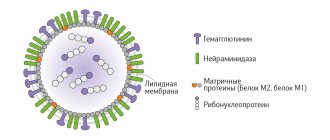
Risk groups for the incidence of pneumococcal infection:
- persons 55 years of age and older;
- children and adults with chronic diseases;
- “organized” contingents (children attending kindergartens, schools, military personnel, residents of nursing homes).
Streptococcus pneumoniae is a representative of the normal microflora of the human upper respiratory tract. Normally, 5 to 70% of people are carriers of one or more types of pneumococci. “Organized” (living or being in groups) children and adults have the highest level of carriage. To date, more than 90 different serotypes (immunological variants) of pneumococci have been identified. All of them are potentially pathogenic, but about two dozen of them cause severe infections.
In response to the use of antibiotics, pneumococci have developed resistance to many of them. Thus, in countries with widespread use of antibiotics, the level of resistance of pneumococci to penicillin is up to 50% of all isolated pneumococci, and to tetracycline and chloramphenicol - about 30%. In addition, due to the rapid development of the disease (2-3 days), there is no time for special Determination of sensitivity to antibiotics is usually not available. Patients with severe infections often die despite the use of standard antibacterial drugs.
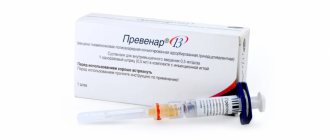
In Russia, vaccination against pneumococcal infection began in 2008, it was a 7-valent vaccine (Prevenar). It is included in the national vaccination calendars of most developed countries and is recommended by WHO for use in all countries of the world. In the USA, it was used in 2000; during the first 5 years, the frequency of severe forms of infection in children under 4 years of age was reduced by 40 times. At the same time, in unvaccinated children aged 5-15 years and adults, the frequency of infections caused by this pathogen significantly decreased. According to American scientists, due to a decrease in the number of pneumococcal carriers, mass vaccination with this vaccine has led to a 2-fold decrease in visits for otitis of any etiology.
Due to the fact that the effectiveness of the vaccine against complicated pneumonia was not so significant, scientists developed a new 13-valent vaccine containing additional strains of pneumococcus, “guilty” of the development of complications of pneumonia.
This vaccine most widely covers the spectrum of pathogens that cause life-threatening diseases such as bacteremia, sepsis, meningitis, pneumonia, acute otitis media and other diseases.
Principles and purposes of vaccination
Pneumococcal disease is one of the leading causes of morbidity and mortality worldwide. Serious diseases often caused by pneumococci include pneumonia, meningitis, and bacteremia with fever. In 2005, WHO estimated that 1.6 million people die each year from pneumococcal disease. Therefore it must be prevented.
By the end of 2013, pneumococcal vaccination had been introduced in 103 countries, and immunization coverage had reached 25%. By order of the Ministry of Health of the Russian Federation No. 125 of March 21, 2014, vaccination against pneumococcal infection was introduced into the National Calendar of Preventive Vaccinations of the Russian Federation.
There are 4 vaccines registered in our country:
- Prevenar (Pfizer, USA/UK) – contains a mixture of purified capsular polysaccharides of 7 serotypes of Streptococcus pneumoniae, individually conjugated to a diphtheria carrier protein, used in children from 2 months to 5 years.
- Prevenar 13 (Pfizer, USA/UK) – the vaccine contains a mixture of purified capsular polysaccharides of pneumococcus 13 serotypes.
- Pneumo 23 (Sanofri Pasteur, France) – contains a mixture of purified capsular polysaccharides of 23 serotypes of pneumococcus, used in children over 2 years old, administered once in frequently ill children, as well as in children with severe oncological and hematological diseases.
The following vaccination schedule with Prevenar vaccines is recommended: if vaccination begins after 6 months, the vaccine is administered 3 times (two injections in the 1st year of life with an interval of 1 month, the 3rd injection in the 2nd year of life). If the vaccine is administered in the 2nd year of life in the period from 12 to 15 months, it is recommended to administer it twice with an interval of 2 months; when vaccinated after 2 years, it is administered once intramuscularly into the shoulder.
