, unusual: it occurs against the background of an ongoing pandemic, when, due to the great similarity of clinical manifestations, it is very difficult to determine which virus (or viruses) caused the disease, especially in an outpatient setting. That is why a rational approach to the treatment of a new coronavirus infection in children, according to experts, is carried out in accordance with the basic principles of the treatment of acute respiratory viral infections.
It should be taken into account that respiratory viruses, including coronavirus, are capable of mutating, changing their antigenic structure, and developing resistance to a number of direct antiviral drugs. Let's take the influenza virus as an example, for which powerful antiviral drugs have been created that have a convincing evidence base. At the same time, today more than 40% of influenza virus strains are insensitive to Rimantadine and other amantadine drugs. There is evidence of the emergence of influenza virus strains resistant to first-line drugs - neuraminidase inhibitors, in particular Oseltamivir. And this only applies to the flu. But we cannot exclude the possibility of combined damage to the respiratory tract, when the disease is caused by several viruses at once, which, of course, greatly complicates the choice of antiviral therapy.
In this regard, in the treatment of respiratory viral infections, it became necessary to use drugs that act not on specific viral proteins, but on the immune mechanisms of antiviral defense. In this situation, the drugs of choice for the treatment of acute respiratory viral infections of various etiologies, including the new coronavirus infection COVID-19, are recombinant interferon drugs.
-Some experts believe that the body needs enough endogenous interferon. How valid is this opinion?
-Unfortunately, this is not entirely true. Of course, viruses are powerful stimulators of the production of endogenous interferon, which does not depend on the taxonomic properties of the pathogen and will continue throughout the period of its replication. From the moment the pathogen comes into contact with the epithelium of the mucous membranes of the respiratory tract, active induction of endogenous (internal) interferon begins. However, this does not mean that its concentration is sufficient for reliable protection against infection, including the new coronavirus infection. In young children, especially newborns, infectious and inflammatory diseases reduce the body's ability to produce interferon alpha and gamma, which reduces the effectiveness of the body's defenses, including local immunity.
Moreover, the results of experimental and clinical studies of our European colleagues indicate that SARS-CoV-2, unlike other respiratory viruses, suppresses the synthesis of its own interferon in the body. When the immune system is weakened, viruses escape the immune mechanisms, the disease prolongs, and complications develop that can even cause an unfavorable outcome. In connection with the above, it is logical in this situation to use recombinant interferon drugs in pediatric practice, the antiviral, immunomodulatory and antioxidant activity of which has been demonstrated in numerous clinical studies. Recombinant interferon-α-2b preparations are effective against infections of various etiologies (viral and bacterial) and localizations (diseases of the respiratory and gastrointestinal tract, nervous and urinary systems, congenital infections). It is important that interferon preparations can be used in patients of completely different age categories: children, including newborns, adolescents, adults, including pregnant women and the elderly. Therefore, the range of indications for the use of recombinant interferon drugs is very wide, without any exaggeration.
The leader among recombinant interferon-α-2b drugs, of course, is VIFERON®, the therapeutic and preventive effectiveness of which has been proven in numerous studies in patients of different age groups with different health conditions. Our clinic also took part in studying the effectiveness and safety of recombinant interferon drugs in the treatment of children with the new coronavirus infection COVID-19.
-Why did VIFERON® become the object of research?
-First of all, because VIFERON® has convenient release forms for children's practice - suppositories (they act systemically, that is, on the entire body as a whole), as well as gel and ointment (they act locally, at the site of the lesion). When applied topically, interferon prevents the fixation and multiplication of viruses at the site of their introduction, thus creating an obstacle for them: the virus cannot penetrate the cell, it has nowhere to multiply. Therefore, local gel or ointment forms can be successfully used not only for treatment purposes, but also for effective prevention.
Administration of recombinant interferon-α-2b from the moment the very first symptoms of the disease appear suppresses viral replication. It is not surprising that not only in Russia, but also in Europe, there is currently an active study of the use of recombinant interferon drugs in the treatment and prevention of the new coronavirus infection COVID-19.
-Tell me more about the study in which you participated.
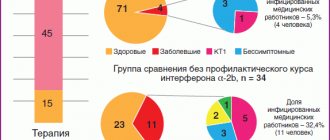
-The study was conducted in three research centers where hospitals were deployed to treat children with COVID-19: in Moscow, Krasnoyarsk and Kazan. The purpose of the study was to study the effectiveness of interferon-α-2b in high doses. After all, SARS-CoV-2 blocks the production of endogenous interferon, and therefore it is advisable to administer high doses of recombinant interferon-α-2b.
The study involved 140 children aged 1 to 17 years, who were divided into two age groups. The first - children from 1 year to 7 years old who received recombinant interferon-α-2b (VIFERON®) in the form of rectal suppositories as etiotropic antiviral therapy in the form of rectal suppositories at a dosage of 1,000,000 IU 2 times a day in combination with VIFERON® Gel 5-6 times per day locally (on the nasal mucosa).
The second group - children from 8 to 17 years old, received rectal suppositories VIFERON® 3,000,000 IU 2 times a day in combination with VIFERON® Gel topically 5-6 times a day.
The control group consisted of 70 children who received the drug with direct antiviral action “Umifenovir” as antiviral therapy in a therapeutic dosage in accordance with the age of the child.
Clinical effectiveness was assessed by reducing the severity of clinical symptoms of the disease: relief of fever, cough, nasal congestion, rhinorrhea, abdominal pain, restoration of the mucous membranes of the eyes, normalization of smell and taste. The study examined the dynamics of coronavirus isolation, the level of IgM and IgG antibodies to SARS-CoV-2 in the blood serum, which allows us to assess the rate of formation of the immune response.
-Can we already talk about the results of the work?
-Yes, the study is almost completed, and its results showed positive dynamics during therapy in patients of the main and control groups. At the same time, it should be noted that in patients of the main group receiving combination therapy with recombinant interferon-α-2b drugs (VIFERON® Rectal Suppositories and Gel), the relief of clinical symptoms of the disease occurred significantly faster compared to children in the comparison group.
