Description of the drug
Imovax Polio vaccine is an inactivated (non-live) vaccine for the prevention of polio.
Poliomyelitis is an acute infectious disease caused by RNA polioviruses (serovars I, II, III), characterized by damage to the central nervous system, lymphatic system, and gastrointestinal tract. The disease is characterized by the appearance of flaccid paralysis, mainly of the lower extremities. In the most severe cases, damage to the spinal cord leads to respiratory arrest and death. Clinically, poliomyelitis is manifested by fever, headaches and muscle pain, followed by the development of paralysis.
The primary goal of the polio vaccine is to eradicate the poliovirus.
The inactivated vaccine was developed to replace the live attenuated polio vaccine (oral polio vaccine - OPV) because OPV can cause a dangerous complication called vaccine-associated polio.
Vaccine-associated polio is a disease similar to polio, but the causative agent is not a virus, but a vaccine strain. The clinical manifestations and outcomes of the two variants of polio are similar. The introduction of at least two injections of the inactivated vaccine Imovax Polio reduces the likelihood of developing vaccine-associated polio to zero.
Vaccination scheme
The course of primary vaccination with Imovax Polio consists of 3 injections with one dose of vaccine (0.5 ml) with an interval of at least 1.5 months, at the age of 3, 4.5 and 6 months, respectively.
Subsequent revaccinations are carried out with live polio vaccine within the periods specified by the National Calendar of Preventive Vaccinations of the Russian Federation: 18 months (or 1 year after the administration of the third vaccine) and 20 months (or 2 months after the first revaccination).
If there are medical contraindications to the use of a live vaccine, revaccinations can be carried out with inactivated polio vaccine according to the following scheme: the first revaccination of Imovax Polio is carried out 1 year after the third administration of the vaccine. Subsequent Imovax Polio revaccination is carried out every 5 years until the patient reaches the age of 18 years and then every 10 years.
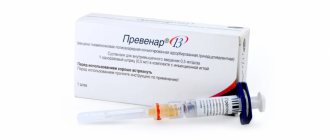
Inactivated polio vaccine “Imovax Polio”
Sanofi Pasteur, France
Release form: 1 syringe / 1 dose / 0.5 ml.
Vaccination schedule: three times (3 months – 4.5 months – 6 months). Revaccination after 1 year, subsequent boosters every five years until age 18 and then every 10 years.
INSTRUCTIONS FOR USE of the vaccine for the prevention of poliomelitis inactivated Imovax Polio.
Latin name: Imovax Polio / Imovax Polio. Composition and release form: Imovax Polio injection solution for intramuscular and subcutaneous administration in syringes or ampoules, 1 dose of 1, 10 and 20 pcs. packaged. 1 dose of vaccine (0.5 ml) Imovax Polio contains: inactivated vaccines for the prevention of poliomyelitis caused by poliovirus type 1, type 2 and type 3; 1 vaccine dose (corresponds to the amount of antigen that meets the standards and requirements of the antigenic activity test described in the French and European pharmacopoeias); 2-phenoxyethanol, formaldehyde. Properties/Action: Imovax Polio is an inactivated vaccine for the prevention of polio. The Imovax Polio vaccine is produced from polio viruses of 3 types, cultivated on the VERO cell line and inactivated with formaldehyde. The Imovax Polio vaccine forms specific immunity against the polio virus. Immunity is acquired after the 3rd injection of the Imovax Polio vaccine, strengthens with subsequent administrations of the drug and persists for at least 5 years after the first revaccination. After administration of 3 doses of the Imovax Polio vaccine, seroconversion is observed in 95-100% of vaccinated people. The Imovax Polio vaccine causes the production of a significant amount of virus-neutralizing antibodies (starting from the second injection), regardless of the general condition of the vaccinated (immunodeficiency, intestinal pathology, dystrophy). Indications: Imovax Polio vaccine is used for the prevention of polio, including in persons with contraindications to the use of “live polio vaccine”; It is possible to use the Imovax Polio vaccine in pregnant women. Method of administration and doses: The regimen for using the Imovax Polio vaccine is determined by the National Preventive Vaccination Calendars. The Imovax Polio vaccine is prescribed in the form of subcutaneous and intramuscular injections. The recommended age for starting vaccination with Imovax Polio is 3 months. Primary vaccination with Imovax Polio: 3 injections (at least 2 injections) of 0.5 ml with a minimum interval of 1 month between them. Revaccination with Imovax Polio: 1 year after the last injection, then every 5-10 years. The used syringe must be destroyed. Contraindications: - acute infectious diseases accompanied by an increase in body temperature (you should wait until you have recovered for vaccination); - hypersensitivity to streptomycin; - early childhood (up to 3 months). Side effects: In rare cases, when using the Imovax Polio vaccine, erythema at the injection site may appear, as well as a slight increase in body temperature. All cases of unusual vaccination reactions should be reported to the doctor or pharmacist, the National Authorities for the Control of Medical Immunobiological Products and the Pasteur Merrier Connaught Representative Office. Drug interactions: Imovax Polio vaccine can be used in combination with other injectable forms of vaccines: for the prevention of diphtheria, tetanus, whooping cough, infections caused by Haemophilus influenzae type b, and hepatitis B. Storage conditions: At temperatures from + 2°C to + 8 °C. Do not freeze. Shelf life: 36 months. Dispensing conditions from pharmacies are by prescription.