Currently, the vaccine Synflorix (Glaxo Smith Klein Biologicals) is being registered - a mixture of purified capsular polysaccharides of 10 serotypes of pneumococcus.
All children from 1-2 years of age to 5 years are subject to vaccination against pneumococcal infection; it is especially important for frequently ill children and elderly people over 55 years of age.
How to vaccinate against pneumonia in Russia, and to whom it is indicated
In November, Pneumonia Day is celebrated around the world. Pneumonia, or pneumonia, remains a terrible disease that claims lives to this day. Children under 5 years of age and the elderly are at particular risk. Infectious pneumonia can be caused by viruses, fungi or bacteria. The most common cause of pneumonia is the bacterium pneumococcus. According to statistics, in Russia, up to 76% of cases of community-acquired pneumonia in adults are due to pneumococcal infection, and in children it is the cause of 94% of cases of complicated community-acquired pneumonia. But fortunately, you can protect yourself from this disease by getting vaccinated. This is much easier than treating it, because available drugs in some cases are powerless against bacteria that are immune to them. We tell you who and when it is indicated, and also answer the most frequently asked questions about the pneumococcal vaccines available in Russia. 1. Who should be vaccinated against pneumococcal infection?
Vaccination against pneumococcus is included in both parts of the Russian vaccination calendar: both for epidemic indications and for age. According to the National Calendar of Preventive Vaccinations for Epidemic Indications of the Ministry of Health of the Russian Federation, such vaccination is mandatory for: children aged 2–5 years; adults at risk, including conscripts for military service; people over 60 years of age suffering from chronic lung diseases. Since 2014, vaccination against pneumococcus has been included in the National Calendar of Preventive Vaccinations, which indicates the timing of vaccination for everyone, regardless of epidemic indications: A child receives his first vaccination against pneumococcus at 2 months. The period of the second vaccination is 4.5 months. Revaccination against pneumococcal infection is given to children who are 15 months old.
2. Who is at high risk?
According to the Union of Pediatricians of Russia, pneumococcus is especially dangerous for the following categories: babies in the first two years of life, including premature babies; children and adults with chronic diseases of the respiratory system, cardiovascular system, kidney disease and endocrine system, liver; persons before and after organ transplantation with HIV infection, cancer and blood diseases; aged people; “heavy” smokers; convalescents (previously suffered) pneumonia, otitis, meningitis; frequently ill children. It should be remembered that pneumococci, when entering the body, can cause not only pneumonia, but also strike other organs, leading to otitis media, conjunctivitis, bronchitis, sinusitis and meningitis.
3. Will the pneumococcal vaccination protect against covid pneumonia?
Pneumococcal and coronavirus pneumonia have different natures. And although each of these diseases has its own type of pathogen, in the first case - the bacterium Streptococcus pneumoniae, and in the second - the SARS-CoV-2 virus, there is scientific evidence showing that those vaccinated against pneumococcus tolerate coronavirus more easily with a lower risk of complications. In any case, if the body is not weakened by the fight against one infection, it will be able to more easily resist another. The recommendation to vaccinate against influenza at the same time as pneumococcus is based on the same principle - this will strengthen long-term T-cell immunity, which is responsible for protecting the body. And strong protection is what you need during a pandemic.
4. Is it necessary to get vaccinated, because there are antibiotics?
On the one hand, effective antimicrobial drugs have actually been developed for the treatment of pneumonia. On the other hand, doctors do not recommend relying too much on them, due to the resistance of many bacteria to antibiotics, which has become a real scourge of our time. In this case, the therapy simply will not work, and the patient will be left alone with the disease, without the protection that a vaccine could provide. “The increasing resistance of infectious disease pathogens to antimicrobial chemotherapy leads to the prescription of additional courses of therapy, increased hospitalization, temporary disability and can lead to social problems. Vaccination against pneumococcal infection plays an important role in the fight against antibiotic-resistant forms of pneumococcal infections,” emphasizes the Honored Scientist of the Russian Federation, Academician of the Russian Academy of Sciences, chief epidemiologist of the Russian Ministry of Health, Head. Department of Epidemiology and Evidence-Based Medicine of the First Moscow State Medical University named after. THEM. Sechenov Doctor of Medical Sciences, Professor Nikolai Briko.
