The RotaTek vaccine is used to prevent rotavirus infection ; it contains subspecies of rotavirus, which most often cause acute gastroenteritis.
RotaTeq is a pentavalent oral live vaccine (made in the USA, Merck Sharp and Dome B.V.).
The RotaTek vaccine does not contain thiomersal or other preservatives.
Forms both humoral (antibody production) and local immunity in the intestine (at the site of potential virus introduction).
The course consists of three doses of the vaccine, with an interval of four to 10 weeks.
Cost of vaccination
| Service | Price | Note |
| RotaTech, USA | 3400 | from 6 to 32 weeks |
Almost every second mother who has one child, and every first mother who has more than one child, knows what “intestinal flu” or rotavirus is.
If the terminology is unfamiliar, then, in any case, absolutely everyone has gone through this disease! In a simplified way, this disease can be characterized as follows: the onset is sudden, the temperature reaches 39 and above, repeated bouts of vomiting, then diarrhea occurs, which lasts on average about 5 days. Naturally, the severity of the disease depends on the general condition of the body and its immunity. The first time the disease is most often quite severe, later, as a rule, it is easier.
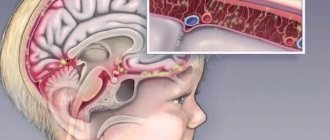
Although the entrance gate for rotavirus infection is the oral cavity, from where viruses enter the underlying parts of the intestinal tract, “rotavirus” received its name for its resemblance to a wheel (translated from Latin rota - “wheel”).
Rotaviruses infect almost every child (!) before they reach the age of 3 - 5 years and are the world's main cause of severe diarrhea with dehydration of the body in children under 5 years of age. In developed countries with high incomes, the first episode of infection usually occurs between 2 and 5 years of age, although most cases occur in infants (65% of cases occur before age 1 year).
Every year in the period before the introduction of the vaccine (1986-2000), more than 2 million children worldwide were hospitalized for rotavirus infection. The widespread spread of rotavirus infection even in conditions of high hygienic standards indicates a high level of transmission of this virus.
Currently, there is no specific therapy for rotavirus infection. As with other childhood diarrheas, the main treatment is to restore fluid loss to prevent dehydration.
Vaccinations against rotavirus infection are currently carried out only in the first year of life.
This causes some difficulties.
Practical medical experience in vaccine prevention shows that parents of their first child often do not vaccinate their baby, because are not fully informed about the existence of rotavirus infection and vaccination against it, and are not sure of this need. The desire to vaccinate with Rotatek usually appears when the child is already 2-3 years old or more. Unfortunately, vaccination against rotavirus infection is not provided at this age.
And most often, in children's medical clinics, vaccination against rotavirus infection is carried out for children who already have older brothers and sisters. In other words, parents have already encountered this problem and want to protect their “new” baby from rotavirus infection. Therefore, the most complete and reliable information about the possibility of vaccination against rotavirus infection is the key to preventing this disease in children. In addition, the vaccination is painless for the baby (drops in the mouth), and can be combined with vaccinations with other vaccines.
In European countries, as well as in the United States, vaccination against rotavirus infection has been introduced into the national calendar and has been provided to children since the 1990s.
, USA (RotaTeq®, Merck& Co., USA).
Complications
The most common complication of RVI is the development of dehydration (dehydration), especially in children of the first years of life, which in the initial stages is manifested by the following symptoms:
- excitement, anxiety;
- thirst;
- dry skin and mucous membranes;
- slight decrease in skin elasticity;
- slight retraction of the large fontanel.
As dehydration progresses, the child becomes lethargic and lethargic, may refuse to drink, the elasticity of the skin decreases (skin folds straighten out slowly), breathing becomes more frequent, there is a decrease in the frequency of urination and a decrease in the volume of urine excreted. The urine becomes dark and has a pungent odor. In the absence of adequate medical care, life-threatening complications may develop.
Other possible complications of rotavirus infection:
- addition of a bacterial infection;
- acute renal failure,
- damage to the central nervous system in the form of impaired consciousness, seizures and other complications.
Vaccination scheme
Three doses of the vaccine are administered. The first dose of the RotaTek vaccine should be administered as soon as possible after the child reaches the age of 1.5 months (6 weeks). Subsequent doses are given 4 to 10 weeks apart, with the final dose of vaccine given before the child reaches 32 weeks (8 months) of age.
Standard recommended RotaTek vaccination schedule: 2-3-4.5 months.
The RotaTek vaccine can be used in premature babies born at at least 25 weeks of gestation. The vaccine should be administered to such children no earlier than 6 weeks after birth.
After the end of the incubation period of the disease, the following symptoms of the disease develop:
- General intoxication: increased body temperature, chills, weakness, headache, dizziness.
- Gastroenteritis: repeated vomiting, abdominal pain, frequent watery foamy stools, flatulence.
- Catarrhal symptoms: redness of the mucous membrane of the oropharynx, sore throat.
The disease can begin either with an increase in body temperature or with repeated vomiting and liquid diarrhea. The child's temperature usually rises to 38-39 degrees Celsius.
If the course of the disease is favorable, its duration is usually 5-7 days. However, in some cases, especially in young children and adults with poor health, various complications may develop.
Treatment
The basis of treatment for rotavirus infection is adequate fluid replacement (rehydration) and diet.
