Ethanol or ethyl alcohol belongs to the group of antiseptics. It is used externally to treat skin surfaces before various medical procedures. Various dosage forms are made on the basis of the product. Most often, they are intended for external use. Internal medications based on ethanol are used in accordance with the instructions and only with medical recommendations.
Content
- 1 Etymology of names
- 2 Preparation 2.1 Fermentation 2.1.1 Industrial production of alcohol from biological raw materials
- 2.1.2 Hydrolysis production
- 3.1 Physical properties
- 4.1 Fuel
- 5.1 Vehicle fleet running on ethanol
- 6.1 Toxicology of ethanol
Receipt
There are 2 main ways to produce ethanol - microbiological (alcoholic fermentation) and synthetic (ethylene hydration):
Fermentation
The method of producing ethanol, known since ancient times, is the alcoholic fermentation of organic products containing carbohydrates (grapes, fruits, etc.) under the action of yeast and bacterial enzymes. The processing of starch, potatoes, rice, corn looks similar; the source of fuel alcohol is raw sugar produced from cane, etc.
The solution obtained as a result of fermentation contains no more than 15% ethanol, since yeast is not viable in more concentrated solutions. The ethanol thus produced needs to be purified and concentrated, usually by distillation.
Industrial production of alcohol from biological raw materials
Modern industrial technology for producing ethyl alcohol from food raw materials includes the following stages:
- Preparation and grinding of starchy raw materials - grain (primarily rye, wheat), potatoes, corn, etc.
- Fermentation. At this stage, the enzymatic breakdown of starch into fermentable sugars occurs. For these purposes, recombinant alpha-amylase preparations obtained by bioengineering are used - glucamylase, amylosubtilin.
- Fermentation. Due to the fermentation of sugars by yeast, alcohol accumulates in the mash.
- Bragorectification. It is carried out on accelerating columns.
Fermentation waste includes carbon dioxide, stillage, ether-aldehyde fraction, fusel alcohol and fusel oils.
The alcohol coming from the bragon rectification unit (BRU) is not anhydrous; the ethanol content in it is up to 95.6%. Depending on the content of foreign impurities in it, it is divided into the following categories:
- 1st grade
- highest purification
- basis
- Extra
- Lux
- Alpha
The productivity of a modern distillery is about 30,000–100,000 liters of alcohol per day.
Hydrolysis production
On an industrial scale, ethyl alcohol is produced from raw materials containing cellulose (wood, straw), which is preliminarily hydrolyzed. The resulting mixture of pentoses and hexoses is subjected to alcoholic fermentation. This technology was not widespread in the countries of Western Europe and America, but in the USSR there was a developed industry of feed hydrolytic yeast and hydrolytic ethanol.
Ethylene hydration
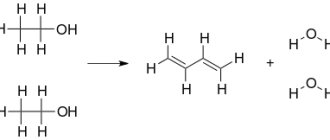
- In industry, along with the first method, ethylene hydration is used. Hydration can be carried out according to two schemes: direct hydration at a temperature of 300 °C, a pressure of 7 MPa, orthophosphoric acid applied to silica gel, activated carbon or asbestos is used as a catalyst.
- hydration through the stage of intermediate sulfuric acid ester, followed by its hydrolysis (at a temperature of 80-90 ° C and a pressure of 3.5 MPa).
This reaction is complicated by the formation of diethyl ether.
Ethanol purification
Ethanol, produced by hydration of ethylene or fermentation, is a water-alcohol mixture containing impurities. For its industrial, food and pharmacopoeial use, purification is necessary. Fractional distillation produces ethanol with a concentration of about 95.6% (wt.); this azeotrope, inseparable by distillation, contains 4.4% water (wt.) and has a boiling point of 78.15 °C.
Distillation frees ethanol from both volatile and heavy fractions of organic substances (bottom residue).
Absolute alcohol
Absolute alcohol is ethyl alcohol containing virtually no water. It boils at 78.39 °C, while rectified spirit containing at least 4.43% water boils at 78.15 °C. Obtained by distillation of aqueous alcohol containing benzene and other methods.[1]
Story
Ethanol is naturally produced by the fermentation of sugary fruits. Since ancient times, man has used this natural substance for intoxication and intoxication. Beer, and later wine, were first produced using natural yeast. The alcohol content of such drinks was lower than today because natural yeasts stop converting sugar into ethanol once a certain concentration is reached. Modern cultured yeasts allow higher concentrations to be achieved.
