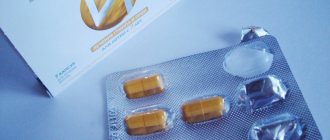
Ingavirin, 10 pcs., 90 mg, capsules
Antiviral drug. Preclinical and clinical studies have shown the effectiveness of the drug Ingavirin® against influenza viruses type A (A(H 1N1), including “swine” A(I-IlNl)pdm09, A(M3N2), A(H5N1)) and type B, adenovirus, parainfluenza virus, respiratory syncytial virus; in preclinical studies: coronavirus, metapneumovirus, enteroviruses, including Coxsackie virus and riovirus. The drug Ingavirin® promotes accelerated elimination of viruses, reduces the duration of the disease, and reduces the risk of complications. The mechanism of action is realized at the level of infected cells due to the stimulation of innate immune factors suppressed by viral proteins. In experimental studies, in particular, it was shown that the drug Ingavirin® increases the expression of the first type interferon receptor IFNAR on the surface of epithelial and immunocompetent cells. An increase in the density of interferon receptors leads to an increase in the sensitivity of cells to endogenous interferon signals. The process is accompanied by activation (phosphorylation) of the transmitter protein STAT1, which transmits a signal to the cell nucleus to induce antiviral genes. It has been shown that under conditions of infection, the drug stimulates the production of the antiviral effector protein MxA, which inhibits the intracellular transport of ribonucleoproteins of various viruses, slowing down the process of viral replication.
The drug Iigavirin® causes an increase in the level of interferon in the blood to the physiological norm, stimulates and normalizes the reduced α-interferon producing ability of blood leukocytes, and stimulates the γ-interferon producing ability of leukocytes. Causes the generation of cytotoxic lymphocytes and increases the content of NK-T cells, which have high killer activity against virus-infected cells.
The anti-inflammatory effect is due to the suppression of the production of key pro-inflammatory cytokines (tumor necrosis factor (TNF-a), interleukins (IL-1 (3 and IL-6)), a decrease in the activity of myeloperoxidase. Experimental studies have shown that the combined use of the drug Ingavirin® with antibiotics increases the effectiveness of therapy in a model of bacterial sepsis, including that caused by penicillin-resistant strains of staphylococcus. Experimental toxicological studies indicate a low level of toxicity and a high safety profile of the drug. According to the parameters of acute toxicity, the drug Ingavirin® belongs to toxicity class 4 - “Low toxic substances” (When determining LD50 in acute toxicity experiments, lethal doses of the drug could not be determined).
Ingavirin
Antiviral drug. Preclinical and clinical studies have shown the effectiveness of the drug Ingavirin® against influenza viruses type A (A(H1N1), including pandemic strain A(H1N1)pdm09 (swine), A(H3N2), A(H5N1)) and type B, adenovirus, parainfluenza virus, respiratory syncytial virus in preclinical studies: coronavirus, metapneumovirus, enteroviruses, including Coxsackie virus and rhinovirus. Ingavirin® reduces the viral load, accelerates the elimination of viruses, shortens the duration of the disease, and reduces the risk of complications. The mechanism of action is realized at the level of infected cells due to the activation of innate immune factors suppressed by viral proteins. In experimental studies, in particular, it was shown that the drug Ingavirin® increases the expression of the first type interferon receptor IFNAR on the surface of epithelial and immunocompetent cells. An increase in the density of interferon receptors leads to an increase in the sensitivity of cells to endogenous interferon signals. The process is accompanied by activation (phosphorylation) of the transmitter protein STAT1, which transmits a signal to the cell nucleus to induce the synthesis of antiviral genes. It has been shown that under conditions of infection, the drug activates the synthesis of the antiviral effector protein MxA (an early factor of the antiviral response that inhibits the intracellular transport of ribonucleoprotein complexes of various viruses) and the phosphorylated form of PKR, which suppresses the translation of viral proteins, thus slowing down and stopping the process of viral reproduction. The effect of the drug Ingavirin® is to significantly reduce the signs of the cytopathic and cytodestructive effects of the virus, reduce the number of infected cells, limit the pathological process, normalize the composition and structure of cells and the morphological picture of tissues in the area of the infectious process, both in its early and late stages . The anti-inflammatory effect is due to the suppression of the production of key pro-inflammatory cytokines (tumor necrosis factor (TNF-α), interleukins (IL-1β and IL-6)), and a decrease in the activity of myeloperoxidase. Experimental studies have shown that the combined use of the drug Ingavirin® with antibiotics increases the effectiveness of therapy in a model of bacterial sepsis, including that caused by penicillin-resistant strains of staphylococcus. Experimental toxicological studies conducted indicate a low level of toxicity and a high safety profile of the drug. According to the parameters of acute toxicity, the drug Ingavirin® belongs to toxicity class 4 - Low toxic substances (when determining LD50 in acute toxicity experiments, lethal doses of the drug could not be determined). The drug does not have mutagenic, immunotoxic, allergenic or carcinogenic properties, and does not have a local irritant effect. The drug Ingavirin® does not affect reproductive function and does not have embryotoxic or teratogenic effects. There is no effect of the drug Ingavirin® on the hematopoietic system when taking an age-appropriate dose in the recommended regimen and course.