Children in the main group developed antibodies to SARSCoV-2 in their blood serum significantly faster (first IgM and then IgG) compared to participants in the control group. This confirms the fact that the immune response to the new coronavirus with the use of recombinant interferon in high doses in a combined regimen was formed more quickly compared to therapy with a direct antiviral drug. Of course, this was reflected in the duration of clinical manifestations of the disease and shortened the length of stay of children in the hospital.
I would like to note that among the patients in the control group there were children (as a rule, teenagers with damage to the lower respiratory tract, pneumonia), who, on the 8th–9th day from the date of prescription of antiviral therapy with Umifenovir, still had clinical manifestations of the disease, in control smears from SARS-CoV-2 continued to be released from the nasopharynx. Prescribing patients a second course of antiviral therapy with recombinant interferon-α-2b drugs (rectal suppositories and gel) led not only to the relief of clinical symptoms of COVID-19, but also to the sanitization of the body from the virus; already on the 5th–7th day the virus was found in smears from the nasopharynx was not determined.
-That is, the use of interferon drugs reduces the period of virus shedding?
-Yes, when using the drug VIFERON®, sanitization of the body occurred faster: in nasopharyngeal swabs in more than half of the children, the virus was not detected already on the 5th–7th day (using the PCR method), unlike the children in the control group. Moreover, its use made it possible to reduce the period of virus isolation from feces, although, according to the literature, virus isolation from feces occurs over a longer period of time compared to its isolation from the mucous membranes of the nasopharynx.
We analyzed the results of a stool PCR study for SARS-CoV-2 in children with a new coronavirus infection, dividing them into three groups. The first group of patients received Umifenovir as antiviral therapy, the second group received VIFERON® in standard low dosages, and the third group received VIFERON® in high dosages (these were children who participated in our study). During therapy, patients in three groups underwent stool testing for SARS-CoV-2 using PCR at the beginning of therapy and at the end of it, on days 11–13. The results showed that with the use of the drug VIFERON®, the release of the virus in feces ceases significantly faster compared to children receiving Umifenovir. Moreover, a relationship was identified between the dose of interferon and the period of virus shedding: in the group of children receiving high dosages of the drug VIFERON®, this period was shorter than in children receiving standard dosages.
Thus, we are talking not only about the clinical effectiveness of recombinant interferon-α-2b with antioxidants in the form of rectal suppositories and gel for external use in the treatment of a new coronavirus infection, but also to a significant extent in reducing the time of viral replication and ongoing viral shedding. This is very important, since the release of the virus into the environment is the cause of the spread of infection. A patient who no longer has clinical symptoms of the disease may consider himself healthy, but in fact may continue to release the virus into the environment, remaining a potential source of infection.
-Can interferons be used to treat adult patients?
-Yes, we studied this issue as well. Many children are hospitalized with mothers who, as a rule, are also sick with coronavirus infection. Most often, they were prescribed Umifenovir as antiviral therapy, but in some cases, despite the therapy, women continued to isolate the virus in nasopharyngeal swabs. In such situations, VIFERON® was recommended, which demonstrated positive results.
Research data and clinical experience confirm that recombinant interferons, in particular VIFERON®, in systemic (suppositories) and local (gel or ointment) forms are advisable to use for the treatment of acute respiratory infections, including coronavirus infection in both children and adults .
Original publication: https://viferon.su/vestnikferona_sp_2020_2/
Author: Marina Izvarina
Interested in the article? You can find out even more in the Expert Opinions section
Compound
Active ingredients: Alpha-glutamyl-tryptophan sodium [Thymogen sodium] (in terms of alpha-glutamyl-tryptophan) 0.15 mg Ascorbic acid 12 mg Bendazole hydrochloride (dibazol) 1.25 mg Excipients: Sucrose 800.0 mg Purified water up to 1 ml
Description:
The syrup is colorless to yellow.
Pharmacotherapeutic group:
Immunostimulating agent.
ATX code: L03AX
Pharmachologic effect:
Pharmacodynamics:
the drug is a means of etiotropic and immunostimulating therapy, has an indirect antiviral effect against influenza A and B pathogens, as well as other viruses that cause acute respiratory diseases. Reduces the severity of the main clinical symptoms of influenza and ARVI, and also shortens the duration of the disease and promotes its uncomplicated course. In in vitro studies, the drug specifically suppresses (inhibits) the reproduction (replication) of the SARS-CoV-2 virus, which is the causative agent of the new coronavirus infection (COVID-19). Increases the content of secretory immunoglobulin A (slgA) in the mucous membrane of the nasopharynx - the entrance gate of infection, increasing the local immunoresistance of the body to respiratory infections of a viral and bacterial nature. With a preventive effect, the drug increases the potential metabolic activity of innate immune cells (neutrophil granulocytes and monocytes), which, in the event of infection, increases their ability to absorb and destroy bacterial and viral agents due to increased enzymatic (oxidative) activity, synthesis of cationic proteins and increased number of phagocytic cells. In this case, the initial state of metabolic activity of innate immune cells, in the absence of infectious agents, does not change, being within normal values. Bendazole induces the body's production of endogenous interferon and has an immunomodulatory effect (normalizes the body's immune response). Enzymes, the production of which is induced by interferon in the cells of various organs, inhibit viral replication. Alpha-glutamyl-tryptophan (thymogen) is a synergist for the immunomodulatory effect of bendazole, normalizing the T-cell component of immunity. Ascorbic acid activates the humoral component of the immune system, normalizes capillary permeability, thereby reducing inflammation, and exhibits antioxidant properties, neutralizing oxygen radicals that accompany the inflammatory process, and increases the body's resistance to infection.
Pharmacokinetics:
When taken orally, the drug is completely absorbed from the gastrointestinal tract. The bioavailability of bendazole is about 80%, alpha-glutamyl tryptophan is no more than 15%, ascorbic acid is up to 70%. Ascorbic acid is absorbed in the gastrointestinal tract (mainly in the jejunum). Communication with plasma proteins – 25%. TCmax after oral administration – 4 hours. Easily penetrates into leukocytes, platelets, and then into all tissues, penetrates the placenta. Gastrointestinal diseases (peptic ulcer of the stomach and duodenum, intestinal motility disorders, helminthic infestation, giardiasis), consumption of vegetable and fruit juices, and alkaline drinking reduce the binding of ascorbic acid in the intestine. Ascorbic acid is metabolized primarily in the liver into deoxyascorbic acid, then into oxaloacetic and diketogulonic acids. It is excreted by the kidneys, through the intestines, with sweat, breast milk unchanged and in the form of metabolites. The products of bendazole biotransformation in the blood are two conjugates formed as a result of methylation and carboethoxylation of the imino group of the imidazole ring of bendazole: 1-methyl-2-benzylbenzimidazole and 1-carboethoxy-2-benzylbenzimidazole. Bendazole metabolites are excreted in the urine. Alphaglutamyl-tryptophan, under the influence of petidases, is broken down into L-glutamic acid and L-tryptophan, which are used by the body in protein synthesis.