At home treatment for initial symptoms of dehydration, rehydration is carried out by introducing additional fluid inside. Preference is given not to ordinary water, but to special solutions for rehydration, which are sold in pharmacies (Rehydron, Hydrovit, etc.). Also during the treatment period, dried fruit compote, tea, and 5% glucose solution can be used. You can alternate giving the patient water and the indicated solutions. The liquid should be at room temperature, and should be given to drink in small increments every 5-10 minutes.
If a patient has developed severe dehydration, he needs timely hospitalization for infusion therapy (intravenous administration of special solutions) and other therapy.
Medications for the treatment of RVI should include drugs from the group of enterosorbents, enzyme preparations, and probiotics, which are prescribed by the attending physician.
Antibacterial therapy for uncomplicated, non-severe forms of RVI is not indicated.
Diet is an important component of the treatment of acute intestinal infections
In case of mild RVI, split meals are recommended (5-6 times a day) with small volumes of food prepared by boiling or steaming, mechanically processed (in liquid or pureed form). Whole milk and other dairy products, fresh fruits and vegetables, and legumes are excluded. When the patient’s condition stabilizes and the symptoms of the disease disappear, the diet is gradually expanded. Nutrition correction for infants is carried out by a pediatrician.
Release form, composition and packaging
Oral solution
in the form of a transparent liquid of pale yellow color, a pink tint is acceptable.
| 1 dose (2 ml) | |
| live reassortants of human and bovine rotaviruses: | |
| reassortant G1 | not less than 2.2×106 IU* |
| reassortant G2 | not less than 2.8×106 IU* |
| reassortant G3 | not less than 2.2×106 IU* |
| reassortant G4 | not less than 2×106 IE* |
| reassortant P1A[8] | not less than 2.3×106 IU* |
* IE - infectious unit
Excipients:
sucrose - 1080 mg, sodium hydroxide - 2.75 mg, sodium citrate dihydrate - 127 mg, sodium dihydrogen phosphate dihydrate - 29.8 mg, polysorbate 80 - from 0.17 to 0.86 mg, culture medium - 15% (v/v).
2 ml - 4 ml low-density polyethylene tubes (1) - cardboard packs 2 ml - 4 ml low-density polyethylene tubes (10) - cardboard packs. 2 ml - tubes made of low-density polyethylene with a volume of 4 ml (1) - individual packaging made of aluminum foil (1) - cardboard packs. 2 ml - tubes made of low-density polyethylene with a volume of 4 ml (1) - individual packaging made of aluminum foil (10) - cardboard packs.
Use with other vaccines
The drug RotaTek® can be administered to children simultaneously with any of the following antigens included in both monovalent and combined vaccines: diphtheria toxoid, tetanus toxoid, acellular pertussis vaccine, conjugate vaccine against Haemophilus influenzae type b, inactivated polio vaccine, vaccine against viral hepatitis B , hexavalent vaccine (containing the above components), conjugate pneumococcal vaccine, conjugate meningococcal serogroup C vaccine. There was no decrease in the immune response with simultaneous administration of several vaccines and the RotaTek® vaccine.
| Name of service | Price |
| Vaccine Rota Tech | RUB 3,150 |
Immunobiological properties
Based on pooled REST and FES data, the reduction in hospitalizations and emergency room visits for rotavirus gastroenteritis within 3 years of vaccination was 94.4% (95% CI: 91.6, 96.2) for genotypes G1-G4. 95.5% (95% CI: 92.8; 97.2) for the GJ genotype, 81.9% (95% CI: 16.1; 98.0) for the G2 genotype, 89.0% (95% CI : 53.3; 98.7) for genotype G3, 83.4% (95% CI: 51.2; 95.8) for genotype G4 and 94.2% (95% CI: 62.2; 99.9) for genotype G9.
Clinical studies have confirmed that to achieve the required level and duration of protection against rotavirus gastroenteritis, a full course of vaccination with 3 doses should be administered. However, a retrospective analysis of the data showed that even before completion of the full course of vaccination, the number of cases of rotavirus gastroenteritis with a severity that would require hospitalization or emergency care was reduced (approximately 14 days after the first dose).
Who produces Rotatek and what is known about it?
The manufacturer of the drug is the American company Merck&Co, Inc. The drug underwent extensive testing and was approved by the FDA in 2006. The trial data is publicly available: it involved more than 71,000 children, it was a randomized, placebo-controlled trial that strictly complied with all the principles of evidence-based medicine.
Contraindications:
- Hypersensitivity to any component of the RotaTek® vaccine, as well as a history of administration of the RotaTek® vaccine.
- History of intussusception.
- Congenital malformations of the gastrointestinal tract predisposing to intussusception.
- Immunodeficiency, suspected immunodeficiency - acute infectious and non-infectious diseases, exacerbation of chronic diseases are temporary contraindications for vaccinations.
- Fructose intolerance, malabsorption of the glucose-galactose complex, and sufficiency of the enzymes sucrase and/or isomaltase.
Carefully:
- For active diseases of the gastrointestinal tract, including chronic diarrhea (lack of clinical data);
- In case of developmental delay (lack of clinical data).
- In an immunocompromised state (for example, as a result of malignancy or immunosuppressive therapy).
- In close contact with immunocompromised persons (for example, persons with malignant neoplasms or persons receiving immunosuppressive therapy).
- When transfusion of blood or blood products, including immunoglobulins, less than 2 days before the scheduled vaccination.