In 1796, Johann Tobias Lowitz first produced pure ethanol by filtering distilled alcohol through activated carbon. Antoine Lavoisier first described ethanol as a compound of carbon, hydrogen and oxygen. In 1808, Nicolas-Theodore de Saussure determined the chemical composition. Fifty years later, Archibald Scott Cooper published the structural formula. Synthetically, the spirit was first produced in 1826 by Henry Hennel and George Simon Cerulla in the United Kingdom of Great Britain.
Today, ethanol is mainly produced by fermentation from biomass. In the context of biofuel production, it is called bioethanol. Farm alcohol is ethanol made from agricultural raw materials.
Properties
Physical properties
Appearance: Under normal conditions it is a colorless volatile liquid with a characteristic odor.
A popular mistake should be avoided: the properties of 95.57% alcohol and absolutized alcohol are often mixed. Their properties are almost the same, but the values begin to differ, starting from the 3rd - 4th significant digit. Properties of ethanol
| Melting temperature | −114.15 °C |
| Boiling temperature | 78.15 °C |
| Solubility | miscible with benzene, water, glycerin, diethyl ether, acetone, methanol, acetic acid, chloroform |
A mixture of 95.57% ethanol + 4.43% water is azeotropic, that is, it does not separate during distillation.
Chemical properties
Reacts with alkali metals to form ethylates (or, in general, alcoholates) and hydrogen.
Narcotic properties
There is a difference between the chemical-pharmacological and legal definitions of the narcotic properties of ethanol. From a chemical and pharmaceutical point of view, ethanol has narcotic properties, while from a legal point of view, ethanol is not recognized as a drug, although indications of narcotic properties are also present in some technical and legal documentation.
Indications of the narcotic properties of ethanol are present in a number of textbooks on pharmacology, in specialized pharmaceutical and chemical dictionaries[2], and in encyclopedias.[3] Thus, the chemical-pharmacological definition, taken from the Textbook of Pharmacology, ed. S.V. Anichkov, M.L. Belenky, says:
Ethyl alcohol - wine alcohol, Spiritus Vini (C2H5OH) according to its pharmacological properties belongs to the narcotic substances of the fatty series.
The narcotic properties of ethanol are also manifested in the characteristic alcoholic excitation, which is followed by depression of the functions of the central nervous system, including blocking the work of inhibition centers.
The narcotic nature of the effects of ethanol is also confirmed by the fact that alcoholism, which is a form of dependence on ethanol, is considered a form of drug addiction [4].
Grain distillate according to GOST
Currently, GOST R 55799-2013 “Grain distillate” is in force in the Russian Federation. Technical specifications" .
Table 3 presents organoleptic indicators for grain distillates.
Table 3
| Indicator name | Characteristic |
| Appearance | Transparent liquid without sediment or foreign matter |
| Color | Colorless liquid or light golden to amber |
| Aroma and taste | Characteristic of a distillate produced from appropriate grain raw materials, without any foreign taste or odor |
In terms of physico-chemical properties, grain distillates must correspond to the values indicated in Table 4.
Application
Fuel
Ethanol can be used as fuel, including for rocket engines and internal combustion engines in its pure form. Limited due to its hygroscopicity (flakes off), it is used in a mixture with classic petroleum liquid fuels. It is used to produce high-quality fuel and a component of gasoline - ethyl tert-butyl ether, which is more independent of fossil organic matter than MTBE.
Chemical industry
- Serves as a raw material for the production of many chemicals, such as acetaldehyde, diethyl ether, tetraethyl lead, acetic acid, chloroform, ethyl acetate, ethylene, etc.;
- Widely used as a solvent (in the paint and varnish industry, in the production of household chemicals and many other areas);
- It is a component of antifreeze and windshield washers.
- In household chemicals, ethanol is used in cleaning products and detergents, especially for the care of glass and plumbing. It is a solvent for repellents.
Medicine
In medicine, ethyl alcohol is primarily used as a solvent, extractant and antiseptic.
