Ethanol (ethyl alcohol, wine alcohol) - an organic compound, a representative of a number of monohydric alcohols with the composition C 2 H5 OH (abbreviated EtOH). Under normal conditions it is a colorless, flammable liquid. According to the National Standard of Ukraine DSTU 4221: 2003 ethanol is a toxic substance with a narcotic effect; in terms of the degree of impact on the human body, it belongs to the fourth class of dangerous substances. Possesses carcinogenic properties.
Ethanol is the main active ingredient in alcoholic beverages, which are usually made by fermenting carbohydrates. For industrial needs, ethyl alcohol is often synthesized from oil and gas feedstocks by catalytic hydration of ethylene. In addition to the production of food products, ethanol is used in large quantities as a fuel, solvent, antiseptic and as a raw material for the production of other industrially important substances.
Story
Ethanol has been used by mankind since ancient times. It played a role in drinks, medicines, as a sedative and an aphrodisiac, and also had a place in religious ceremonies.
In Ancient Egypt it was extracted by fermentation of plant materials. In this way, only a dilute alcohol solution was obtained. In order to increase concentration, a distillation method was invented in China. As evidenced by paintings on Chinese ceramics, drinks from a fermented mixture of rice, fruit and honey were made 9,000 years ago. Around the same time, in the Middle East, alcohol was obtained from grapes and barley, as evidenced by records on clay tablets in Mesopotamia.
In the Middle Ages, ethyl alcohol played the role of a basis for the preparation of numerous medicines and tinctures. Alchemists always used ethanol in their works, giving it the name lat. Aqua vitae, that is, living water.
Pure ethanol was first obtained in 1796 by the Russian-German chemist Toviy Egorovich Lovitz. According to the description of the leading scientist of the time, Antoine Laurent Lavoisier, the compound under study consisted of the chemical elements carbon, hydrogen and oxygen. In 1808, Swiss biochemist Nicolas Théodore de Saussure established the chemical formula of ethanol, and fifty years later Scottish chemist Archibald Scott Cooper proposed its structure.
The first synthetic method for producing ethylene was developed independently by the English chemist Henry Hennel and the French pharmacist Georges-Simon Serulla in 1826. And in 1828, the English physicist and chemist Michael Faraday obtained ethanol by catalytic hydration of ethene, a by-product of oil and gas refining. This method formed the basis of many methods that are used in ethanol production to this day.
Examples of problem solving
EXAMPLE 1
| Exercise | Determine the simplest formula for the compound of potassium with manganese and oxygen, if the mass fraction of potassium is 24.7%, manganese 34.8%. |
| Solution | The mass fraction of element X in a molecule of the composition NX is calculated using the following formula: ω (X) = n × Ar (X) / M (HX) × 100%. Let's calculate the mass fraction of oxygen in the compound: ω (P) = 100% - ω(K) - ω(Mn) = 100% - 24.7% - 34.8% = 40.5%. Let us denote the number of moles of elements included in the compound as “x” (potassium), “y” (manganese) and “z” (oxygen). Then, the molar ratio will look like this (the values of relative atomic masses taken from D.I. Mendeleev’s Periodic Table are rounded to whole numbers): x:y:z = ω(K)/Ar(K) : ω(Mn)/Ar(Mn) : ω(O)/Ar(O); x:y:z= 24.7/39 : 34.8/55 : 40.5/16; x:y:z= 0.63: 0.63:2.53 = 1: 1: 4. This means that the formula for the compound of potassium, manganese and oxygen will be KMnO4. This is potassium permanganate. |
| Answer | KMnO4 |
EXAMPLE 2
| Exercise | Derive the simplest formula of a compound in which the mass fraction of phosphorus is 43.66%, and the mass fraction of oxygen is 56.34%. |
| Solution | The mass fraction of element X in a molecule of the composition NX is calculated using the following formula: ω (X) = n × Ar (X) / M (HX) × 100%. Let us denote the number of phosphorus atoms in the molecule by “x”, and the number of oxygen atoms by “y” Let's find the corresponding relative atomic masses of the elements phosphorus and oxygen (the values of the relative atomic masses taken from D.I. Mendeleev's Periodic Table are rounded to whole numbers). Ar(P) = 31; Ar(O) = 16. We divide the percentage content of elements into the corresponding relative atomic masses. Thus we will find the relationship between the number of atoms in the molecule of the compound: x:y = ω(P)/Ar(P) : ω (O)/Ar(O); x:y = 43.66/31 : 56.34/16; x:y: = 1.4: 3.5 = 1: 2.5 = 2: 5. This means that the simplest formula for combining phosphorus and oxygen is P2O5. It is phosphorus(V) oxide. |
| Answer | P2O5 |
structure
Both carbon atoms in the ethanol molecule, including the atom that is associated with the hydroxyl group, are in a state of sp 3 hybridization. The CC distance is 1.512 angstroms.
