Ingavirin - use of capsules for adults
The use of the drug in capsule form (30 mg) is allowed from 3 years of age. But children under 6 years of age do not always know how to swallow a capsule whole. For this reason, it is permitted to open the form. Pour the powder into a cup, pour in 50-70 ml of apple juice or water (the temperature of the liquid should be room temperature), and then immediately give it to the baby to drink.
Dosages according to age:
- adult patients - 90 mg once a day;
- patients 7-17 years old - 60 mg once a day;
- patients 3-6 years of age - 30 mg once a day.
Course therapy for patients over 7 years of age is 5-7 days (flu - 7 days). Patients aged 3-6 years are indicated to be treated for 5 days (flu - 5 days).
If the symptoms of the disease are severe, patients are advised to take a double dosage in the first 3 days. This will ensure a rapid reduction in the pathogen population. The following days you can take the standard dose.
If therapy is not effective, the condition becomes worse within 5 days, you should consult a doctor. The doctor will conduct an examination and prescribe other therapy.
Ingavirin caps 60 mg children N10 (Valenta)
Antiviral drug. Preclinical and clinical studies have shown the effectiveness of the drug Ingavirin® against influenza viruses type A (A(H1N1), including “swine” A(H1Nl)pdm09, A(H3N2), A(H5N1)) and type B, adenovirus, parainfluenza virus, respiratory syncytial virus; in preclinical studies: coronavirus, metapneumovirus, enteroviruses, including Coxsackie virus and rhinovirus. The drug Ingavirin® promotes accelerated elimination of viruses, reduces the duration of the disease, and reduces the risk of complications. The mechanism of action is realized at the level of infected cells due to the stimulation of innate immune factors suppressed by viral proteins. In experimental studies, in particular, it was shown that the drug Ingavirin® increases the expression of the first type interferon receptor IFNAR on the surface of epithelial and immunocompetent cells. An increase in the density of interferon receptors leads to an increase in the sensitivity of cells to endogenous interferon signals. The process is accompanied by activation (phosphorylation) of the transmitter protein STAT1, which transmits a signal to the cell nucleus to induce antiviral genes. It has been shown that under conditions of infection, the drug stimulates the production of the antiviral effector protein MxA, which inhibits the intracellular transport of ribonucleoproteins of various viruses, slowing down the process of viral replication. The drug Ingavirin® causes an increase in the level of interferon in the blood to the physiological norm, stimulates and normalizes the reduced α-interferon producing ability of blood leukocytes, stimulates γ-interferon producing ability of leukocytes. Causes the generation of cytotoxic lymphocytes and increases the content of NK-T cells, which have high killer activity against virus-infected cells. The anti-inflammatory effect is due to the suppression of the production of key pro-inflammatory cytokines (tumor necrosis factor (TNF-α), interleukins (IL-1β and IL-6)), a decrease in the activity of myeloperoxidase. Experimental studies have shown that the combined use of the drug Ingavirin® with antibiotics increases the effectiveness of therapy on a model of bacterial sepsis, including that caused by penicillin-resistant strains and staphylococcus. Experimental toxicological studies conducted indicate a low level of toxicity and a high safety profile of the drug. According to the parameters of acute toxicity, the drug Ingavirin® belongs to toxicity class 4 - “Low toxic substances” (when determining LD50 in acute toxicity experiments, lethal doses of the drug could not be determined). The drug does not have mutagenic, immunotoxic, allergenic or carcinogenic properties, and does not have a local irritant effect. The drug Ingavirin® does not affect reproductive function, does not have embryotoxic or teratogenic effects. Efficacy in children. In a double-blind, randomized, placebo-controlled, multicenter study assessing the clinical efficacy and safety of the drug Ingavirin® at a daily dose of 60 mg for the treatment of influenza and other acute respiratory viral infections in 180 children aged 13-17 years, it was shown that the drug Ingavirin® was significantly superior to placebo, faster normalizing body temperature, relieving intoxication, fever, and catarrhal symptoms. A double-blind, randomized, placebo-controlled, multicenter study assessing the clinical efficacy and safety of the drug Ingavirin® at a daily dose of 60 mg for the treatment of influenza and other acute respiratory viral infections in 310 children aged 7-12 years showed that the drug Ingavirin® is significantly more effective than placebo and provides a faster (on average 18 hours) decrease in body temperature and disappearance of intoxication symptoms (sore throat, sore throat, pain when swallowing, nasal congestion, runny nose).