5. What types of pneumococcal vaccines are there?
Today, two types of vaccines are used in the world to prevent pneumococcal infection: polysaccharide (23-valent vaccine) and conjugate vaccines (10- and 13-valent). They have different usage patterns and schedules. The doctor will help you choose the right one, taking into account the information contained in the instructions for the drug and the health characteristics of his patient.
6. Is it true that the higher the valence of a vaccine, the better it is?
It would seem obvious that a vaccine that copes with a large number of strains provides better protection. But it's not that simple. In the case of pneumococcal vaccines, we are talking about different production technologies. Without delving into terminology, we can say that the basis of polysaccharide vaccines is the mechanism of protection due to the production of B lymphocytes, which trigger IgM antibodies. This has an effect, but it is short-lived and does not contribute to the development of immune memory. “A significant disadvantage of polysaccharide vaccines is the low efficiency of the immune response in children under 2 years of age, since B-dependent antigens are difficult to recognize by the immature immune system of newborns and infants,” the clinical guidelines say. Therefore, doctors recommend using such vaccines for vaccination of designated risk groups. As for conjugate vaccines, when polysaccharides are combined with a carrier protein, a qualitatively different, T-dependent immune response is formed. Unlike simpler polysaccharide vaccines, this type uses T cells, which are responsible for a long-lasting immune response. And when they are used, the vaccinated person produces predominantly IgG class antibodies, which are responsible for the production of long-term immune memory cells. The T-dependent immune response is considered more effective in young children. In addition, scientists have proven that the 13-valent conjugate vaccine has a population effect - it reduces the incidence of disease in unvaccinated children and adults not directly, but through the formation of stable collective immunity. In addition, the 13-valent vaccine reduces the prevalence of antibiotic-resistant bacteria, which is also a huge plus. This vaccine can also be used in combination with the main vaccines of the National Preventive Vaccination Calendar, so it is ideal for children.
7. What vaccines are available in Russia?
Russians can be vaccinated against pneumococcus with any of the above vaccines. For those who are indicated for a polysaccharide 23-valent vaccine, there is the drug Pneumovax 23 from the American company Merck Sharp & Dohme (MSD). The 10-valent conjugate vaccine “Synflorix” is produced by the British GlaxoSmithKline, and the 13-valent “Prevenar 13” is produced in Russia under a license from Pfizer in Russia. Also currently undergoing the third stage of clinical trials is a 13-valent polysaccharide vaccine against pneumococcus, developed by the Russian pharmaceutical company. In addition, scientists from the N.D. Institute of Organic Chemistry are currently working on a new type of pneumococcal vaccine. Zelinsky RAS together with immunologists from the Research Institute of Vaccines and Serums named after I.I. Mechnikov. This drug, unlike existing ones, will work not on polysaccharides, but on synthetic oligosaccharides. The new technology is expected to reduce side effects and enhance the protective response to certain strains of pneumococcus.