← Back
Compatibility of Isovax Polio with other vaccines
Imovax Polio can be used with an interval of 1 month or simultaneously (on the same day) with other vaccines (with the exception of the BCG vaccine - in accordance with the National Calendar of Preventive Vaccinations of the Russian Federation), provided that they are administered to different parts of the body using different syringes.
The use of the Imovax polio vaccine in combination with other vaccinations does not affect their immunogenicity (ability to develop immunity). Tolerability of vaccines does not deteriorate, and the number of adverse reactions does not increase. Administering several vaccines on the same day does not place an excessive burden on the immune system. Imovax polio can be used to continue and complete a vaccination course started with other polio vaccines, both live and inactivated. All vaccines in the Russian national vaccination calendar are interchangeable.
The problem of polio and the prospects for using inactivated polio vaccine
Abortive form. Occurs most often. In the classic version, it occurs in the form of a febrile disease with disorders of the respiratory and gastrointestinal tract. The disease resolves within 2–3 days, with complete recovery and without any neurological consequences or symptoms [1–7].
Non-paralytic form (“aseptic, or serous, meningitis”). There are signs of an abortive form of poliomyelitis, but more pronounced - with a 2-phase course. The disease goes away without a trace after 3–5 days [1–7].
Paralytic form (acute flaccid peripheral paresis/paralysis). Its appearance is preceded by a prodromal period (catarrhal phenomena and vegetative disorders), then a pre-paralytic (febrile) period begins - with a sharp increase in body temperature to 38–39 ° C (it is accompanied by headache, sleep disturbances, meningeal symptoms). In this case, the following symptoms are common: increased sweating, hyperemia of the skin, pain along the projection of nerve trunks, asymmetry and decreased tendon reflexes. Peripheral paralysis (proximal muscles, usually lower, less often upper limbs; usually asymmetrical) appears suddenly, increasing in severity from paresis to paralysis over several days. Laboratory studies can detect an increase in the number of leukocytes in the blood, an acceleration of ESR, and in the cerebrospinal fluid an opalescent hue, an increase in the number of mononuclear cells (up to 400), and an increase in protein content (up to 500 mg) [1–7].
In the USA, there are 3 leading syndromes of paralytic poliomyelitis, depending on the dominant damage to one or another locus of the central nervous system: spinal paralytic poliomyelitis, bulbar poliomyelitis, polioencephalitis [5].
Pathogenesis of polio. Under the influence of polioviruses, swelling of the brain and its membranes occurs, as well as degenerative changes in the cells of the anterior horns of the spinal cord and sometimes the brain stem. These pathological changes in the central nervous system cause flaccid paralysis of the peripheral type, characteristic of poliomyelitis [1–9].
Vaccine-associated paralytic poliomyelitis can develop in children who have received multiple intramuscular injections. In this case, retrograde axonal transport along the injured nerve plays a role in the pathogenesis of the disease, facilitating the entry of the vaccine poliovirus into the neurons of the spinal cord [1–9].
Vaccine-associated paralytic polio (VAPP). Acute flaccid paralysis is diagnosed by a pediatric neurologist, although it may initially be suspected by a pediatrician. VAPP in recipients is most often caused by type 3 vaccine poliovirus (alone or in combination with another poliovirus) [1–9].
VAPP usually develops 4–30 days after oral polio vaccine (OPV) administration [1–7]. In children who have been in contact with vaccinated OPV, the incubation period can reach 60 days (rarely more). Finally, in immunocompromised children, manifestations of VAPP can occur even after 6 months [10].
The criteria for VAPP are relatively simple: the presence of residual paresis after 60 days; lack of contact with a polio patient; vaccine virus types 1 or 2 in stool samples; negative (absent) result for wild virus in 2 stool samples [1–7].