Is it possible to give Ingavirin to a child - rules for taking syrup?
Ingavirin for children 60 mg
The syrup is used more in pediatric practice, but is also acceptable in adult patients. To draw up the medicine, insert a special measuring syringe into the neck of the bottle. Next, the container is turned over, the syringe piston is pulled down to the required mark. A measuring syringe allows you to accurately measure the dosage of the drug. After using the syringe, it is recommended to rinse it with warm water and dry it so that the piston does not stick. Washing is also necessary to prevent the accumulation of bacteria.
Medication dosages depending on age:
- adult patients - 15 ml once a day;
- 7-17 years old - 10 ml once a day;
- 3-6 years of age - 5 ml once a day.
The duration of therapy in patients over 7 years of age is 5-7 days. In patients under 6 years of age - 5 days.
Composition and release form
Capsules - 1 capsule:
- Active substance: pentanedioic acid imidazolylethanamide (vitaglutam) - 90 mg;
- Excipients: lactose monohydrate - 90 mg, potato starch - 35.6 mg, colloidal silicon dioxide (aerosil) - 2.2 mg, magnesium stearate - 2.2 mg;
- Composition of the capsule shell: titanium dioxide (E171) - 1.3333%, crimson dye [Ponceau 4R] (E124) - 0.0008%, azorubine dye (E122) - 0.3066%, quinoline yellow dye (E104) - 0.4207%, gelatin - up to 100% ;
- Logo ink composition: shellac, propylene glycol (E1520), titanium dioxide (E171).
7 or 10 capsules in a blister pack made of polyvinyl chloride film and printed varnished aluminum foil. 1 blister pack along with instructions for use is placed in a pack.
Similar medicines
The drug has non-structural analogues that are active against viral agents. These include:
- Viferon - a medication that enhances immune defense mechanisms, is produced in the form of rectal suppositories, contains interferon alpha-2b; allowed for use by newborns, premature babies, pregnant and lactating women, as it does not cause complications and is safe to use; The medication is quite effective against different strains of viral agents.
- Anaferon - acceptable for use in children from 1 month and adults; less effective than Viferon and Ingavirin.
- Arbidol - allowed for use only from 2 years of age, indicated no later than 2 days from the onset of the disease; There is a form of tablets and syrup; fights viruses, preventing them from entering the cell; can be used to treat coronavirus infection.
- Kagocel - is a stimulator of the production of endogenous interferons, indicated for use in patients over 3 years of age; works well against any strains of viral agents.
Anaferon for children, lozenges, 20 pcs.
MATERIA MEDICA HOLDING NPF, Russia
Price from 191₽
Arbidol, 100 mg, capsules, antiviral against influenza and ARVI, 40 pcs.
OTC Pharm, Russia
Price from 813₽
Kagocel, 12 mg, tablets, 20 pcs.
Nearmedic Plus, Russia
Price from 458₽
There are contraindications. Specialist consultation is required.
The cost of these drugs is much lower.
The antiviral drug Ingavirin has become very popular today. The drug can be used for various viral diseases, including coronavirus infection, due to its wide spectrum of effects. The peculiarity of the drug’s action is to prevent further formation of new virions, reduce inflammation and increase immune defense. The medication is indicated for both adults and children over 3 years of age. The drug should be used as recommended by a doctor, despite its over-the-counter sale.