8. How high is the production of the Russian pneumococcal vaccine?
Since other Russian vaccines against pneumococcus are still only in the testing and development stages, the PharmMedProm portal asked the manufacturer of the only vaccine manufactured in Russia and registered, Prevenar 13, to answer several questions about its quality and production methods. The Prevenar-13 vaccine is produced in a full cycle in Russia. What are the requirements for its quality (to what extent does the production comply with GMP standards) and how is Pfizer’s control carried out? Indeed, in partnership with Pfizer, we implemented the first project in the history of the Russian pharmaceutical industry to localize the 13-valent conjugate pneumococcal vaccine Prevenar 13 with the introduction of full-cycle production technology for the finished dosage form. In 2014, Petrovax began producing the vaccine at its plant in the Moscow region and supplying the drug to the Russian market, incl. for the National Preventive Vaccination Calendar. Vaccine supplies over the years have exceeded 35 million doses. The production process of such a drug is high-tech and includes several stages: formulation, bottling, secondary packaging and release quality control. Preparatory work for the launch of production lasted 4 years. During this time, we installed new equipment at our production complex, modernized many technological processes, and transferred all control methods. In the process of technology transfer, over fifty PetrovaxPharm specialists were trained, 10 of whom completed internships in the USA. Our employees visited Pfizer factories to get a closer look at the technologies and processes for producing the vaccine. Together with our partners, we translated and implemented technological documentation. After the technology transfer, we assessed the readiness of the equipment and all systems, and released the first pilot batches of drugs. It took at least six months to study the stability of samples from this series. Pfizer accompanied and advised us at all stages of this labor-intensive process. Today, our partner continues to conduct regular audits at the production site, which from year to year confirm the compliance of production processes with international GMP standards and the strict requirements of partners, as well as the quality and safety of the produced pneumococcal vaccine Prevenar 13. The vaccine is prepared in pre-filled syringes. How convenient is this form compared to an ampoule that needs to be drawn into a syringe? What other problems, besides convenience for medical staff, can this method of filling solve? The main advantage of this form of release for the patient is the accuracy of the administered dose of the vaccine and the additional safety of the vaccination procedure. The absence of the need to open the ampoule and draw the drug into the syringe makes the injection procedure comfortable for the patient and medical personnel, which generally increases the public’s confidence in immunization. Do you have any plans to develop your own vaccine against pneumococcal disease, perhaps in the future? The company has no such plans.
Link to publication: pharmmedprom.ru
Side effects, drug interactions and complications
The following side effects have been identified with the use of these vaccines:
- From the skin: burning sensation, redness, swelling of the skin in the injection area.
- From the immune system: allergic reactions of varying severity.
- From the nervous system: fatigue, headache, drowsiness.
- Other: slight increase in temperature.
Complications when using these vaccines occur rarely and, as a rule, are associated with violations of antiseptics, storage of the drug, improper patient behavior, or ignoring contraindications. Such complications include:
- local allergic reaction such as urticaria;
- general allergic reaction with a malignant course (Quincke's edema, anaphylactic shock);
- convulsions;
- symptoms of general intoxication with fever, weakness, headache and aching joints.
When you vaccinate children against pneumococcal infection, take the following precautions (to avoid complications):
- Avoid contact of your child with people with infectious diseases.
- Eliminate from your child’s diet foods and medications that cause even minor allergies.
- Avoid overheating and hypothermia of the child.
- Within 30 minutes after administration of the vaccine, the patient should be under the supervision of a physician for possible assistance with reactions such as anaphylactic shock.
Contraindications to vaccination
The Pneumo-23 and Prevenar vaccines are generally well tolerated. However, there are a number of contraindications.
Relative:
- infectious diseases, acute and chronic in the acute stage;
- fever of any origin;
- hyperthermia;
- pregnancy (no studies have been conducted);
- simultaneous vaccination with incompatible vaccines.
Absolute:
- allergy to any component of the drug.
Vaccines
Pneumo-23
This vaccine is produced in France. Release form: individual disposable syringe with a capacity of 0.5 ml. This vaccine contains antigens of 23 strains in a phenol buffer solution.
Prevenar
This vaccine is produced in the USA. Release form: individual disposable syringe with a capacity of 0.5 ml. This vaccine contains antigens of 23 strains, as well as the CRM197 protein in a buffered sodium chloride solution.
Pneumococcal vaccine "Prevenar 20": effectiveness of protection against pneumonia
The preventive effectiveness of Prevenar 20, firstly, is based on the results of a clinical trial of the previous vaccine, Prevenar 13, and, secondly, was established in three separate clinical studies.
Thus, the CAPiTA (NCT00744263) phase IV clinical trial (randomized, double-blind, placebo-controlled, multicenter) enrolled Dutch volunteers (n=84,496) aged 65 years or older who were assigned to a single dose of Prevenar 13 or placebo.
The primary endpoint was the prophylactic efficacy of Prevenar 13 in preventing a first episode of vaccine-type community-acquired pneumonia (VT-CAP), confirmed by the presence of at least two clinical criteria and chest radiography, as well as a positive vaccine-specific urinary antigen test. type, or by isolation of vaccine-type S. pneumoniae from blood or other sterile site.