Indications for use:
Prevention and complex therapy of influenza and acute respiratory viral infections in children over one year of age.
Contraindications:
hypersensitivity to the components of the drug, diabetes mellitus, pregnancy, children under 1 year of age. If necessary, please consult your doctor before taking the drug.
Precautions for use.
Consult your doctor before taking the drug if you have hypotension.
Use during pregnancy and breastfeeding:
During pregnancy, taking the drug is not recommended due to insufficient clinical data. During breastfeeding, use is possible if the expected benefit to the mother outweighs the potential risk to the child. If you need to take the drug during breastfeeding, please consult your doctor.
Directions for use and dosage:
Orally 30 minutes before meals. For treatment:
Children aged 1 to 3 years – 2 ml 3 times a day;
children aged 3 to 6 years – 4 ml 3 times a day; children aged 6 to 10 years – 8 ml 3 times a day; children over 10 years of age – 12 ml 3 times a day. Course of application – 4 days. If after 3 days of treatment there is no improvement or the symptoms worsen or new symptoms appear, you should consult your doctor. For prevention:
- in direct contact with a patient with influenza and other acute respiratory viral infections: Children aged 1 to 3 years - 2 ml 3 times a day; children aged 3 to 6 years – 4 ml 3 times a day; children aged 6 to 10 years – 8 ml 3 times a day; children over 10 years of age – 12 ml 3 times a day. Course of application – 4 days. Preventive courses, if necessary, are repeated after 3 weeks (until the epidemic situation normalizes).
Side effect:
a short-term decrease in blood pressure is possible. Possible allergic reactions: urticaria. In these cases, the use of the drug is stopped and symptomatic treatment and antihistamines are prescribed. Consult your doctor if you experience any of the side effects mentioned or other side effects not listed in the instructions. If the side effects listed in the instructions get worse, tell your doctor.
Overdose:
Cases of overdose have not been described.
Interaction with other drugs:
No interaction of alpha-glutamyl-tryptophan with drugs has been identified. Bendazole prevents the increase in total peripheral vascular resistance caused by the use of non-selective beta-blockers. Strengthens the hypotensive (lowering blood pressure) effect of antihypertensive and diuretic drugs. Phentolamine enhances the hypotensive effect of bendazole. Ascorbic acid increases the concentration in the blood of antibacterial drugs of the tetracycline series and benzylpenicillin. Improves the absorption of iron (Fe) preparations in the intestine. Reduces the effectiveness of heparin and indirect anticoagulants. Acetylsalicylic acid (ASA), oral contraceptives, fresh juices and alkaline drinks reduce its absorption and absorption. When used simultaneously with ASA, the urinary excretion of ascorbic acid increases and the excretion of ASA decreases. ASA reduces the absorption of ascorbic acid by approximately 30%. Ascorbic acid increases the risk of developing crystalluria when using drugs containing acetylsalicylic acid (ASA) and short-acting sulfonamides, slows down the excretion of acids by the kidneys, increases the excretion of drugs with an alkaline reaction (including alkaloids), and reduces the concentration in the blood of oral contraceptives. When used simultaneously, it reduces the chronotropic effect of isoprenaline. Reduces the therapeutic effect of antipsychotic drugs (neuroleptics) - phenothiazine derivatives, tubular reabsorption of amphetamine and tricyclic antidepressants. Barbiturates and primidone increase the excretion of ascorbic acid in the urine. Possible simultaneous use with antiviral drugs and symptomatic treatment of influenza and ARVI. Consult your doctor if you are taking the medications listed in this section or other medications.
Special instructions:
After a repeated course, monitoring of blood glucose concentrations is recommended.
Impact on the ability to drive vehicles and machinery:
the drug does not affect the ability to drive vehicles, operate moving machinery, or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Release form:
Syrup [for children]. 50 ml in a dark glass bottle. The bottle is sealed with a plastic cap with tamper evident or a plastic cap with tamper evident and childproof. One bottle is placed in a cardboard pack. Instructions for use are placed in a pack or in the form of a folded sheet placed under the opening part of the label. A dosing device is placed in the pack: a measuring cup or a dosing spoon, or a dosing pipette.
Storage conditions:
Store at a temperature not exceeding 25 Сo. Keep out of the reach of children.
Best before date:
2 years. Do not use after the expiration date stated on the packaging.
Vacation conditions:
available without a prescription.
Manufacturer / legal entity in whose name the registration certificate was issued / organization receiving complaints regarding the quality of the drug: Joint Stock Company Medical-Biological Research and Production Complex Cytomed (JSC MBNPK Cytomed). Address: Russia, 194356, St. Petersburg, Orlovo-Denisovsky prospect, building 14, building 1, tel., cytomed.ru Production site address: Russia, St. Petersburg, Orlovo-Denisovsky prospect, building 14, building 1.
General Director A.N. Khromov JSC Medical-Biological Research and Production Complex Cytomed
Treatment of acute respiratory diseases in children
Acute respiratory diseases (ARI) are a large group of infections that have much in common in pathogenesis and transmission routes: we are talking mainly about airborne infections, although contact (through dirty hands) transmission route plays an equally important role. This term is used to combine acute nonspecific infections, regardless of their location - from rhinitis to pneumonia. However, as a clinical diagnosis of acute respiratory infections, it requires deciphering: there must be an indication of either organ damage (otitis media, bronchitis, pharyngitis, etc.), for which the spectrum of pathogens is known, or the possible etiology of the disease (viral, bacterial acute respiratory infections). Since up to 90% of acute respiratory infections are caused by respiratory viruses and influenza viruses, in the absence of signs of bacterial infection, the term “acute respiratory viral infection” (ARVI) and the prescription of antiviral therapy are justified.