- In terms of its action, ethyl alcohol can be classified as an antiseptic;
- as a disinfectant and drying agent, externally;
- the drying and tanning properties of 96% ethyl alcohol are used to treat the surgical field or in some techniques for treating the surgeon’s hands;
- solvent for medicines, for the preparation of tinctures, extracts from plant materials, etc.;
- preservative for tinctures and extracts (minimum concentration 18%);
- defoamer when supplying oxygen, artificial ventilation;
- in warm compresses;
- for physical cooling during fever (for rubbing)[5];
- possible use as a component of parenteral nutrition (in the form of a 5% solution) in weakened patients, taking into account the high energy value of the substance
- component of general anesthesia in situations of drug shortage
- As an antifoam agent for pulmonary edema in the form of inhalation of a 33% solution.
- Ethanol is an antidote for poisoning with certain toxic alcohols such as methanol and ethylene glycol. Its action is due to the fact that the enzyme alcohol dehydrogenase, in the presence of several substrates (for example, methanol and ethanol), carries out only competitive oxidation, due to which, after timely
(almost immediate, following methanol/ethylene glycol) intake of ethanol, the current concentration of toxic metabolites decreases (for methanol - formaldehyde and formic acid, for ethylene glycol - oxalic acid).
Perfumes and cosmetics
It is a universal solvent for various substances and the main component of perfumes, colognes, aerosols, etc. It is part of a variety of products, including even toothpastes, shampoos, shower products, etc.
Food industry
Along with water, it is a necessary component of alcoholic drinks (vodka, whiskey, gin, etc.). It is also found in small quantities in a number of drinks obtained by fermentation, but not classified as alcoholic (kefir, kvass, kumiss, non-alcoholic beer, etc.). The ethanol content in fresh kefir is negligible (0.12%), but if it has stood for a long time, especially in a warm place, it can reach 1%. Kumis contains 1-3% ethanol (in strong ethanol up to 4.5%), kvass - from 0.6 to 2.2%.
Solvent for food flavorings. Can be used as a preservative for bakery products, as well as in the confectionery industry.
Registered as a food additive E1510
[6].
The energy value of ethanol is 7.1 kcal/g.
Description
Ethanol (C2 H5 OH) is a linear alkanol. “Alcohol or alcohol” is a well-established colloquial address. The term "alcohols" refers to a group of organic chemical compounds that, in addition to a hydrocarbon skeleton, have at least one hydroxyl group as an additional functional group. In this case, the carbon atom containing the hydroxyl group does not have a higher order substituent.
The alcohol is a natural product of alcoholic fermentation in ripe fruits and juices. Many products contain small amounts of ethanol. For example, non-alcoholic beer contains up to 0.5%. Fruit juices can have an alcohol content of about 0.38%. The exception is grape juice, which can contain up to 1% ethanol, as well as ripe banana and kefir. The physiological alcohol content in human blood ranges from 0.02 to 0.03%.
The use of ethanol as automobile fuel
Different countries have different government programs for the use of ethanol in transport:
| A country | Requirements |
| USA | produces 28 billion liters of ethanol annually by 2012, 85% ethanol/gasoline blend (E85) |
| European Union | 2% in 2005, 5.75% biofuels by 2010 (ethanol + biodiesel) |
| China | produce 3 million tons annually by 2010 |
| Thailand | 10% blend in Bangkok, 5% blend nationwide since 2007 |
In the United States, the Energy Bill, signed by President Bush in August 2005, provides for the annual production of 30 billion liters of ethanol from grain and 3.8 billion liters from cellulose (corn stalks, rice straw, forest waste) by 2012.
Ethanol mixes well with water, unlike gasoline. The problem of segregation of a mixture of gasoline and ethanol has not yet been solved.
Vehicle fleet running on ethanol
A mixture of ethanol and gasoline is designated by the letter E. The number next to the letter E indicates the percentage of ethanol. E85 means a mixture of 85% ethanol and 15% gasoline.
Blends up to 20% ethanol can be used on any vehicle. However, some car manufacturers limit the warranty when using blends containing more than 10% ethanol. Blends containing more than 20% ethanol in many cases require modifications to the vehicle's ignition system.
Automakers produce cars that can run on both gasoline and E85. Such cars are called "Flex-Fuel". In Brazil, such cars are called “hybrid”. There is no name in Russian. Most modern cars either natively support the use of such fuel, or optionally, upon request.