Ingavirin capsules 90 mg, 10 pcs.
Manufacturer
Valenta Pharma, Russia
Compound
One capsule contains:
active ingredient: pentanedioic acid imidazolylethanamide (vitaglutam) in terms of 100% substance - 90.00 mg;
excipients: lactose monohydrate, potato starch, colloidal silicon dioxide (aerosil), magnesium stearate;
hard gelatin capsules: titanium dioxide E 171, crimson dye [Ponceau 4 R] E 124, azorubine dye E 122, quinoline yellow dye E 104, gelatin;
ink composition for the logo: shellac, propylene glycol E 1520, titanium dioxide E 171.
pharmachologic effect
Antiviral agent. Anti-inflammatory agent.
ATX code: [J05AX].
Pharmacological properties
Pharmacodynamics
Antiviral drug.
Preclinical and clinical studies have shown the effectiveness of the drug Ingavirin® against influenza viruses type A (A(H1N1), including the pandemic strain A(H1N1)pdm09 (“swine”), A(H3N2), A(H5N1)) and type B, adenovirus, parainfluenza virus, respiratory syncytial virus; in preclinical studies: coronavirus, metapneumovirus, enteroviruses, including Coxsackie virus and rhinovirus.
Ingavirin® reduces the viral load, accelerates the elimination of viruses, shortens the duration of the disease, and reduces the risk of complications.
The mechanism of action is realized at the level of infected cells due to the activation of innate immune factors suppressed by viral proteins. In experimental studies, in particular, it was shown that the drug Ingavirin® increases the expression of the first type interferon receptor IFNAR on the surface of epithelial and immunocompetent cells. An increase in the density of interferon receptors leads to an increase in the sensitivity of cells to endogenous interferon signals.
The process is accompanied by activation (phosphorylation) of the transmitter protein STAT1, which transmits a signal to the cell nucleus to induce the synthesis of antiviral genes. It has been shown that under conditions of infection, the drug activates the synthesis of the antiviral effector protein MxA (an early factor of the antiviral response that inhibits the intracellular transport of ribonucleoprotein complexes of various viruses) and the phosphorylated form of PKR, which suppresses the translation of viral proteins, thus slowing down and stopping the process of viral reproduction.
The effect of the drug Ingavirin® is to significantly reduce the signs of the cytopathic and cytodestructive effects of the virus, reduce the number of infected cells, limit the pathological process, normalize the composition and structure of cells and the morphological picture of tissues in the area of the infectious process, both in its early and late stages.
The anti-inflammatory effect is due to the suppression of the production of key pro-inflammatory cytokines (tumor necrosis factor (TNF-α), interleukins (IL-1β and IL-6)), and a decrease in the activity of myeloperoxidase.
Experimental studies have shown that the combined use of the drug Ingavirin® with antibiotics increases the effectiveness of therapy in a model of bacterial sepsis, including that caused by penicillin-resistant strains of staphylococcus.
Experimental toxicological studies conducted indicate a low level of toxicity and a high safety profile of the drug.
According to the parameters of acute toxicity, the drug Ingavirin® belongs to toxicity class 4 - “Low toxic substances” (when determining LD50 in acute toxicity experiments, lethal doses of the drug could not be determined).
The drug does not have mutagenic, immunotoxic, allergenic or carcinogenic properties, and does not have a local irritant effect. The drug Ingavirin® does not affect reproductive function and does not have embryotoxic or teratogenic effects.
There is no effect of the drug Ingavirin® on the hematopoietic system when taking an age-appropriate dose in the recommended regimen and course.
Pharmacokinetics
Absorption and distribution.
An experiment using a radioactive label established that the drug quickly enters the blood from the gastrointestinal tract, distributing throughout the internal organs. Maximum concentrations in the blood, blood plasma and most organs are reached 30 minutes after administration of the drug. The AUC values (area under the pharmacokinetic concentration-time curve) of the kidneys, liver and lungs are slightly higher than the AUC of blood (43.77 mcg.h/g). AUC values for the spleen, adrenal glands, lymph nodes and thymus are lower than blood AUC. MRT (mean drug retention time) in the blood is 37.2 hours.