Secondary endpoints include prevention of the first episode of confirmed nonbacterial and noninvasive VT-CAP (culture negative for S. pneumoniae) and the first episode of confirmed invasive pneumococcal disease (with S. pneumoniae present in sterile sites).
After almost 4 years of observation, the group of subjects who received Prevenar 13 was statistically significantly ahead of the placebo group in preventing the first episode of infection caused by the S. pneumoniae serotypes included in this vaccine.
Thus, community-acquired pneumonia developed in 49 people in the vaccine group versus 90 in the placebo group: the effectiveness of Prevenar 13 was 45.6% (95.2% CI: 21.8–62.5).
Non-bacterial and non-invasive community-acquired pneumonia occurred in 33 subjects - versus 60: the effectiveness of Prevenar 13 reached 45.0% (95.2% CI: 14.2–65.3).
Invasive pneumococcal infection was recorded in 7 participants versus 28: the effectiveness of Prevenar 13 was 75.0% (95.2% CI: 41.4–90.8).
Three phase III clinical trials assessed the safety and immunogenicity of Prevenar 20 among more than 6,000 adult participants (including seniors aged 65 years and older) who had and had not previously been vaccinated against pneumonia:
Compatibility with other vaccines
"Prevenar" is combined with any other vaccines included in the immunization schedule for children in the first years of life. "Prevenar" can be administered to children simultaneously (on the same day) with any of the following antigens included in both monovalent and combined vaccines: diphtheria, tetanus, acellular or whole-cell pertussis, Haemophilus influenzae type b, inactivated polio, hepatitis B, measles, mumps, rubella and chickenpox - without changing reactogenicity and immunological parameters.
When simultaneous vaccination with Prevenar and other vaccines, injections are made in different parts of the body.
Vaccination schedule against pneumococcus
It is useful to know that since 2015, vaccination against pneumococcus has been included in the mandatory vaccination calendar of the Russian Federation, as a result of which vaccination against pneumococcus has become mandatory.
The vaccination schedule against pneumococcus involves a three-time administration of the vaccine with one revaccination every other year when starting vaccination for a child under 6 months of age. A two-dose vaccination regimen without revaccination is carried out when the first dose of the vaccine is administered in the interval from 12 to 23 months, and if a child is vaccinated for the first time between the ages of 2 and 5 years, then the primary vaccination is carried out with only one dose of the vaccine without subsequent revaccination.
Can the pneumococcal vaccine protect against Covid?
In late summer and fall 2022, reports emerged that the pneumococcal vaccine could protect against Covid-19 infection. Actually, no—it only prevents pneumococcal infections. On the other hand, many scientists and doctors believe that the pneumococcal vaccine can protect the patient from secondary complications of coronavirus infection.
In any case, vaccination against other respiratory diseases is still highly recommended during the Covid-19 pandemic.
There are no complications after vaccination with Prevenar. The patient may experience increased temperature and redness at the injection site. However, such a reaction usually occurs in people who have already had pneumococcus.
To get vaccinated, please make an appointment with our vaccination doctor by phone or online chat on the website.
Do I need to be vaccinated against hemophilus influenzae and with what?
There is still the issue of HIB, Haemophilus influenza, which until recently was considered necessary only for babies at increased risk of the disease. Unfortunately, this is no longer the case. In the current situation, for a child whose parents follow the National Calendar, immunization with Pentaxim or Infanrix Hex is optimal. This allows you to simultaneously protect him from several diseases and at the same time get rid of it with only one injection. According to the National Calendar, the first dose is administered at the age of 3 months, subsequent doses at 4.5 months and 6 months. Another injection, which consolidates the effect, is given at 18 months (one and a half years).
If a young patient has previously been given DPT or ADS-M for tetanus and diphtheria, you should consult your pediatrician. The fact is that, unlike the imported Infanrix Hex and Pentaxim, they do not contain antigens that protect against HIB. Perhaps it would be optimal to include Hiberix or another analogue of it in an individual schedule, which fights directly against Haemophilus influenzae.