In 2005, more than 5 million vehicles in the United States had Flex-Fuel engines. At the end of 2006, 6 million vehicles with Flex-Fuel engines were in use in the United States. The total vehicle fleet is 230 million vehicles.
1200 gas stations sell E85 (May 2007). In total, about 170,000 gas stations sell automobile fuel in the United States.
In Brazil, about 29,000 gas stations sell ethanol.
Economical
The cost of Brazilian ethanol (about US$0.19 per liter in 2006) makes its use economically viable.
Environmental aspects
Bioethanol as a fuel is neutral as a source of greenhouse gases. It has a zero carbon dioxide balance because its production through fermentation and subsequent combustion releases the same amount of CO2 as was previously taken from the atmosphere by the plants used to produce it.
In 2006, the use of ethanol in the United States reduced emissions of about 8 million tons of greenhouse gases (CO2 equivalent), which is approximately equal to the annual emissions of 1.21 million cars.
Safety and Regulation
Denatured alcohol
- Ethanol is a flammable substance; a mixture of its vapors and air is explosive.
- Synthetic ethyl alcohol, technical and food grade, unsuitable for the production of alcoholic beverages, is included in the list of toxic substances for the purposes of Article 234 and other articles of the Criminal Code of the Russian Federation[7]
- Since 2005, retail sales of alcohol have been prohibited in Russia (with the exception of the Far North) [8]
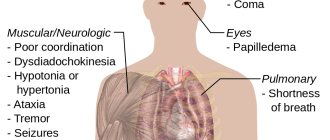
Toxicology of ethanol
Depending on the dose, concentration, route of entry into the body and duration of exposure, ethanol can have narcotic, anesthetic and toxic effects. In certain doses to body weight and concentrations it leads to acute poisoning and death (a lethal single dose is 4-12 grams of ethanol per kilogram of weight). However, ethanol is a natural metabolite of the human body, and in certain doses it is used in medicine as an independent medicine, as well as as a solvent for pharmaceuticals, extracts and tinctures.
Long-term consumption of ethanol can cause diseases such as gastritis, stomach ulcers, stomach cancer and esophageal cancer.
Ethanol consumption can cause oxidative damage to brain neurons.
Excessive consumption of alcoholic beverages can lead to alcoholism.
Health effects
Ethanol is absorbed throughout the digestive tract. The first stage begins to a small extent already in the oral mucosa. The alcohol absorbed there goes directly into the blood and is thus distributed throughout the body, including the brain. About 20% is absorbed in the stomach, the rest in the small intestine.
Ethanol absorbed in the stomach and intestines first enters the liver along with the blood, where it is partially decomposed. The absorption of alcohol is increased by various factors such as heat (Irish coffee, grog), sugar (liqueur) and carbon dioxide (sparkling wine).
Approximately 2-10% of alcohol consumed is excreted in urine, sweat and exhaled air.
In the liver, most ethanol (as well as other water-soluble toxins) is broken down into acetic acid by alcohol dehydrogenase (ADH) enzymes. Acetic acid is then converted to CO2 through the Krebs cycle. Intermediate ethanal is responsible for the so-called “hangover” symptoms - headache, nausea and vomiting. The decomposition of ethane is inhibited by sugar, so hangovers are especially intense when drinking sweet alcoholic drinks, especially liqueur and some types of champagne.
The rate of degradation by alcohol dehydrogenase is constant within certain limits. For men it is about 0.1 g per hour and kg of body weight, for women it is 0.085
Notes
- Article “Absolute alcohol” in TSB.
- Pharmaceutical reference book Khimik.ru
- Great Soviet Encyclopedia, article “Ethyl alcohol”
- TSB, article “Alcoholism”
- Drugs affecting the central nervous system
- E1510 - ethyl alcohol
- Decree of the Government of the Russian Federation of December 29, 2007 N 964 Moscow “On approval of lists of potent and toxic substances for the purposes of Article 234 and other articles of the Criminal Code of the Russian Federation, as well as large amounts of potent substances for the purposes of Article 234 of the Criminal Code of the Russian Federation”
- Article 26 of the Federal Law of November 22, 1995 N 171-FZ (as amended on April 5, 2010) (Consultant+)