According to the authors of a series of works carried out under the auspices of WHO, in different countries - both developed and developing - young children suffer 5-8 acute respiratory infections annually, and in rural areas they get sick less often than in cities, where a child can suffer 10- 12 infections per year. Children, who in early childhood have less contact with sources of infection and therefore get sick less during this period, “get the missing infections” in primary school. Statement of this fact, of course, should not be the reason for the development of fatalism regarding ARVI - children should be hardened and, if possible, protected from sources of infection, adequately fed and treated for diseases (chronic tonsillitis, allergies), against the background of which ARVI develops especially often. At the same time, it is necessary to protect sick children in every possible way from unnecessary therapeutic interventions, since acute respiratory infections are the reason for unnecessary treatment and the most common cause of side effects of drugs.
Antiviral agents
Strictly speaking, antiviral therapy is indicated for any respiratory viral disease. Unfortunately, the antiviral drugs at our disposal often do not provide a pronounced effect, and the mildness of most episodes of ARVI, limited to 1-3 feverish days and catarrhal syndrome for 1-2 weeks, does not justify chemotherapy. But in more severe cases, especially with influenza, antiviral drugs have a certain effect and should be used more widely than is considered appropriate today.
The basic rule for the use of antiviral chemotherapy drugs is their administration in the first 24–36 hours of illness; at a later date, their effect is not visible. The main anti-influenza drug, which also acts on a number of other viruses [1], is rimantadine, which suppresses the reproduction of all strains of influenza type A. Rimantadine also inhibits the reproduction of respiratory syncytial (RS) and parainfluenza viruses. Recommended; 5-day course at the rate of 1.5 mg/kg/day in 2 divided doses for children 3–7 years old; 50 mg 2 times for children 7–10 years old - 3 times a day - over 10 years old [2]. At an early age, rimantadine is used in the form of algirem (0.2% syrup): in children 1–3 years old, 10 ml; 3-7 years - 15 ml: 1st day 3 times, 2nd-3rd days - 2 times, 4th - 1 time a day. The effectiveness of rimantadine increases when taken with the drug no-shpa (drotaverine) orally, at a dose of 0.02–0.04 g for children 4–6 years old and 0.04–0.1 g for patients 7–12 years old. especially when heat transfer is impaired (cold extremities, marbling of the skin) [3].
Arbidol has a similar antiviral effect, inhibiting the fusion of the lipid membrane of influenza viruses with the membrane of epithelial cells. It is also an interferon inducer. This low-toxic drug can also be prescribed for moderate ARVI from the age of 2: for children 2–6 years old, 50 mg per dose, 6–12 years old, 100 mg, over 12 years old, 200 mg per dose 4 times a day. Both rimantadine and arbidol reduce the febrile period by an average of 1 day in both influenza A2, mixed infections, and non-influenza ARVI [1].
Ribavirin (ribamidil, virazole) is an antiviral drug originally used (mainly in the USA) as having activity against the RS virus in bronchiolitis in the most severely ill patients with an unfavorable premorbid background (premature infants, with bronchopulmonary dysplasia). The drug is used for this purpose in the form of continuous (up to 18 hours a day) inhalations through a special inhaler at a dose of 20 mg/kg/day; Due to the high price and side effects, it is practically not used in Europe. It also turned out that this drug is active against influenza viruses, parainfluenza, herpes simplex, adenoviruses, as well as coronavirus, the causative agent of severe acute respiratory syndrome (SARS). For influenza in adolescents over 12 years of age, it is used orally at a dose of 10 mg/kg/day for 5–7 days. For SARS, ribavirin is administered intravenously.
Progress in the treatment of influenza caused by both type A and type B viruses may be due to the use of the neuraminidase inhibitors oseltamivir-Tamiflu and zanamivir-Relenza. These drugs, when taken early, reduce the duration of fever by 24–36 hours and have a preventive effect, but there is little experience in their use in children (from 12 years of age) in Russia, and in recent years there is practically no mention of them in reference books. Relenza is used in the form of powder inhalations (in the USA from 7 years of age) - 2 inhalations (5 mg each) per day with an interval of at least 2 hours (on the 1st day) and 12 hours (from the 2nd to the 5th day treatment). Tamiflu (75 mg capsules and 12 mg/ml suspension) in adults and children over 12 years old is used at 75 mg once a day for 5 days (in the USA, doses for children 1–12 years old: weighing up to 15 kg - 30 mg 2 times a day, 15-23 kg - 45 mg 2 times a day, 23-40 kg - 60 mg 2 times a day). This drug is the only one to which H5N1 avian influenza is sensitive, and a number of countries are currently stockpiling it in case of an epidemic, which apparently limits its use with relatively small production (Hoffman-La Roche, Switzerland, produces 7 million doses of Tamiflu in year).
The drugs Florenal 0.5%, oxolinic ointment 1–2%, bonafton, lokferon and others used locally (in the nose, in the eyes) have some antiviral activity; they are indicated, for example, for adenovirus infection. Although their effect is difficult to assess, the low toxicity justifies the use of these agents.
Proteolytic processes occurring during the synthesis of viral polypeptides, as well as the fusion of viruses with cell membranes, can inhibit aprotinins - contrical, gordox, etc., as well as amben. These drugs can be used for severe forms of respiratory infections with high inflammatory activity, usually with signs of disseminated intravascular coagulation (as fibrinolysis inhibitors) and microcirculatory disorders. Ambien is part of hemostatic sponges. Contrical is used at a dose of 500–1000 IU/kg/day. Olifen and Erisod, used in adults and included in this group of drugs, have not yet been tested in children.
Interferons and their inducers have universal antiviral properties, suppressing the replication of both RNA and DNA, while simultaneously stimulating the immunological reactions of the macroorganism. Early use of interferons can, if not interrupt the course of the infection, then mitigate its manifestations.