Depending on the position of the hydroxyl group in relation to the other part of the molecule, gauche (French Gauche) and trans forms are distinguished. The trans form is characterized by the position of the OH bond of the hydroxyl group in the same plane with the CC bond and one of the CH bonds. In the gauche form, the hydrogen atom in the hydroxyl group is facing away. The dipole moment for the gauche form is 1.68 D, and for the trans form it is 1.44 D.
Ethyl alcohol for children
There are no absolute contraindications to the use of this solution in children. However, be careful, alcohol has a systemic toxic effect, which is especially pronounced in children. Therefore, even when used externally, do not get carried away with rubbing and lotions.
There are some tinctures and preparations based on alcohol, which in some cases are approved for use by children. Be careful and careful, carefully read the composition of the drug and instructions for use
Pay attention to your child's condition and behavior. The use of such drugs, and especially in high dosages, can cause serious symptoms of alcohol poisoning in a child.
Distribution in nature
Ethanol is a waste product of some fungi. Among them, the main ones are the genera Saccharomyces, Schizosaccharomyces, and Kluyveromyces. One of the most famous representatives of these classes is the species Saccharomyces cerevisiae, which has the common name brewer's yeast. Other common species include Saccharomyces pastorianus, Saccharomyces anamensis, Schizosaccharomyces pombe, Candida utilis, etc. Ethanol is also produced by some bacteria, for example Zymomonas mobilis.
In 1975, astronomers reported finding significant accumulations of ethanol in the gas and dust cloud Sagittarius B2. According to scientists, the number of ethanol molecules present there significantly exceeds the amount of alcohol produced in the entire history of mankind. The ethanol found had a trans-form of molecules, and in 1996 it was also recorded in the gauche form.
Among the possible ways for the formation of ethanol in the interstellar medium, in particular, its synthesis from methane and methyl cation under the influence of radiation is given:
Another potential way is to react the methyl cation with formaldehyde, which is also common in space:
The harm and benefits of alcohol
Let's not touch this huge iceberg called “to drink or not to drink.” Let us remember the words of everyone’s beloved Semyon Semenovich Gorbunkov about the benefits of alcohol. Alcohol can slightly prevent the formation of blood clots, reduce cholesterol levels in the blood, and dilate blood vessels, which will lead to more intense blood circulation. And the medicine, as you know, loves a clear dosage, so 50 ml heals and invigorates. But the benefits that alcohol brings are too small compared to its harm: alcohol makes changes to a person’s genetic structure, which can lead to deviations in the mental development of descendants, and has a detrimental effect on a person’s moral behavior. It is worth remembering that it is alcohol that encourages a person to commit rash, stupid and cruel acts. To prevent this from happening, you should strictly follow the norm. A complete abstinence from a variety of alcoholic beverages will also be useful.
Remember that if you exceed the limit, alcohol will become a poison that will harm organs and even entire systems of your body.
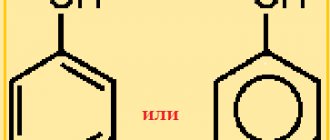
Actually, this is where we can finish our discussion on how to distinguish methyl from ethyl alcohol.
fb.ru
physical properties
Ethanol is a colorless liquid with a faint “alcoholic” odor. It is volatile and flammable. Mixes in any proportions with water, ethers, acetone, benzene. Ethyl alcohol is a good solvent for many organic as well as inorganic substances.
With water it forms an azeotropic mixture: 95.6% alcohol and 4.4% water.
Anhydrous ethanol is slightly hygroscopic: to achieve stability, it is able to absorb 0.3-0.4% water. Ethanol viscosity, mPa * s
| -25 °C | 0 °C | 25 °C | 50°C | 75 °C |
| 3,262 | 1,786 | 1,074 | 0,694 | 0,476 |
Notes
- Article “Absolute alcohol” in TSB.
- Pharmaceutical reference book Khimik.ru
- Great Soviet Encyclopedia, article “Ethyl alcohol”
- TSB, article “Alcoholism”
- Drugs affecting the central nervous system
- E1510 - ethyl alcohol
- Decree of the Government of the Russian Federation of December 29, 2007 N 964 Moscow “On approval of lists of potent and toxic substances for the purposes of Article 234 and other articles of the Criminal Code of the Russian Federation, as well as large amounts of potent substances for the purposes of Article 234 of the Criminal Code of the Russian Federation”
- Article 26 of the Federal Law of November 22, 1995 N 171-FZ (as amended on April 5, 2010) (Consultant+)
receiving
ethylene hydration
There are two main ways to produce ethanol from ethylene. Historically, the first was the indirect hydration method, invented in 1930. Another, developed in the 1970s, was designed as an acid-free method (eliminating the use of sulfuric acid).
indirect hydration
The production of ethanol from ethylene using sulfuric acid occurs in three stages. First, ethylene is absorbed by concentrated acid, forming the esters ethyl sulfate or diethyl sulfate:
Absorption is carried out with a 95-98% acid solution at a temperature of 80 ° C and a pressure of 1.3-1.5 MPa. This interaction is exothermic, so the reactor walls must be cooled. The presence of ethyl sulfate in the acid solution can significantly increase the absorption rate, since the solubility of ethylene in ethyl sulfate is much higher than in pure acid.