VAPP requires virological examination as early as possible (examination of two stool samples with an interval of 1 day). In addition, an immunological and biochemical examination is carried out to exclude an immunodeficiency state (the content of immunoglobulins of the main classes and protein fractions in the blood) [1–7]. Treatment of VAPP is carried out in a specialized hospital.
Although modern medical reference books and guidelines mention various groups of drugs for the treatment of polio (wild and vaccine-associated) - gamma globulin, Proserin, galantamine, securinin, etc., etiotropic drugs for effective treatment of the disease currently do not exist.
In relation to polio, an almost unique situation has arisen: there are no known treatments for the disease, but there is a highly effective measure of its prevention (vaccines).
Recent history of vaccination against polio. Since the mid-1950s. has found application for prophylactic purposes (live) OPV Sabin, including in the republics of the USSR. Its trials were carried out in 1959 in the Estonian SSR, due to the extremely high incidence of polio in this region. The result was a demonstration of the possibility of stopping the circulation of a wild virus in a single territory, and the experience gained was transferred to other republics of the Soviet Union and picked up in some countries of Eastern Europe (Hungary, Poland). Sabin's OPV is still used in the Russian Federation.
Jonas Salk's name is associated with the development and use of inactivated polio vaccine (IPV). For the first time in Europe, immunization using the Salk vaccine began in Denmark (1955), and after just a few years, almost all countries in the European Region began to use IPV.
Current state of the polio problem in the Russian Federation. The use of OPV, which continues in our country, is associated with cases of VAPP, leading to a decrease in the quality of life, and often to direct disability of children.
The last case of endemic polio in the European Region was reported on November 26, 1998 in Turkey (in an unvaccinated boy aged 33 months). In general, in 1998, an outbreak of wild polio (26 cases) caused by polioviruses types 1 and 3 was observed in Turkey [3].
The World Health Organization (WHO) certified the entire European region (including the Russian Federation) as a territory free from wild polio on June 21, 2002. In 1994, “poliomyelitis free” was declared in the American region, and after 6 years (2000 .) in the Western Pacific region [2].
As for VAPP, 15 cases of VAPP were registered in the Russian Federation in 2004 (1 case per 8.7 million population). In previous and subsequent years, official statistics indicated comparable numbers of VAPP among Russian children. According to international data, the risk of developing VAPP is 1 in 14 million (or 1 in 500,000–1,000,000 first doses of OPV) [3]. It is possible that VAPP in the Russian Federation occurs more often than expected: with a frequency of 1 case per 100–200 thousand first OPV administrations. During the period 1998–2004. 86 cases of VAPP have been reported in the country.
Attitude to the problem of polio. In fact, there are 2 possibilities: continue using OPV (Sabin) or use IPV (Salka). Let us quote the position of Rospotrebnadzor: “Cases of VAPP cast a shadow on the vaccine prevention program as a whole, giving parents a reason to refuse to vaccinate their children” [11].
Refusal to vaccinate against polio cannot be considered acceptable, although this attitude is often propagated by public and religious organizations, and is also often supported by representatives of the media. We should not forget that polio is one of the 9 decreed infections against which mandatory immunoprophylaxis is provided in our country [3].
Benefits of IPV. High technology product; The production time for IPV (including quality control) is about 18 months. A guaranteed supply of the vaccine dose to the body is ensured through the parenteral route of administration. The composition of IPV allows one to achieve a stable level of specific immunity against all three types of polioviruses [12]. There are no complications of immunoprophylaxis in the form of VAPP. The latter position reliably secures the advantages of IPV, in particular, Imovax Polio.
IPV has a pronounced revaccination effect that exceeds that of OPV. When using OPV, local immunity formed by primary vaccination partially neutralizes reintroduced viruses. IPV cannot cause intestinal dysbiosis even theoretically.
Vaccine "Imovax Polio" (0.5 ml doses), the composition of which is represented by the following ingredients: inactivated poliovirus type 1 - 40 D-antigen units, type 2 - 8 D-antigen units, type 3 - 32 D-antigen units; preservative: 2-phenoxyethanol—max. 0.005 ml) [3].
Compared to the original (“classical”) Salk IPV, the modern Imovax Polio vaccine has an improved composition (increased antigenic concentration for all three types of polioviruses). Therefore, it is more correct to call Imovax Polio an enhanced version of IPV (uIPV), more immunogenic than the 1st generation of IPV. The modern version of uIPV was developed in 1977 and created in 1982. In the early 1990s. The preventive efficacy of IPV has been shown to be higher than that of live OPV [13]. IPV is more resistant to environmental influences (easier to store, vaccine remains effective). IPV can be stored at a temperature of +2–8 °C and does not require transportation on ice, like OPV.