Native leukocyte interferon α (1000 IU/ml - 4-6 times a day in the nose in a total dose of 2 ml on the 1st-2nd day of illness) is less effective than recombinant interferon preparations [4]. Among the latter, the use of influenza feron - interferon α-2β (10,000 IU/ml) with thickeners is promising; it is administered in the form of drops in the nose - 5 days, for children under one year old - 1 drop 5 times a day (single dose 1000 IU, daily dose - 5000 IU), for children from 1 to 3 years old 2 drops 3-4 times a day (single dose 2000 IU, daily dose - 6000-8000 IU), from 3 to 14 years - two drops 4-5 times a day (single dose - 2000 IU, daily dose - 8000-10 000 IU). The administration of interferon drugs parenterally, practiced, for example, for the treatment of chronic hepatitis, is hardly justified in the vast majority of respiratory infections. However, a number of studies have shown the effectiveness of rectal suppositories Viferon - interferon α-2β + vitamins E and C for influenza and ARVI. Viferon-1 (150,000 IU) is used in children under 6 years of age, Viferon-2 (500,000 IU) in children older 7 years - they are prescribed 2-3 times a day for 5 days. Viferon is also used prophylactically in frequently ill children [3].
Laferon - interferon α-2β powder - is used in the form of nasal drops, and in children over 12 years of age it is administered intramuscularly at a dose of 1-3 million IU.
In addition to arbidol, a number of drugs are used as interferon inducers. Amiksin (Tilorone) has gained the most popularity among children over 7 years of age - it is administered at the first symptoms of acute respiratory infections or flu orally after meals, 60 mg 1 time per day on the 1st, 2nd, and 4th day from the start of treatment. Children's anaferon - homeopathic doses of affinity-purified antibodies to interferon α, it is used 1 tablet every 30 minutes for 2 hours, then 3 times a day, but there is little convincing data on its effectiveness so far.
In children with ARVI, it is often necessary to treat a primary herpesvirus infection that occurs as severe febrile stomatitis. Children with atopic dermatitis often develop Kaposi's eczema, a herpesvirus infection of the affected skin that is also severe. In older children, ARVI is the most common cause of reactivation of herpes viruses in the form of specific rashes on the lips, wings of the nose, and less often on the genitals. This infection responds well to treatment with acyclovir - it is used at a dose of 20 mg/kg/day in 4 doses, in severe cases - up to 80 mg/kg/day or intravenously at 30-60 mg/kg/day. Valacyclovir does not require divided administration; its dose for adults and adolescents over 12 years of age is 500 mg 2 times a day.
For the treatment of acute respiratory viral infections, a significantly larger number of drugs are used in practice, including those of plant origin (adaptogens, dietary supplements, tinctures, etc.). There is no data on the effectiveness of the vast majority of them, but side effects are often encountered.
Antibacterial agents
Bacterial acute respiratory infections in children, as in adults, are relatively few in number, but they pose the greatest threat in terms of the development of serious complications. Making a diagnosis of bacterial acute respiratory infections at the bedside of an acutely ill child is very difficult due to the similarity of many of their manifestations with those of acute respiratory viral infections (fever, runny nose, cough, sore throat), and rapid methods of etiological diagnosis are practically unavailable. And the identification of a microbial pathogen in the material of the respiratory tract does not indicate its etiological role, since most bacterial diseases are caused by pathogens that constantly grow in the respiratory tract.
Under these conditions, naturally, the doctor, at the first contact with the child, tends to overestimate the possible role of the bacterial flora and use antibiotics more often than necessary. Our data show that in Moscow antibiotics are prescribed to 25% of children with ARVI; in some Russian cities this figure reaches 50–60%. The same trend is typical for other countries: antibiotics for ARVI are used in children in 14–80% of cases [6, 7]. Indicators close to our data are given by authors from France (24% [8]) and the USA (25% [9]). In developing countries, antibacterial drugs for acute respiratory infections are also used too widely, although this process is constrained by their lower availability. In China, 97% of children with acute respiratory infections who seek medical help receive antibiotics [10]. It is obvious that with a viral etiology of the disease, antibiotics are at least useless and, most likely, even harmful, since they disrupt the biocenosis of the respiratory tract and thereby contribute to the colonization of them by unusual, often intestinal, flora [11].
Antibiotics in children with ARVI more often than in bacterial diseases cause side effects - various rashes and other allergic manifestations. During bacterial processes in the body, a powerful release of a number of mediators (for example, cyclic adenosine monophosphate) occurs, which prevent the manifestation of allergic manifestations. This does not happen with viral infections, so allergic reactions occur much more often.
Another danger of excessive use of antibiotics is the spread of drug-resistant strains of pneumotropic bacteria, which is observed in many countries around the world. It is obvious that the unjustified use of antibiotics also leads to unnecessary treatment costs.
The effect of antibiotics on the development of the child’s immune system should not be ignored. The predominance of the immune T-helper response type 2 (Th-2), characteristic of a newborn, is inferior to the more mature T-helper response type 1 (Th-1), largely under the influence of stimulation by endotoxins and other products of bacterial origin. Such stimulation occurs both during a bacterial infection and during an acute respiratory viral infection, since a viral infection is accompanied by increased (albeit non-invasive) proliferation of pneumotropic flora [11]. Naturally, the use of antibiotics weakens or even suppresses this stimulation, which, in turn, contributes to the preservation of the Th-2 direction of the immune response, which increases the risk of allergic manifestations and reduces the intensity of anti-infective protection.
Indications for antibacterial treatment of acute respiratory infections
Recommendations from professional pediatric societies in most countries emphasize the importance of not using antibacterial agents in children with uncomplicated respiratory viral infection. The recommendations of the US Academy of Pediatrics emphasize that antibiotics are not used not only for uncomplicated ARVI, but also mucopurulent runny nose is also not an indication for the prescription of antibiotics if it lasts less than 10–14 days [8]. The French consensus allows the use of antibiotics for ARVI only in children with a history of recurrent otitis media, in infants under 6 months of age, if they attend a nursery, and in the presence of immunodeficiency [9].
The recommendations of the Union of Pediatricians of Russia indicate that for uncomplicated ARVI, systemic antibiotics are not indicated in the vast majority of cases [4]. This document lists symptoms observed in the first 10–14 days that do not justify the administration of antibiotics.
The question of prescribing antibiotics in a child with ARVI arises if he has a history of recurrent otitis media, an unfavorable premorbid background (severe malnutrition, congenital malformations) or if there are clinical signs of immunodeficiency.