Ingavirin syrup for children over 3 years old 6 mg/ml 90 ml
Pharmacological group:
Antiviral drug ATC code: J05AX
Pharmacodynamics:
Antiviral drug. Preclinical and clinical studies have shown the effectiveness of the drug Ingavirin® against influenza viruses type A (A(H1N1), including the pandemic strain A(H1N1)pdm09 (“swine”), A(H3N2), A(H5N1)) and type B, adenovirus, parainfluenza virus, respiratory syncytial virus; in preclinical studies: coronavirus, metapneumovirus, enteroviruses, including Coxsackie virus and rhinovirus.
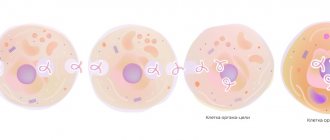
Ingavirin® reduces the viral load, accelerates the elimination of viruses, shortens the duration of the disease, and reduces the risk of complications. The mechanism of action is realized at the level of infected cells due to the activation of innate immune factors suppressed by viral proteins. In experimental studies, in particular, it was shown that the drug Ingavirin® increases the expression of the first type interferon receptor IFNAR on the surface of epithelial and immunocompetent cells. An increase in the density of interferon receptors leads to an increase in the sensitivity of cells to endogenous interferon signals. The process is accompanied by activation (phosphorylation) of the transmitter protein STAT1, which transmits a signal to the cell nucleus to induce the synthesis of antiviral genes. It has been shown that under conditions of infection, the drug activates the synthesis of the antiviral effector protein MxA (an early factor of the antiviral response that inhibits the intracellular transport of ribonucleoprotein complexes of various viruses) and the phosphorylated form of PKR, which suppresses the translation of viral proteins, thus slowing down and stopping the process of viral reproduction.
The effect of the drug Ingavirin® is to significantly reduce the signs of the cytopathic and cytodestructive effects of the virus, reduce the number of infected cells, limit the pathological process, normalize the composition and structure of cells and the morphological picture of tissues in the area of the infectious process, both in its early and late stages. The anti-inflammatory effect is due to the suppression of the production of key pro-inflammatory cytokines (tumor necrosis factor (TNF-alpha), interleukins (IL-1beta and IL-6)), and a decrease in the activity of myeloperoxidase.
Experimental studies have shown that the combined use of the drug Ingavirin® with antibiotics increases the effectiveness of therapy in a model of bacterial sepsis, including that caused by penicillin-resistant strains of staphylococcus.
Experimental toxicological studies conducted indicate a low level of toxicity and a high safety profile of the drug.
According to the parameters of acute toxicity, the drug Ingavirin® belongs to toxicity class 4 - “Low toxic substances” (when determining LD50 in acute toxicity experiments, lethal doses of the drug could not be determined).
The drug does not have mutagenic, immunotoxic, allergenic or carcinogenic properties, and does not have a local irritant effect. The drug Ingavirin® does not affect reproductive function and does not have embryotoxic or teratogenic effects.
There is no effect of the drug Ingavirin® on the hematopoietic system when taking an age-appropriate dose in the recommended regimen and course.
Pharmacokinetics:
Absorption and distribution In an experiment using a radioactive label, it was established that the active substance quickly enters the blood from the gastrointestinal tract, distributing throughout the internal organs. In a study in healthy volunteers, with a single dose of 90 mg, the maximum concentration (Cmax) was 441.45 ± 252.99 ng/ml; the time to achieve it (Tmax) is 1.30 ± 0.41 hours. In preclinical studies, it was found that with a course of taking the drug once a day, it accumulates in internal organs and tissues. At the same time, the qualitative characteristics of the pharmacokinetic curves after each administration of the drug are identical: a rapid increase in the concentration of the drug after each administration 0.5-1 hour after administration and then a slow decrease by 24 hours. The AUC values (area under the pharmacokinetic concentration-time curve) of the kidneys, liver and lungs are slightly higher than the AUC of blood. AUC values for the spleen, adrenal glands, lymph nodes and thymus are lower than blood AUC.
Metabolism The drug is not metabolized in the body and is excreted unchanged.