In the second stage, the resulting reaction products undergo hydrolysis and decompose to form alcohol and acid. However, the interaction of two basic esters is switched off, which leads to the formation of a third, diethyl:
After treating sulfuric acid with absorbed ethyl and diethyl sulfate in a sufficient amount of water, the solution acquires a concentration of about 50-60%. The hydrolysis products are sent to columns for separation: the diluted acid remains at the bottom of the tank, and the gassed alcohol-Eterna mixture is at the top. The target mixture is washed with water or a dilute sodium hydroxide solution and then purified by distillation.
The final step is to restore the concentration of the dilute acid. This stage is one of the most expensive in the entire synthesis. Using an acid evaporation system, it is possible to increase the acid concentration to 90%. This indicator is increased to the required 98% by mixing with oleum (concentration 103%).
A serious problem for the indirect hydration method is the formation of carbonaceous substances in the acid, which have a significant effect on its concentration. The use of concentrated acid also causes corrosion on the equipment, so some parts of the equipment are made from silicon, tantalum alloys, lead, etc.
direct hydration
Synthesis according to the direct hydration scheme is carried out using catalysts. There are two forms of interaction here:
- Gaseous reagents come into contact with a solid or liquid catalyst (gas-phase process)
- Both liquid and gaseous reagents come into contact with a solid or liquid catalyst (zmishanophase process).
Ethanol is synthesized primarily through a gas-phase process. The output ethylene and water are passed over a carbon catalyst, saturated with phosphoric acid:
At normal temperatures, only a small amount of ethanol can be present in the gas phase, and increasing temperature will lead to a decrease in its concentration. The reaction equilibrium can be leveled by applying the Le Chatelier-Brown principle, increasing the pressure in the reaction mixture and reducing the number of molecules in the system. The optimal conditions for the interaction are a temperature of 250-300 ° C and a pressure of 6.1-7.1 MPa.
The reaction product may undergo intermolecular dehydration, leading to the formation of diethyl ether:
If the carbohydrate raw material contains an admixture of acetylene, it is hydrated to ethanal:
The presence of ethanal is undesirable, since it produces crotonaldehyde, which negatively affects the quality of ethanol, even in parts per million:
obtained by fermentation
Ethanol production by fermentation of sugary substances is the oldest. For its production, any product containing sugar or substances from which it can be obtained (for example, starch) can be used. Fruit and cane sugar, sugar beets, molasses are used as sugar-containing products, and starch-containing products are potatoes, grains of wheat, rye, and corn. Cellulose (from agricultural waste, pulp and paper industry, etc.) is also used as a raw material.
Extracts from starch and sugar
To convert starch into sugary substances, it is first subjected to hydrolysis. For this purpose, raw materials (mashed potatoes or flour) are brewed with hot water to speed up the swelling of starch. An enzyme is also added to the raw material, under the influence of which starch is excoriated, that is, it is converted into glucose.
Diastase contained in sprouted grains or other amylases of fungal origin are used as an enzyme.
The second stage, which is similar to the production of alcohol from sugars, consists of anaerobic fermentation, that is, conversion into alcohol and carbon dioxide:
Here the reaction occurs under the influence of microorganisms: fungi (yeast) or bacteria.
Comparison of the characteristics of some representatives of bacteria and yeast
| characteristic | Zymomonas mobilis | Saccharomyces carlsbergensis |
| Quantity doubling time, h | 2,51 | 5,64 |
| Ethanol production, g/(g h) | 5,44 | 0,82 |
| Product yield, g/g | 0,465 | 0,460 |
Among the yeasts used in the process, Saccharomyces cerevisiae (the so-called brewer's yeast) occupies an active place. When using them, acidity and temperature are important - they affect yeast growth, ethanol yield, by-product formation and bacterial contamination. Typically, such fermentation in industrial production is carried out at a pH of 4-6. At a pH value less than 5, bacterial growth in the medium is greatly suppressed; for the growth of the yeast Saccharomyces cerevisiae, acidity must be maintained in the range of 2.4-8.6 with an optimal value of 4.5, and the fermentation process is more intense in the range of 3.5-6.
Most yeasts used in ethanol production have an optimal temperature for growth of about 39-40 ° C, and the maximum value observed in the species Kluyveromyces marxianus is 49 ° C. Since the fermentation process is exothermic (586 J of heat is released from 1 g of absorbed glucose), Using yeast with a higher optimal growth temperature allows you to save money on cooling the reaction system. An important point is the supply of small amounts of oxygen for the yeast to synthesize unsaturated fatty acids and ergosterol, which contribute to their growth and good cell permeability. In the absence of oxygen, the lack of acids and sterols will cause changes in yeast physiology within a few generations.
Bacteria are also used in the synthesis of ethanol, in particular the common species Zymomonas mobilis, which have a high growth rate, high yield of the final product and do not depend on the supply of oxygen.