All components of Imovax Polio are included in the Pentaxim vaccine (+DTP+filamentous hemagglutinin). It is important that Imovax Polio and Pentaxim are produced in individual doses (accurate dosing of vaccines is ensured). There is absolutely no risk of reversal of the virulence properties of IPV. IPV is not contraindicated in patients with various types of immunodeficiency (endogenous or induced).
Practice of using IPV. In accordance with the national calendar of preventive immunization of the Russian Federation (order of the Ministry of Health and Social Development of the Russian Federation No. 673 of January 30, 2007), vaccination against polio is provided in the following decreed periods: in the first year of life (starting from 3 months of age, 3 times, at intervals of 45 days , together with DPT); revaccinations 1 and 2 (in the 2nd year of life) - at the ages of 18 and 20 months; subsequently, revaccination (3rd) at the age of 14 years [14].
The current situation with IPV in the Russian Federation. Imovax Polio has been actively used in the Russian Federation since the 1990s. Since 2005, Imovax Polio has been used for routine vaccination of children 1 year of age in a number of regions of Russia (there are currently about 70 such regions).
On January 17, 2006, Order No. 27 of the Ministry of Health and Social Development of the Russian Federation “On Amendments to Appendix No. 1 to the Order of the Ministry of Health of Russia of June 27, 2001 No. 229 “On the National Calendar of Preventive Vaccinations and the Calendar of Preventive Vaccinations for Epidemic Indications” was put into effect, which opened new prospects for the use of IPV in our country, and from January 1, 2008, Order of the Ministry of Health and Social Development of the Russian Federation No. 673 [14–16]. In particular, in the period 2006–2007. additional immunization of the population was carried out against a number of infectious diseases, including polio (other diseases: hepatitis B, rubella, influenza).
Vaccination against polio with an inactivated vaccine is intended for the following groups of children: young children with clinical signs of an immunodeficiency state; HIV-infected or born from HIV-infected mothers; with an established diagnosis of oncohematological diseases and/or receiving immunosuppressive therapy for a long time; children who are at the 2nd stage of nursing and have reached 3 months of age; children from orphanages (regardless of health status); children from families where there are patients with immunodeficiency diseases [14].
The Federal Service for Surveillance in the Sphere of Consumer Rights Protection and Welfare drew up an order “On the use of inactivated polio vaccine” (dated February 24, 2005), addressed to the heads of territorial departments of the Federal Service for Surveillance in the Sphere of Consumer Rights Protection and Human Welfare in the constituent entities of the Russian Federation . Along with possible vaccination regimens in which all 4 doses of DPT are combined with IPV, 4 more immunization regimens have been proposed that provide for the prevention of VAPP at lower financial costs [11]. In conditions of “limited funding” for the “purchase of IPV in order to reduce the risk of VAPP”, priority groups of children who are indicated for a combined immunization regimen were identified: children who are in hospital at the time of vaccination or are planning to be hospitalized within 60 days after vaccination; children from orphanages; children in whose families there are patients suspected of immunodeficiency [11].
Note that the scheme of combined use of IPV with OPV has a significant drawback - the risk of VAPP remains in persons in contact with those vaccinated with live vaccine.
Among children with neurological pathology, there are representative groups of patients for whom the use of IPV can be considered indicated, effective and safe (perinatal lesions of the nervous system; demyelinating diseases of the central nervous system, including MS, etc.; epilepsy, congenital hydrocephalus, cerebral palsy, etc.). These multifactorial types of pathology of the nervous system, different in their etiostructure, severity and clinical manifestations, have been traditionally considered for many years as direct contraindications for vaccination with live vaccines.
Tatochenko V.K. (2005) points out: “The main problem after the eradication of polio in the world, that is, after the complete cessation of the circulation of wild viruses, will be the abolition of vaccination” [3]. This problem is associated not only with the danger of using polio viruses for the purpose of bioterrorism, but also with the possibility of reversal of virulence in vaccine viruses used in OPV.
It seems most appropriate to switch to IPV at the final stage of polio eradication, since the poliovirus circulates among the immune population for a relatively short time—only a few weeks. Almost (with the exception of Russia, Portugal, Greece and Spain) all developed countries of the world community have already made the transition to IPV. In Russia, the need for such a step has already been recognized at the federal level.
In the event of the return of wild poliovirus, the control measure should be mass vaccination with an oral vaccine (preferably monovalent), which more strongly stimulates the local immunity of the intestinal mucosa. Currently, such a vaccine is used in residual foci of polio [3].