Elimination In a study in healthy volunteers with a single dose of 90 mg, the half-life (T1/2) was 1.82 ± 0.23 hours. In preclinical studies, it was found that the main elimination process occurs within 24 hours. During this period, 80% of the dose taken is excreted: 34.8% is excreted in the time interval from 0 to 5 hours and 45.2% in the time interval from 5 to 24 hours. Of these, 77% is excreted through the intestines and 23% through the kidneys.
Sources
- ON THE. Malyshev, L.N. Merkulova, E.I. Burtseva, M.Yu. Shchelkanov // Study of the effectiveness and safety of the new antiviral drug Ingavirin in the treatment of patients with influenza // Russian Medical Journal // 2008
- V.V. Zarubaev, A.V. Slita // Experimental study of the antiviral activity of Ingavirin against human adenovirus // journal “Antibiotics and Chemotherapy” // 2010.
- A.G. Malyavin, N.I. Krikheli, I.V. Rogova, P.O. Asadulin, S.A. Kucher, V.A. Kharkovsky // Therapy of patients with COVID-19: results of assessing the effectiveness and safety of including the drug Ingavirin® in the recommended standard therapy regimen in real clinical practice // Journal “Therapy” // 2022.
Instructions and dosage of the drug Ingavirin
Ingavirin®
Antiviral drug.
Preclinical and clinical studies have shown the effectiveness of the drug Ingavirin® against influenza viruses type A (A(H1N1), including the pandemic strain A(H1N1)pdm09 (“swine”), A(H3N2), A(H5N1)) and type B, adenovirus, parainfluenza virus, respiratory syncytial virus; in preclinical studies: coronavirus, metapneumovirus, enteroviruses, including Coxsackie virus and rhinovirus.
Ingavirin® reduces the viral load, accelerates the elimination of viruses, shortens the duration of the disease, and reduces the risk of complications. The mechanism of action is realized at the level of infected cells due to the activation of innate immune factors suppressed by viral proteins. In experimental studies, in particular, it was shown that the drug Ingavirin® increases the expression of the first type interferon receptor IFNAR on the surface of epithelial and immunocompetent cells. An increase in the density of interferon receptors leads to an increase in the sensitivity of cells to endogenous interferon signals. The process is accompanied by activation (phosphorylation) of the transmitter protein STAT1, which transmits a signal to the cell nucleus to induce the synthesis of antiviral genes. It has been shown that under conditions of infection, the drug activates the synthesis of the antiviral effector protein MxA (an early factor of the antiviral response that inhibits the intracellular transport of ribonucleoprotein complexes of various viruses) and the phosphorylated form of PKR, which suppresses the translation of viral proteins, thus slowing down and stopping the process of viral reproduction.
The effect of the drug Ingavirin® is to significantly reduce the signs of the cytopathic and cytodestructive effects of the virus, reduce the number of infected cells, limit the pathological process, normalize the composition and structure of cells and the morphological picture of tissues in the area of the infectious process, both in its early and late stages. The anti-inflammatory effect is due to the suppression of the production of key pro-inflammatory cytokines (tumor necrosis factor (TNF-a), interleukins (IL-lß and IL-6)), and a decrease in the activity of myeloperoxidase.
Experimental studies have shown that the combined use of the drug Ingavirin® with antibiotics increases the effectiveness of therapy in a model of bacterial sepsis, including that caused by penicillin-resistant strains of staphylococcus. Experimental toxicological studies conducted indicate a low level of toxicity and a high safety profile of the drug.
According to the parameters of acute toxicity, the drug Ingavirin® belongs to toxicity class 4 - “Low toxic substances” (when determining LD50 in acute toxicity experiments, lethal doses of the drug could not be determined).
The drug does not have mutagenic, immunotoxic, allergenic or carcinogenic properties, and does not have a local irritant effect.
The drug Ingavirin® does not affect reproductive function and does not have embryotoxic or teratogenic effects. There is no effect of the drug Ingavirin® on the hematopoietic system when taking an age-appropriate dose in the recommended regimen and course.