The following are signs of a bacterial infection that require antibacterial treatment:
- purulent processes (sinusitis with swelling of the face or orbit, lymphadenitis with fluctuation, paratonsillar abscess, descending laryngotracheitis);
- acute tonsillitis with culture of group A streptococcus;
- anaerobic sore throat - usually ulcerative, with a putrid odor;
- acute otitis media, confirmed by otoscopy or with suppuration;
- sinusitis - if clinical and radiological changes in the sinuses persist 10–14 days after the onset of acute respiratory viral infection;
- respiratory mycoplasmosis and chlamydia;
- pneumonia.
More often than these obvious lesions, the pediatrician sees only indirect symptoms of a probable bacterial infection, among which the most common are persistent (3 days or more) febrile temperature, shortness of breath in the absence of obstruction (respiratory rate above 60 per 1 min in children 0–2 months of age , more than 50 per 1 minute at the age of 3–12 months and more than 40 in children 1–3 years old), asymmetry of auscultatory data in the lungs. Such symptoms force one to prescribe an antibiotic, which, if the diagnosis is not confirmed during subsequent examination, should be immediately discontinued.
For initial treatment of bacterial acute respiratory infections, a small set of antibiotics is used. For otitis and sinusitis, amoxicillin 45–90 mg/kg/day is prescribed orally to suppress the main pathogens - pneumococcus and Haemophilus influenzae. In children who have recently received antibiotics, amoxicillin/clavulanate 45 mg/kg/day is used, which suppresses the growth of probably resistant Haemophilus influenzae and Moraxella in these patients.
Acute tonsillitis requires differential diagnosis between adenoviral tonsillitis, infectious mononucleosis and streptococcal tonsillitis. Viral tonsillitis is characterized by cough and catarrhal syndrome, streptococcal tonsillitis is characterized by absence of cough, and mononucleosis is characterized by blood changes. Antibiotics (penicillin vau, cephalexin, cefadroxil) are indicated for streptococcal tonsillitis; the use of amoxicillin is undesirable, since in mononucleosis it can cause toxic rashes. Although adenoviral tonsillitis does not require antibiotics, the presence of severe leukocytosis (15–25x109/l) and increased levels of C-reactive protein justify their use in many cases.
Bronchitis is usually a viral disease that does not require antibacterial treatment. The exception is bronchitis caused by mycoplasma; when they are detected, the use of macrolides (azithromycin, midecamycin, etc.) is indicated. Clinical signs of mycoplasma bronchitis are:
- age (preschool and older);
- high temperature without severe toxicosis;
- an abundance of crepitating wheezing (as with bronchiolitis in infants);
- asymmetry of wheezing;
- mild “dry” catarrh of the upper respiratory tract;
- hyperemia of the conjunctiva (“dry conjunctivitis”);
- local enhancement of the bronchovascular pattern on the radiograph.
The choice of antibacterial agents for the initial treatment of community-acquired pneumonia is also not very large, since most of the “typical” pneumonias are caused by pneumococcus or Haemophilus influenzae (with the exception of the first months of life, when the causative agent can be staphylococci and intestinal flora), while “atypical” forms are treatable macrolides. The choice of starting antibiotic for pneumonia is determined taking into account the likely causative agent of the disease.
For typical pneumonia (febrile, with a focus or homogeneous infiltrate), the following is used:
- 1–6 months (the most likely pathogens are E. coli, staphylococcus) - amoxicillin/clavulanate orally, intravenously; cefuroxime, ceftriaxone or cefazolin + aminoglycoside intravenously, intramuscularly;
- 6 months–18 years: mild (the most likely pathogens are pneumococcus, H. influenzae) — amoxicillin orally; severe (the most likely pathogens are pneumococcus, in children under 5 years old - H. influenzae type b) - cefuroxime, ceftriaxone or cefazolin + aminoglycoside intravenously, intramuscularly.
For atypical (with inhomogeneous infiltrate) pneumonia:
- 1–6 months (the most likely pathogens are C. trachomatis, U. urealyticum, rarely P. carinii) - macrolide, oral azithromycin, co-trimoxazole;
- 6 months–15 years (the most likely pathogens are M. pneumoniae, C. pneumoniae) — macrolide, azithromycin, doxycycline (> 12 years) orally.
Pathogenetic methods of treatment
These methods include interventions used for acute laryngitis and obstructive forms of bronchitis.
Acute laryngitis and croup are conditions that require assessment of the degree of stenosis, as judged by the intensity of inspiratory retractions of the chest, pulse and respiration rates. Grade 3 croup requires emergency intubation, grade 1 and 2 croup is treated conservatively. Antibiotics are not administered to a patient with laryngitis; according to global consensus, intramuscular dexamethasone 0.6 mg/kg is most effective, which stops the progression of stenosis. Further treatment is continued with inhaled steroids (dosed or through a nebulizer - Pulmicort) in combination with antispasmodics (salbutamol, Berotec, Berodual in inhalations).
Laryngeal stenosis can be caused by epiglottitis (H. influenzae type b plays a major role in its etiology) - it is characterized by high temperature and increased stenosis in the supine position; Prescribing an antibiotic (cefuroxime, ceftriaxone) in this case is mandatory.
Difficulty breathing and expiratory shortness of breath are often observed with bronchiolitis and obstructive bronchitis, as well as with an asthma attack against the background of ARVI. Since bacterial infection is rare in such cases, antibiotics are not justified. Treatment - inhaled sympathomimetics (in young children it is better in combination with ipratropium bromide) and the use of steroids in refractory cases - makes it possible to cope with obstruction in 1-3 days.
Symptomatic treatment of acute respiratory infections
As stated above, acute respiratory infections are the most common reason for the use of drugs, in particular symptomatic drugs, which occupy most of the pharmacy shelves. It is important, however, to clearly understand that the mere presence of a particular symptom should not be the basis for intervention; one must first of all assess the extent to which this symptom interferes with life and whether the treatment will be more dangerous than the symptom.
Fever accompanies most acute respiratory infections and is a protective reaction, so reducing its level with antipyretics is justified only in certain situations. Unfortunately, many parents and doctors consider fever the most dangerous manifestation of the disease and strive to normalize the temperature at all costs. According to our research [12], 95% of children with ARVI, including 92% of children with low-grade fever, receive antipyretics. This tactic cannot be considered rational, since fever, as a component of the body’s inflammatory response to infection, is largely protective in nature.