Pulp extracts
Both cellulose and starch are polysaccharides, polymers of carbohydrates, but the synthesis of ethanol from cellulose is much more difficult due to its low tendency to hydrolysis. Its structure is more similar to crystalline, which makes it more difficult to break the bonds inside the polymer, and in plants it is protected from hydrolytic decomposition by a layer of lignin (after treating cellulose with acid, only 15% of the total mass is hydrolyzed). Waste raw materials also contain hemicellulose, which consists mainly of pentoses.
Preoperative processing includes grinding and soaking the raw material for swelling. Subsequently, it is heated in autoclaves with 0.3-0.5% acid under a pressure of 7-10 atm. The acid most often used is sulfuric, less often hydrochloric. At the end of the process, the acid is concentrated in a separate tank and put back into production, and the lignin is filtered and purified by washing.
The ethyl alcohol obtained in this way is called hydrolytic. It is used only for technical purposes, because it contains a number of harmful impurities, including methyl alcohol, acetone, etc.
Also, unlike acid hydrolysis, the enzymatic method is used. Here hydrolysis occurs under the action of fungi like Trichoderma viride. Pre-treatment involves removing the lignin shell with the solvent cadoxene (a solution containing 5-7% cadmium oxide and 28% ethylenediamine) and treating with liquid ammonia under high pressure, which agitates the fibers in the cellulose, facilitating the penetration of enzymes. In some cases, it is possible to achieve 100% recycling of cellulose.
other methods
Hydrolysis of halogenated hydrocarbons
Ethanol is formed by the hydrolysis of halogenated ethane. It is carried out in water or in an aqueous solution of alkalis. In the first case, the reaction is reverse, and in the second, elimination (elimination) of the hydrogen halide may occur:
Syngas conversion
The production of ethanol from synthesis gas is similar to the method for producing methanol using the Fischer-Tropsch process:
The reaction occurs at a temperature of 125-175 °C and a pressure of 1.42 MPa, using a catalyst such as powdered iron.
Recovery of organic compounds
The reduction of aldehydes and acids is a fairly common method for producing alcohols, including ethanol:
Catalytic reduction is carried out over Raney nickel, platinum; In laboratory conditions, lithium aluminum hydride and sodium borohydride are used.
ethanol purification
Synthesized ethanol is usually a water-alcohol mixture. Its purification and dehydration begins with distillation (rectification), which can achieve a concentration of 95.6% vol. The resulting mixture is azeotropic and cannot be purified by subsequent distillation. For additional dehydration, benzene, cyclohexane or heptane are used. Their presence creates new azeotropic mixtures with a low boiling point, which makes it possible to obtain anhydrous ethanol.
On an industrial scale, molecular sieves can be used for dehydration, whose pores are permeable to water molecules, but not to ethanol. Such sieves can be artificial or zeolites of natural origin (for example, clinoptilolite). 75% of the adsorbed molecules are water, the remaining 25% are ethanol, which is then returned to the distillation system.
The membrane method is also used, which consists of separating a water-alcohol mixture heated to 60 ° C with a semi-permeable membrane that does not allow ethanol to pass through. This operation is performed under pressure less than 1 kPa. As a result of separation, ethanol is formed with a concentration of 99.85% and a solution that has passed through the membrane with a concentration of 23%. The condensed membrane solution can be rectified again.
Is it possible to drink
The only alcohol from the mentioned category that can be drunk “with a stretch” is first-grade ethyl (medicinal), and even then only occasionally, in small quantities and always diluted with water. A burn to the gastrointestinal mucosa can lead to gastritis, ulcers, and oncology. Do not forget that this product, albeit conditionally, belongs to the food category.
It is strictly forbidden to use technical alcohols for internal consumption. This threatens severe poisoning and even death. You can remain disabled forever, lose your sight, develop cirrhosis or cancer of the liver or pancreas.
Methyl alcohol is a terrible thing. Accidentally drunk, it literally “corrodes” from the inside, and the ambulance doesn’t even have time to get there. Methanol destroys the visual, vascular and nervous systems. To get poisoned, it is enough to drink 5-10 ml; death occurs from 30 ml.
Carefully. Sometimes “veterans” remember that they drank denatured alcohol in the 70s and 80s, and nothing happened.
Perhaps it was so
But today, new standards and what is added to denatured alcohol are truly dangerous. In addition, the added ingredients cannot be chemically separated. Therefore, we don’t even recommend trying!
Perhaps it was so. But today, new standards and what is added to denatured alcohol are truly dangerous. In addition, the added ingredients cannot be chemically separated. Therefore, we don’t even recommend trying!