When taking a course of the drug once a day, it accumulates in internal organs and tissues. At the same time, the qualitative characteristics of the pharmacokinetic curves after each administration of the drug are identical: a rapid increase in the concentration of the drug after each administration 0.5-1 hour after administration and then a slow decrease by 24 hours.
Metabolism.
The drug is not metabolized in the body and is excreted unchanged.
Excretion.
The main elimination process occurs within 24 hours. During this period, 80% of the dose taken is excreted: 34.8% is excreted in the time interval from 0 to 5 hours and 45.2% in the time interval from 5 to 24 hours. Of these, 77% is excreted through the intestines and 23% through the kidneys.
Indications
Treatment and prevention of influenza A and B and other acute respiratory viral infections (adenoviral infection, parainfluenza, respiratory syncytial infection, rhinovirus infection) in adults and children over 3 years of age.
Use during pregnancy and breastfeeding
The use of the drug during pregnancy has not been studied.
The use of the drug during lactation has not been studied, therefore, if it is necessary to use the drug during lactation, breastfeeding should be stopped.
Contraindications
- Hypersensitivity to the active substance or any other component of the drug.
- Lactase deficiency, lactose intolerance, glucose-galactose malabsorption.
- Pregnancy.
- Breastfeeding period.
- Children under 3 years of age.
Side effects
Allergic reactions (rare).
If any of the side effects indicated in the instructions get worse or you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Drug interactions with Ingavirin® have not been described.
How to take, course of administration and dosage
Inside. Regardless of food intake.
For children aged 3 to 6 years who have difficulty swallowing the capsule, the contents of the capsule can be diluted in water or apple juice. To do this, you need to carefully open the capsule over a container with a small amount (50-70 ml) of boiled water or apple juice at room temperature, pour the contents of the capsule into water or juice, stir the mixture for 20 seconds and drink it whole. Added sugar is allowed. The mixture should be prepared immediately before use; The finished mixture cannot be stored.
For the treatment and prevention of influenza and acute respiratory viral infections, adults are prescribed 90 mg once a day, children over 7 years old - 60 mg once a day, children from 3 to 6 years old - 30 mg once a day.
The duration of treatment for influenza and acute respiratory viral infections in adults and children over 7 years of age is 5-7 days (depending on the severity of the condition). The duration of treatment for influenza and acute respiratory viral infections in children from 3 to 6 years is 5 days. The drug should be taken from the moment the first symptoms of the disease appear, preferably no later than 2 days from the onset of the disease.
Adults and children with severe symptoms, as well as in the presence of concomitant diseases (diseases of the respiratory and cardiovascular systems, diabetes mellitus, obesity) should take a double dose of the drug in the first three days of the disease, then continue taking it at the usual dosage for 2-4 days .
For the prevention of influenza and acute respiratory viral infections after contact with sick persons, adults and children over 7 years old are prescribed the drug for 7 days, for children from 3 to 6 years old - for 5 days.
If after 5 days of treatment there is no improvement or the symptoms worsen or new symptoms appear, you should consult your doctor. Use the drug only according to the indications, method of administration and in the doses indicated in the instructions.
Overdose
Cases of overdose of the drug Ingavirin® have not been reported to date.
Description
Capsules No. 2 or No. 4 are blue (for a dosage of 30 mg), capsules No. 2 or No. 3 are red (for a dosage of 90 mg). On the cap of the capsule there is a white logo in the form of a ring and the letter I inside the ring.
The contents of the capsules are granules and white or almost white powder; the formation of conglomerates is allowed, easily crumbling under light pressure.
Special instructions
It is not recommended to take other antiviral drugs at the same time without first consulting a doctor.
Save the instructions. It may be needed again. If you have any questions, consult your doctor.
Impact on the ability to drive vehicles and machinery
It has not been studied, however, given the mechanism of action and the profile of adverse reactions, it can be assumed that the drug does not affect the ability to drive vehicles or operate machinery.
Release form
Capsules 90 mg.
7 or 10 capsules in a blister pack or in a blister pack with perforation made of polyvinyl chloride film and printed varnished aluminum foil.
1 contour package (for a dosage of 90 mg) together with instructions for use are placed in a pack.
Storage conditions
In a place protected from light at a temperature not exceeding 25 ° C.
Keep out of the reach of children.
Best before date
3 years
Do not use after the expiration date stated on the package.
Active substance
Pentanedioic acid imidazolylethanamide
Dosage form
capsules
Information in the State Register of Medicines
Go
Barcode and weight
Barcode: 4602193012837, -, 0104602193012 Weight: 0.010 kg