Antipyretics do not affect the cause of fever and do not shorten its duration; they increase the period of viral shedding in acute respiratory infections [12, 13]. With most infections, the maximum temperature rarely exceeds 39.5°. This temperature does not pose any threat to a child older than 2–3 months; Usually, in order to feel better, it is enough to lower it by 1–1.5°. Indications for reducing temperature [4]:
- For previously healthy children over 3 months of age - at a temperature > 39.0°–39.5°, and/or with discomfort, muscle aches and headaches.
- Children with a history of febrile seizures, severe heart and lung diseases, and from 0 to 3 months of life - with a temperature > 38°–38.5°.
The safest antipyretic for children is paracetamol, its single dose is 15 mg/kg, daily dose is 60 mg/kg. Ibuprofen (5–10 mg/kg per dose) more often produces side effects (with a similar antipyretic effect); it is recommended for use in cases where an anti-inflammatory effect is required (arthralgia, muscle pain, etc.).
For acute respiratory infections in children, acetylsalicylic acid (aspirin) is not used - due to the development of Reye's syndrome, metamizole sodium (analgin) orally (danger of agranulocytosis and collaptoid state), amidopyrine, antipyrine, phenacetin. Nimesulide is hepatotoxic; unfortunately, its childhood forms have been registered in Russia, although they are not used anywhere else in the world.
Treatment of a runny nose with vasoconstrictor drops improves nasal breathing only in the first 1–2 days of illness; with longer use, they can worsen a runny nose and also cause side effects. At an early age, due to pain, only 0.01% and 0.025% solutions are used. Convenient (after 6 years) nasal sprays, which allow the drug to be evenly distributed at a lower dose (dlyanos, vibrocil). But the most effective way to cleanse the nose and nasopharynx, especially with thick exudate, is saline solution (or its analogues, including a solution of table salt prepared at home: add salt to 1/2 cup of water on the tip of a knife) - 2-3 pipettes in each nostril 3-4 times a day, lying on your back with your head hanging down and back. Orally administered remedies for the common cold containing sympathomimetics (phenylephrine, phenylpropanolamine, pseudoephedrine) are used after 12 years of age; from 6 years of age, Fervex for children, which does not contain these components, is prescribed. Antihistamines, including second generation, effective for allergic rhinitis, are not recommended by WHO for use in acute respiratory infections [15].
The indication for prescribing antitussives (non-narcotic centrally acting drugs - glaucine, butamirate, oxeladin) is only a dry cough, which usually quickly becomes wet in case of bronchitis. Expectorants (their cough-stimulating effect is similar to an emetic) are of questionable effectiveness and can cause vomiting in young children, as well as allergic reactions, including anaphylaxis. Their purpose is more a tribute to tradition than a necessity; expensive remedies from this group have no advantages over conventional herbal remedies; WHO generally recommends limiting oneself to “home remedies” [15].
Among the mucolytics, acetylcysteine is the most active, but in acute bronchitis in children there is practically no need for its use; carbocisteine is prescribed for bronchitis - based on its beneficial effect on mucociliary clearance. Ambroxol for thick sputum is used both orally and inhalations. Aerosol inhalations of mucolytics are used for chronic bronchitis; Aerosol inhalations of water, saline, etc. are not indicated for acute respiratory infections.
For long-lasting cough (whooping cough, persistent tracheitis), anti-inflammatory drugs are indicated: inhaled steroids, fenspiride (erespal). Emollient lozenges and sprays for pharyngitis usually contain antiseptics and are used from 6 years of age; starting from 30 months, a local antibiotic, fusafyungin, is used, produced in an aerosol (bioparox) and used both nasally and orally.
Mustard plasters, cups, and burning patches, which are still popular in Russia for bronchitis, should not be used in children; In case of acute respiratory infections, there are rarely indications for physiotherapy. Surprising is the popularity of halochambers, the purpose of which is to “inhale table salt vapors,” as in a salt mine. But in a salt mine, the patient is not exposed to salt (which is not a volatile substance), but to clean air, free of dust and other allergens; In addition, they are not there for 15 minutes. Treatment in a halochamber is not included in the consensus on asthma, however, many clinics spend a lot of money on their construction.
The means indicated in this section, with a few exceptions, cannot be considered mandatory for ARVI; Moreover, we often encounter side effects resulting from such treatment. Therefore, one should make it a rule to minimize drug loads in cases of mild ARVI.
The problem of acute respiratory infections in childhood remains relevant not only because of their prevalence, but also due to the need to revise and optimize treatment tactics. Accumulated data show that the approaches prevailing in pediatric practice at least do not contribute to the development of the child’s immune system, therefore, a revision of tactics should be primarily aimed at modifying therapeutic activity, in particular at reducing cases of unjustified prescriptions of antibacterial and antipyretic drugs.
Literature
- Drinevsky V.P. Assessment of the safety and effectiveness of new drugs for etiotropic treatment and specific prevention of influenza in children. M., 1999.
- Drinevsky V.P., Osidak L.V., Natsina V.K. et al. Chemotherapy in the treatment of influenza and other acute respiratory viral infections in children // Antibiotics and chemotherapy. M., 1998. T. 43. Issue. 9. pp. 29–34.
- Ministry of Health of the Russian Federation, Russian Academy of Medical Sciences, Research Institute of Influenza. Standardized principles for diagnosis, treatment and emergency prevention of influenza and other acute respiratory infections in children. St. Petersburg, 2004.
- Union of Pediatricians of Russia, International Foundation for Mother and Child Health: Scientific and practical program “Acute respiratory diseases in children. Treatment and prevention." M., 2002.
- Mainous A., Hueston W., Love M. Antibiotics for colds in children: who are the high prescribers? Arch. Pediatr. Adolesc. Med. 1998; 52:349–352.
- Pennie R. Prospective study of antibiotic prescribing for children. Can. Fam. Physician 1998; 44: 1850–1855.
- Nyquist A., Gonzales R., Steiner GF, Sande MA Antibiotic prescribing for children with colds, upper respiratory infections, and bronchitis. JAMA 1998; 279:875–879.
- Chalumeneau M., Salannave B., Assathiany R. et al. Connaissance et application par des pediatres de ville de la conference de concensus sur les rhinopharyngites aigues de l'enfant. Arch. Pediatr. 2000; 7(5), 481–488.