Ethanol classification
The resulting alcohol is conventionally divided into four classes according to its composition:
- industrial ethanol (96.5% vol.) is a product for industrial and technical use: as a solvent, fuel, etc. To prevent its use, substances with an unpleasant odor are usually added to it, for example, pyridine in an amount of 0.5-1% (denaturation is carried out). Also, for easier identification, it can be lightly stained with methyl violet;
- denatured alcohol is a technical product with an ethanol concentration of 88% vol., which is a significant amount of impurities. It is denatured and colored accordingly. Used in lighting and heating;
- high-quality alcohol (96.0-96.5% vol.) - purified ethanol, used for pharmaceutical needs, in the manufacture of cosmetics for food consumption;
- absolute ethanol (99.7-99.8% vol.) - very pure ethanol, used in pharmaceuticals and the production of aerosols.
In Ukraine, the grades of rectified ethanol produced are regulated by the standard DSTU 4221: 2003 “Rectified ethyl alcohol”.
Depending on the degree of purification, four varieties are distinguished: “Wheat Tear”, “Lux”, “Extra” and “Highest Purification”. Standards for types of alcohol according to GOST 4221: 2003
| index | "Wheat Tear" | "Lux" | "Extra" | "Highest purification" |
| Volume fraction of ethyl alcohol, at a temperature of 20 ° C,%, not less | 96,3 | 96,3 | 96,3 | 96,0 |
| Mass concentration of aldehydes, converted to acetaldehyde in anhydrous alcohol, mg/dm³, no more | 2,0 | 2,0 | 2,0 | 2,0 |
| Mass concentration of fusel oil: propyl, isopropyl, butyl, isobutyl and isoamyl alcohols in terms of a mixture of propyl, isobutyl and isoamyl alcohols (3: 1: 1) in anhydrous alcohol, mg/dm³, no more | 2,0 | 2,0 | 2,0 | 2,0 |
| Mass concentration of fusel oil in terms of a mixture of isobutyl and isoamyl alcohols (1: 1) in anhydrous alcohol, mg/dm³, no more | 2,0 | 2,0 | 2,0 | 2,0 |
| Mass concentration of ethers, in terms of ethyl acetate in anhydrous alcohol, mg/dm³, no more | 1,5 | 2,0 | 3,0 | 5,0 |
| Volume fraction of methyl alcohol in terms of anhydrous alcohol,%, no more | 0,005 | 0,01 | 0,02 | 0,03 |
| Mass concentration of free acids (without CO2), in terms of acetic acid in anhydrous alcohol, mg/dm³, no more | 8,0 | 8,0 | 12,0 | 15,0 |
Content
- 1 Etymology of names
- 2 Preparation 2.1 Fermentation 2.1.1 Industrial production of alcohol from biological raw materials
- 2.1.2 Hydrolysis production
- 3.1 Physical properties
- 4.1 Fuel
- 5.1 Vehicle fleet running on ethanol
- 6.1 Toxicology of ethanol
Chemical properties
Ethanol is a monohydric primary alcohol and the hydroxyl group accounts for most of its chemical properties. Thus, ethanol can take part in dehydration reactions - both intranitrous and intermolecular:
When interacting with other alcohols, a mixture of three esters is formed:
Ethanol forms esters with carboxylic acids in the presence of concentrated sulfuric acid:
As a result of the addition of ethanol to acetylene, vinylethyl ether is synthesized:
Displaying its acidic properties, ethanol reacts with alkali metals (for example, sodium) and alkalis to form ethoxide:
This reaction is carried out in an anhydrous environment because hydroxide is formed faster than ethoxide.
Less active metals - aluminum and magnesium - also react with ethanol, but only in the presence of a mercury catalyst:
The hydroxyl group present in the molecule can be replaced by halide acids to form ethane halogen derivatives:
Ethanol is oxidized to ethanal and then to acetic acid. The result of complete oxidation (for example, burning ethanol) is carbon dioxide and water:
By treating ethanol with ammonia at 300 °C in an acidic environment, substituted amines are formed: primary, secondary, tertiary or even quaternary ammonium salts (depending on the ratio of the reagents):
Ethanol is the raw material for the synthesis of butadiene. The reaction is carried out at a temperature of 370-390 ° C and in the presence of catalysts - MgO-SiO 2 or Al 2 O 3 -SiO 2 (with a selectivity of 70%):
Ethyl alcohol according to GOST
GOST is, first of all, a document regulating what qualities goods sold in a certain country should have. There are several GOST standards for ethanol, which describe the purpose, storage conditions, transportation and much more.
But such a complex issue as drinking alcohol can generate hours of debate. And that’s not the point, because each person must decide issues of this level for himself. But let's turn to GOST 1972: “Ethyl alcohol is a flammable, colorless liquid with a characteristic odor. Refers to potent drugs that first cause excitement and then paralysis of the nervous system.”
Now we read GOST 1982: “Ethyl alcohol is a flammable, colorless liquid with a characteristic odor, classified as a potent drug.” And finally, 2000: “Ethyl alcohol is a colorless, flammable liquid with a characteristic odor.” These same regulatory documents regulate such a substance as technical ethyl alcohol.
biological effect
metabolism
Almost all consumed alcohol (90-98%) is metabolized by the body and only a small part of it (2-10%) is excreted unchanged: with urine, air, sweat, saliva. Ethanol consumption leads to excessive urination: every 10 g of alcohol causes the body to lose 100 ml of fluid, but does not help remove alcohol from the body. The main part of the ethanol entering the body enters the liver, where it undergoes biological transformation in microsomes.