I would like to hope that in the coming years we will witness not only the complete eradication of endemic polio from the globe, but also the absence of cases of VAPP. The latter achievement is possible subject to the final transition to the use of exclusively IPV for preventive purposes.
Literature
- Jenson HB, Baltimore RS eds. Pediatric infectious diseases. 2nd ed. London. Elsevier Saunders. 2001. 1230 p.
- Red book. Report of the Committee on Infectious Diseases (2004–2006). 27 th ed. Elk Grove Village (Il, USA). American Academy of Pediatrics. 992 p.
- Immunoprophylaxis-2005 (reference book) / Ed. Tatochenko V.K., Ozeretskovsky N.A.M.: Silver Threads Publishing House. 2005. 192 p.
- Roos KL (ed). Principles of neurologic infectious diseases. New York-Chicago. McGraw Hill/Medical Publ. Div. 2005. 572 p.
- Feigin R.D., Cherry J., Demmler G.J., Kaplan S.eds. Textbook of pediatric infectious diseases. 5 th ed. Elsevier Saunders. Philadelphia-London. 2003. 2880 p.
- Katz S., Gershon A.A., Hotez P.J. eds. Krugman's infectious diseases in children. 11 th ed. Philadelphia. Elsevier Mosby. 2003.
- Long SS, Pickering LK, Prober CG Principles and practice of pediatric infectious diseases. 2nd ed. Philadelphia. Elsevier Mosby. 2002. 1392 p.
- Koike S. Molecular mechanism of tissue-specific infection of poliovirus // Uirisu. 2004. Vol.54. P. 205–212.
- Ohka S., Nomoto A. The molecular basis of poliovirus neurovirulence // Dev. Biol. (Basel). 2001. Vol. 105. P. 51–58.
- Parvaneh N., Schahmahmoudi S., Tabatabai H., Zahraei M. et al. Vaccine-associated paralytic poliomyelitis in a patient with MHC class II deficiency // J. Clin. Virol. 2007. Vol. 39. P.145–148.
- On the use of inactivated polio vaccine//Letter of Rospotrebnadzor No. 0100/1248–05–32 dated February 24, 2005.
- Wahid R., Cannon MJ, Chow M. Virus-specific CD4+ and CD8+ cytotoxic T cell responses and long-term T cell memory in individuals vaccinated against polio // J. Virol. 2005. Vol. 79. P.5988–5995.
- Patriarca PA, Wrigh PF, John TJ Factors, affecting the immunogenicity of oral poliovirus vaccine in developing countries: review// Rev. Infect. Dis. 1991. Vol. 13. P.926–939.
- On introducing amendments and additions to Order of the Ministry of Health of the Russian Federation No. 229 of June 27, 2001 “On the national calendar of preventive vaccinations and the calendar of preventive vaccinations for epidemic indications” // Order of the Ministry of Health and Social Development of the Russian Federation No. 673 of January 30, 2007
- On amendments to Appendix No. 1 to Order of the Ministry of Health of Russia dated June 27, 2001 No. 229 “On the national calendar of preventive vaccinations and the calendar of preventive vaccinations for epidemic indications” // Order of the Ministry of Health and Social Development of the Russian Federation No. 27 of January 18, 2006
- On the national calendar of preventive vaccinations and the calendar of preventive vaccinations for epidemic indications // Order of the Ministry of Health of the Russian Federation No. 229 of June 27, 2001.
V. M. Studenikin , Doctor of Medical Sciences, Professor of the Scientific Center for Children's Diseases of the Russian Academy of Medical Sciences, Moscow
How is vaccination carried out?
Vaccination is carried out in a vaccination room, in compliance with all sanitary requirements. All drugs are certified. A certificate for the drug is provided upon request.
Without reminders, before vaccination, the medical worker must show the drug and the expiration date of the vaccine.
Only sterile and disposable instruments are used. The vaccination must be carried out using disposable medical gloves.
On the day of vaccination, the child is examined by a pediatrician and the temperature is measured. In the absence of contraindications, vaccination is carried out. Information about the vaccination performed is entered into the card, vaccination certificate, and detailed recommendations for caring for the child in the post-vaccination period are given.
Before vaccination, the doctor will answer all your questions. Be sure to bring information about previous vaccinations to your appointment!
Please note that vaccination of a child, Mantoux test, Diaskintest can only be carried out in the presence of parents or legal representatives of the child (guardians), or if the accompanying person has a NOTARIZED power of attorney to carry out the manipulation (indicating the drug planned for administration) . Otherwise, vaccination will be denied. We comply with the laws of the Russian Federation.
Only here!