- Jacobs RF Judicious use of antibiotics for common pediatric respiratory infections. Pediatr. Infect. Dis. J. 2000; 19(9):938–943.
- Li Hui, Xiao–Song Li, Xian–Jia Zeng et al. Pattern and determinants of use of antibiotics for acute respiratory tract infections in children in China. Pediatr. Infect. Dis J. 1997; 16 (6): 560RZR–564.
- Acute pneumonia in children/Ed. V. K. Tatochenko. Cheboksary: Publishing house. Chuvash University, 1994.
- Shokhtobov H. Optimization of the management of patients with acute respiratory infections in the pediatric area: Dis. ...cand. honey. Sci. M., 1990. 130 p.
- Romanenko A.I. Course and outcomes of acute respiratory diseases in children: Abstract of thesis. dis. ...cand. honey. Sci. M., 1988.
- Stanley ED, Jackson GG, Panusarn C. et al. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA 1975; 231:1248.
- World Health Organization. Cough and cold remedies for the treatment of acute respiratory infections in young children. WHO/FCH/CAH/01.02. WHO. 2001.
V. K. Tatochenko , Doctor of Medical Sciences, Professor of the Scientific Center for Children's Diseases of the Russian Academy of Medical Sciences, Moscow
Composition and release form
5 ml (1 teaspoon) of syrup contains vitamin B1 4 mg (thiamine 5 mg), vitamin B2 1 mg (riboflavin 1.32 mg), vitamin B6 (pyridoxine hydrochloride) 2 mg, vitamin B12 5 mcg, niacin (nicotinamide, vitamin B3) 8 mg, folic acid 150 mcg, vitamin C (ascorbic acid) 10 mg, pantothenic acid 2 mg, calcium glycerophosphate 10 mg, iron 7 mg (iron ammonium citrate 35 mg), zinc (sulfate) 5 mg, manganese 0 .25 mg (manganese sulfate 21.99 mg), copper 0.25 mg (copper sulfate 0.91 mg), iodine 40 mcg (potassium iodide 52.32 mcg), lysine hydrochloride 40 mg, honey 100 mg, malt extract 500 mg; in dark glass bottles of 200 ml, 1 bottle in a box.
pharmachologic effect
Pharmacological action - replenishes iron deficiency, replenishes deficiency of vitamins and minerals, normalizes hemoglobin biosynthesis.
Vitamins B1, B2, B3, B6, B12, folic and pantothenic acids, zinc are necessary for the biosynthesis of hemoglobin and other proteins, energy metabolism, and maintaining the central nervous system in a normal state. Iron is the main component of hemoglobin. Vitamin C and copper increase the efficiency of iron absorption. Manganese regulates carbohydrate metabolism. Iodine is necessary for the formation of thyroxine and the regulation of thyroid function, especially in children and during pregnancy. Calcium glycerophosphate and lysine maintain the normal state of the body as a whole.
Instructions for use of Cytovir-3 powder
Pharmacodynamics:
the drug is a means of etiotropic and immunostimulating therapy, has an indirect antiviral effect against influenza A and B pathogens, as well as other viruses that cause acute respiratory diseases.
In in vitro studies, the drug specifically suppresses (inhibits) the reproduction (replication) of the SARS-CoV-2 virus, which is the causative agent of the new coronavirus infection (COVID-19).
The degree of inhibition of the pathogen increases with increasing concentration of the drug (the linearity of the dose-effect relationship is shown).
Reduces the severity of the main clinical symptoms of influenza and ARVI, and also shortens the duration of the disease and promotes its uncomplicated course. Increases the content of secretory immunoglobulin A (sIgA) in the mucous membrane of the nasopharynx - the entrance gate of infection, increasing the local immunoresistance of the body to respiratory infections of a viral and bacterial nature.
With a preventive effect, the drug increases the potential metabolic activity of innate immune cells (neutrophil granulocytes and monocytes), which, in the event of infection, increases their ability to absorb and destroy bacterial and viral agents due to increased enzymatic (oxidative) activity, synthesis of cationic proteins and increased number of phagocytic cells. In this case, the initial state of metabolic activity of innate immune cells, in the absence of infectious agents, does not change, being within normal values.
Bendazole induces the body's production of endogenous interferon and has an immunomodulatory effect (normalizes the body's immune response). Enzymes, the production of which is induced by interferon in the cells of various organs, inhibit viral replication. Alpha-glutamyl-tryptophan (thymogen) is a synergist for the immunomodulatory effect of bendazole, normalizing the T-cell component of immunity.
Ascorbic acid activates the humoral component of the immune system, normalizes capillary permeability, thereby reducing inflammation, and exhibits antioxidant properties, neutralizing oxygen radicals that accompany the inflammatory process, and increases the body's resistance to infection.
Pharmacokinetics:
When taken orally, the drug is completely absorbed from the gastrointestinal tract. The bioavailability of bendazole is about 80%, alpha-glutamyl-tryptophan is no more than 15%, ascorbic acid is up to 70%. Ascorbic acid is absorbed in the gastrointestinal tract (mainly in the jejunum). Communication with plasma proteins – 25%. Tmax after oral administration – 4 hours. Easily penetrates into leukocytes, platelets, and then into all tissues, penetrates the placenta.
Gastrointestinal diseases (peptic ulcer of the stomach and duodenum, impaired intestinal motility, helminthic infestation, giardiasis), consumption of vegetable and fruit juices, and alkaline drinking reduce the binding of ascorbic acid in the intestine.
Ascorbic acid is metabolized primarily in the liver into deoxyascorbic acid, then into oxaloacetic and diketogulonic acids.
It is excreted by the kidneys, through the intestines, with sweat, breast milk unchanged and in the form of metabolites. The products of bendazole biotransformation in the blood are two conjugates formed as a result of methylation and carboethoxylation of the imino group of the imidazole ring of bendazole: 1-methyl-2-benzylbenzimidazole and 1-carboethoxy-2benzylbenzimidazole. Bendazole metabolites are excreted in the urine. Alphaglutamyl-tryptophan, under the influence of peptidases, is broken down into L-glutamic acid and L-tryptophan, which are used by the body in protein synthesis.