At the first stage of metabolism, acetaldehyde is formed from ethanol. This occurs under the action of alcohol dehydrogenase (ADH), an enzyme whose cofactor is nicotinamide (NAD). Subsequently, acetaldehyde, formed from ethanol, is oxidized into acetate in the mitochondria by the enzyme aldehyde dehydrogenase, which uses NAD as a coenzyme, which, by adding a proton, is reduced to NAD H. At this stage, the interaction occurs much faster than at the previous one. Acetate enters the Krebs cycle, where it is destroyed to CO 2 and H 2 O. Aldehyde dehydrogenase is found not only in the liver, but also in other organs, including the brain. In an adult, healthy person, ADH destroys about 10 g of alcohol per hour.
In addition to the main metabolic process, ethanol is also oxidized in two other ways. One of them involves microsomal oxidase in combination with reduced nicotinamide adenine dinucleotide phosphate (NADP), while the other involves catalase in combination with hydrogen peroxide. Both paths lead to the formation of toxic aldehyde, which has carcinogenic properties and is ten times more toxic than ethanol.
Impact on the body
Symptoms depending on the dose of alcohol received
| symptom | Ethanol content in blood,% |
| confusion | 0,06—0,08 |
| slow thinking | 0,10 |
| stupor | 0,11—0,15 |
| intoxication | 0,16 |
| significant poisoning | 0,2—0,4 |
| death | 0,4—0,5 |
Entering the human body through the esophagus, ethanol is quickly absorbed. 20% of the initial ethanol is absorbed in the stomach, and 80% in the small intestine. After absorption, it enters the blood within 5 minutes, spreading through the bloodstream throughout the body.
Central nervous system. Ethanol depresses the functions of the central nervous system like other anesthetics. Despite popular belief, ethanol does not stimulate the action of the nervous system: if excitations occur, their appearance is due to counteraction to inhibitory processes. In normal doses, ethanol acts mainly on activating the function of the reticular formation of the brainstem, and only large doses directly suppress the function of the cerebral cortex.
Chronic ethanol consumption causes serotonin deficiency. A functional decrease in the activity of this system prevents the development of tolerance and, conversely, an increase in its activity and an increase in serotonin levels accelerate the development of tolerance to alcohol. Under the influence of ethanol, the metabolism of dopamine, which is involved in the synthesis of norepinephrine and coordinates movements, emotional and mental states, is disrupted. Ethanol also has a negative effect on physical and mental capabilities: it reduces visual and hearing acuity, impairs muscle coordination and stability, and slows down the reaction time to irritation.
Respiratory system. Ethanol has a pronounced toxic effect on the respiratory system. Damage to the lungs affects the development of bronchopulmonary infection due to a decrease in the protective functions of the body. The negative effects of alcohol are associated with inhibition of phagocytosis and antibody formation, facilitating the penetration of bacteria into the respiratory tract, and the like. Bronchopulmonary pathologies can develop into acute pneumonia, which has a significant percentage of fatal cases.
The cardiovascular system. Under the influence of ethanol, lipids of cell membranes, in particular myocardial cells, dissolve. As a result, membrane permeability increases and the exchange of sodium, potassium, magnesium and calcium ions is disrupted. This weakens the contractility of the heart muscle.
Digestive system. A single dose leads to acute hemorrhagic erosive gastritis; ethanol has a similar effect on the mucous membrane of the duodenum. Within a minute after entering the stomach of rats, ethanol caused diffuse hyperemia of the gastric mucosa.
Liver. The degree of liver damage from ethanol directly depends on the amount of alcohol consumed. As a result of its action, steatosis, fibrosis, alcoholic hepatitis and cirrhosis may appear, often ending in the development of hepatocellular carcinoma. Thus, according to the International Agency for Research on Cancer, ethanol is carcinogenic.
One of the results of long-term exposure to ethanol on the body is an increase in the volume of red blood cells - macrocytosis, caused by the toxic effect of acetaldehyde, folic acid deficiency and hyperlipidemia.
alcoholism
Ethanol is the basis of alcoholic beverages. Their long-term use causes alcoholism.
Alcoholism is a set of phenomena that characterize the clinical picture of dependence on alcohol (that is, ethanol-containing products). Among the symptoms and manifestations of such dependence are the following: the body’s tolerance to alcohol, physical dependence, withdrawal syndrome when stopping or reducing consumption, uncontrolled and time-consuming excessive consumption.