Adult patients are recommended to be vaccinated against HIB if they have a pathology accompanied by a severe decrease in the body's natural defenses: for example, they have had their spleen removed, they need to undergo chemotherapy, or they have HIV.
One way or another, your attending physician can give you a recommendation based on the benefits of immunization for you. MAKE AN APPOINTMENT PRICES
Contraindications
For pneumococcal conjugate vaccines: increased sensitivity to previous vaccination (severe generalized allergic reactions); hypersensitivity to diphtheria toxoid and/or excipients; acute infectious or non-infectious diseases, exacerbations of chronic diseases. Vaccinations against pneumococcal infection are carried out after recovery or during remission. Pneumococcal polysaccharide vaccines: pronounced reaction to the previous vaccination, PPV23 vaccination less than 3 years before the intended PPV23 vaccination.
Let's compare vaccines with dogs?
For even greater clarity, we offer you another entertaining analogy.
A wild virus is a wolf:
The weakened virus is a domestic dog:
An inactivated virus is a dead wolf:
An artificial antigen is a paw from a plush dog toy:
What components may be contained in modern vaccines?
There are different classifications of drugs for active immunization:
Live with an artificially weakened pathogen
The immune system sees the pathogen in much the same way as a real virus or bacterium. The difference is that it cannot cause disease, because to the extent that it has ceased to be pathogenic. This type of “live” vaccines includes drugs against the following infections: polio (oral vaccine), tuberculosis (BCG vaccine), rubella, measles, mumps, chickenpox, brucellosis, Q fever, etc.
Killed (inactivated)
This case differs from the first only in that the infectious agent is no longer alive and contains killed whole microorganisms (bacteria and viruses) or their parts.
Note:
When it comes to viruses, it is incorrect to talk about a “live” or “dead” virus, because, from the point of view of science, viruses are not something living. It is more correct to talk about virulent ones, i.e. capable of infecting and causing a full-fledged disease, and inactivated - not capable of causing a disease, but sufficient to develop an immune response. For convenience, we will call them alive/killed, especially since this expression has already become firmly established in everyday life.
Examples of inactivated vaccines: against whooping cough (whole cell), against polio (IPV), etc.
Anatoxins
These drugs contain neutralized (inactivated) bacterial toxins that are immunogenic and non-toxic. They are no longer poisonous to the body, but are still capable of causing an immune response. Vaccines are made based on toxoids, for example, against diphtheria, tetanus, whooping cough (a vaccine with an acellular pertussis component).
Note:
An interesting fact is that with natural infection with tetanus, immunity to it is not formed. Because the toxin content in the blood is not enough to form immune memory, and a higher concentration leads to death. In this case, an inactivated toxin is the only opportunity to gain immunity and not be afraid of this infection.
Artificial antigens
The material for creating artificial antigens is recombinant proteins or their fragments synthesized in laboratories using genetic engineering methods. In this case, the vaccine developer acts as an engineer of the design that will be administered to the patient.
To create such a vaccine, it is necessary to go through several stages of development: - first, one of the pathogen proteins is selected to which the immune system responds well; — in the laboratory, a specially “trained” cell culture is created, which will produce this protein according to instructions (gene modification is carried out by inserting a sequence encoding the desired protein into the genome of the producing cells); — provide this culture with everything necessary for the modified cell to actively multiply and produce antigens for the vaccine;
- after some time, they “harvest” by isolating the desired protein from the solution.
An analogy can be drawn with the process of conventional fermentation. That is, “yeast” will act as that specially trained cell culture, and “alcohol” will act as the desired substance that we want to obtain from these cells. Fruit or sugar is food for yeast. The difference is that yeast “knows how” to produce alcohol, but antigens, for example, for a vaccine against viral hepatitis B, do not.
The peculiarity of vaccines with artificial antigens is that they do not contain the real infectious agent at all. Relatively speaking, part of the virus was printed many times on a 3D printer, i.e. cloned.
Examples of such vaccines: against the hepatitis B virus, against the human papillomavirus (HPV).