There are three stages of progression of alcoholism:
- the person has no desire for alcohol, there is a loss of control when consuming, a transition to systematic consumption, an increase in tolerance to alcohol, there are initial mental disorders;
- there is physical dependence with loss of measure, the formation of a psychopathic-like syndrome, disruption of the body systems (cardiovascular, genitourinary, respiratory) and organs (the appearance of gastritis, hepatitis)
- alcohol dependence is mental, there is a strong physical attraction as a manifestation of withdrawal syndrome, the appearance of hallucinations, irreversible damage to internal organs (liver cirrhosis, heart disease, encephalopathy, etc.).
Effect on pregnancy
The risk of abnormalities in fetal development is directly proportional to the amount of alcohol consumed during pregnancy.
Ethanol easily penetrates the placenta, so its content in the blood of the mother and fetus quickly reaches the same level. It accumulates in fetal tissues rich in phospholipids, in the brain, and also in red blood cells. The removal of alcohol from the body is carried out with the help of liver enzymes, and in the unborn child it is formed only in the second half of the mother’s pregnancy. The harmful effects of ethanol on the fetus are associated with the immaturity of the protective mechanism and increased vascular permeability, etc. Of particular importance are the critical periods of embryonic development, when the sensitivity of the embryo and fetus to foreign substances reaches its maximum level. The toxic effect of ethanol causes a slowdown in development or even death of the embryo.
Maternal consumption of ethanol during pregnancy is associated with fetal (fertility) teratogenic effects. The influence of alcohol is manifested in disruption of the general development of the fetus, the birth of a child with less than normal body weight and height, and mental disability. In particular, children affected by the teratogenic effect of ethanol have modified facial features: narrow palpebral fissures, thin upper lip, the appearance of microcephaly and retrognathia, absence of filters and various ear anomalies. Physical changes are complemented by underdevelopment of the brain, a tendency to seizures, cerebral edema, poor coordination of movements, decreased intelligence and congenital heart defects. This effect of ethanol is called fetal alcohol syndrome, FAS (or fetal alcohol syndrome).
Interaction with medications
Ethanol has the ability to enhance the effect of antibiotics, antihistamines, barbiturates, muscle relaxants, and also cause a negative reaction in the body.
Interaction of medications with ethanol
| drug class | a drug | Type of interaction with ethanol, consequences |
| analgesics | aspirin Acetaminophen | Aspirin increases gastric emptying, which leads to rapid sorption of alcohol in the small intestine, and can slow down the action of alcohol dehydrogenase in the stomach. Ethanol increases the metabolism of acetaminophen, which produces toxic substances that damage the liver. You may experience increased heart rate, abdominal pain, stomach ulcers, |
| antibiotics | Erythromycin Isoniazid Ketoconazole metronidazole | Erythromycin increases gastric emptying, which leads to rapid sorption of alcohol in the small intestine; Together with isoniazid, alcohol increases the risk of liver disease. Accompanied by headaches, nausea, sudden changes in blood pressure |
| antihistamines | Diphenhydramine Clemastine Promethazine | Ethanol enhances the effect of drugs on the central nervous system, causing lethargy and decreased motor skills; the combined effect has a stronger effect on older people. |
| barbiturates | phenobarbital | Weakness of the body, dizziness, risk of a convulsive attack. Chronic alcohol consumption increases the level of cytochrome P-450 barbiturate metabolism |
| Sleeping pills (benzodiazepines) | Diazepam lorazepam Oxazepam | Ethanol enhances the effect of drugs on the central nervous system, causing memory problems, lethargy, decreased motor skills, slowing or difficulty breathing; |
| anti-inflammatory drugs | Diclofenac Ibuprofen Naproxen | Ethanol consumption increases the risk of stomach bleeding and peptic ulcers |
| H2 receptor blockers | Nizatidine Ranitidine Cimetidine | The drugs inhibit the action of alcohol dehydrogenase and promote gastric emptying, leading to increased ethanol levels in the blood. |
Areas of use of alcohol
Users have found many more ways to use inexpensive industrial alcohol in everyday life:
- cleaning paints and stains from different materials;
- dissolves almost all chemicals;
- cleans kerosene heaters well;
- can serve as a filler for drugs;
- in flexographic printing;
- alcohol is used as a biofuel substitute.
Industrial alcohol is sold in any Moscow stores specializing in chemical compounds. The price depends on the packaging and manufacturer, and can range from 47 rubles per liter and above.
All packages put up for sale must have an inscription stating that this product is dangerous for the human body, because it causes not only poisoning, but also death. It is strictly prohibited to use it internally; this alcohol is used only for technical work. All substances included in technical alcohol are very dangerous for the human body. It can only be used for scientific experiments and disinfection of products.
The use of technical alcohol in enterprises is currently very common. This includes operation, mechanical maintenance of machines, equipment, and carrying out scientific experiments.
Each product has its own meaning. Thus, technical alcohol is needed for the production of paints and varnishes, detergents, and fuel additives. Taking it internally is strictly prohibited. Those people who drink diluted alcohol to get the pleasure of intoxication have mental illnesses. These people should seek help from a medical specialist, namely a narcologist. Because there is also a huge chance that they are addicted to alcohol. The cost of industrial alcohol is much less than that of alcoholic beverages in the store. Alcoholics most of all want to save money on their addiction in order to pay less and get more pleasure.
So, why can’t you use technical alcohol? It is never used for oral administration, because it contains toxic substances that are dangerous to the human body. Alcohol intoxication is one of the most terrible consequences of taking technical alcohol as a drink. But what's worse is that it's fatal. Even a small dose can simply kill an adult.
application
Ethanol has a wide range of uses, among which the most significant are the production of alcoholic beverages, use as a solvent, fuel, and the synthesis of other chemicals.
fuel
The first car that was capable of running on ethanol was designed by Henry Ford in 1920 - the Ford Model T. However, then this innovation did not receive the necessary development due to technical and economic problems: the production of pure ethanol was too expensive, and the use of unrefined alcohol mixed with hydrocarbon fuel was limited to a certain extent - at low temperatures, water insoluble in gasoline froze, corroding the fuel tank.
Now, with the technology to produce cheap ethanol, replacing traditional gasoline or diesel fuel with ethanol, or using it as an additive, has become widespread throughout the world. World production of ethanol for the needs of the fuel industry in 2014 amounted to 24750000000. Gallons.
solvent
Ethanol is the most important solvent after water. Its main application is the production of cosmetics, perfumes, surfactants and disinfectants, pharmaceuticals, and various coatings. For these purposes, ethanol of both synthetic and enzymatic origin is used.
antiseptic
Ethanol is the oldest antiseptic known to mankind. Its ability to disinfect wounds was noted by the ancient Greek physician Claudius Galen, and later by the medieval French surgeon Guy de Chauliac.
Ethanol exhibits bactericidal effects at concentrations of 30% or higher, depending on the type of bacteria, water content and time of action. According to research, the effect of ethanol is most effective at its concentration of 60-70% - both in the presence of water and in its absence. This is the ethanol content of household hand sanitizers. Using a high concentration (for example, a 90% solution) to disinfect skin is impractical, since at such concentrations ethanol exhibits its tanning properties, while the antiseptic properties decrease.
The principle of ethanol's action on microorganisms is probably its effect on their membranes and rapid denaturation of proteins, which leads to disruption of bacterial metabolism and further cell destruction. Ethanol demonstrates a high biocidal effect against vegetative bacteria (including mycobacteria), viruses, fungi, but not spores.
Due to the lack of sporicidal effects, ethanol cannot be used for sterilization, however, its properties are sufficient for the preventive disinfection of surfaces, skin treatment, etc.
Precipitation of nucleic acids
Ethanol is widely used in molecular biology to precipitate and concentrate DNA and RNA. It is used in conjunction with buffer solutions of salts containing simple singly charged cations (for example, sodium cations). It is typical to use a 0.3 mol/L sodium acetate buffer with a pH of 5.2 (at 4 °C) and ethanol - absolute and 70% (at -20 °C).
To precipitate nucleic acids, the sample is mixed with a buffer solution and absolute ethanol and cooled at -20 °C for an hour, after which it is centrifuged. After separating excess liquid from the surface with a pipette, add a 70% ethanol solution and repeat centrifugation and liquid separation. The residue is evaporated at a temperature of 37 ° C in a water bath and thus a concentrated substance is obtained.
antidote
Due to its ability to form esters when interacting with alcohols, ethanol is used as an available antidote for poisoning with methanol, ethylene glycol and diethylene glycol. Ethanol is administered orally or intravenously into the body, and the dose for administration is calculated based on the consideration that its concentration in the blood serum should reach 10-15 mg/l.
The risk of using ethanol is the inhibition of the central nervous system, the appearance of hypoglycemia (due to decreased gluconeogenesis) and nausea. When administered intravenously, phlebitis, hypertension, and hyponatremia may occur. The use of such an antidote requires constant monitoring of serum ethanol and venous blood glucose levels.
Synthesis of other substances
In industry, ethanol is used to produce ethanal, butadiene, diethyl ether, ethyl acetate, ethylamine, and the like.
Benefit or harm
It is impossible to say unequivocally whether ethyl alcohol brings benefit or harm.
Today it is difficult to find an area of human activity where it is not used. It brings invaluable benefits in medicine as a disinfectant. It helps maintain sterile cleanliness in operating rooms. It is indispensable in the preparation of various types of medicines.
For humans, taking small doses of alcohol-containing drinks is also very useful. When entering the human body, they help to dilate blood vessels, thin the blood, thereby improving blood circulation.
It happens that only thanks to the psychotropic effect of alcohol a person manages to dull severe pain.
The pronounced antiseptic properties of alcohol solutions have long been appreciated by cosmetologists. Most cleansing lotions are made from them.
From the point of view of harm to the human body, alcohol is the most dangerous drug. Frequent use can cause severe psychological and physical dependence; it is not for nothing that alcoholism has long been considered a disease.
It has already been proven that alcohol has a detrimental effect on the brain, liver, kidneys, heart and all body systems. But alcoholic drinks are made from food grade alcohol.