Forms of the produced drug
Just like adults, children also have digestive problems.
Motilium for children is produced in the form of a suspension, which has a neutral taste and is easily accepted by the child’s body without causing refusal to take it. The viscous liquid has a white tint, the main component of which is domperidone in an amount of 0.005 g. Additional components of the drug include:
- Microcrystalline cellulose
- Sodium saccharin
- Sorbitol
- Carboxymethylcellulose
- Parahydroxybenzoate
- Sodium hydroxide
- Polysorbate
- Distilled water
In addition, the drug is produced in the form of self-absorbing tablets, which contain 0.01 g of the active substance domperidone, which can be placed in the oral cavity on the tongue without additional use of liquid. Inactive substances in tablet form include:
- Aspartame
- Mannitol
- Gelatin
- Flavoring flavor
Motilium is used depending on the age category of the child, as well as individual preferences, providing the best internal effect on the body.
How Motilium works
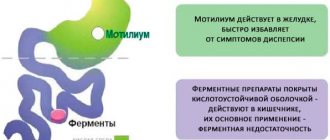
The drug belongs to the group of antiemetics, which have a central effect in the form of blocking dopamine receptors. In addition to the antiemetic effect, the drug activates the contraction of the smooth muscles of the small intestine, stimulating its peristalsis. The drug is similar to Cerucal and neuroleptics, but is characterized by poor permeability through the blood-brain barrier, eliminating a number of negative effects, but it promotes the activation of the removal of prolactin from pituitary cells.
The effect of the drug lengthens the contractility of the intestine during internal absorption, accelerating the time of gastric emptying. In a healthy state, there is a transition of homogenized semi-liquid fractions to subsequent sections of the intestine, determining the tonic state and pressure of the sphincter entering the stomach. In patients with a pathological disorder in the activity of the digestive tract - solid fractions, determining the inhibition of the process of food exiting the stomach into the initial part of the intestine. Motilium does not change the state of the internal environment of the gastric cavity.
Structure of internal reactions
After taking Motilium, in a state of an empty stomach, the following chain of reactions occurs:
- Absorption. The highest level in blood plasma, after administration on an empty stomach, is achieved after 60 minutes. Low sensitivity to the active component of the drug may be due to a reduced degree of diffusion ability of the intestinal walls and liver. An increase in activity is observed in healthy people with subsequent meals. Disturbances in the motor activity of the digestive tract limit food intake to half an hour after consuming Motilium. Reduced gastric acidity reduces the diffusion capacity of domperidone.
- Transportation. Domperidone does not regulate its own metabolic process. When taken continuously for 14 days in an amount of no more than 0.3 g, the maximum content in the blood plasma is maintained within a constant level 1.5 hours after the initially taken dosage. The connection with the protein structure of plasma is at least 92%. The total content of the active component in breast milk has a ratio 4 times lower than that of the blood plasma component.
- Diffusion. Metabolism of the main substance occurs in liver cells in the form of dealkylation and hydroxylation reactions. Excretion routes are through the urinary tract and intestines in percentages of 30 and 65%, respectively. In unchanged form, domperidone is eliminated up to 10% in feces and up to 1% in urine.
In pathological disorders of the urinary system, an increase in creatinine clearance is observed, determining a low level of the active component in the blood plasma.
Motizhekt
Manufacturer: TATHIMPHARMPREPARATY OJSC (Russia)
◊ tab., cover. film-coated, 10 mg: 30 pcs. Reg. No.: LP-001281
Clinical and pharmacological group:
Centrally acting antiemetic drug that blocks dopamine receptors
Release form, composition and packaging
◊ Film-coated tablets
white or almost white, round, biconvex in shape; on a cross section, two layers are visible: the shell is white or almost white, the core is white or white with a yellowish tint.
| 1 tab. | |
| domperidone | 10 mg |
Excipients:
potato starch 16.8 mg, lactose monohydrate (milk sugar) 78.9 mg, microcrystalline cellulose 5 mg, colloidal silicon dioxide (Aerosil) 1.2 mg, medium molecular weight polyvinylpyrrolidone (povidone) 1.4 mg, magnesium stearate 1.2 mg, sodium lauryl sulfate 1.5 mg.
Film shell composition:
opadry II 4 mg (hydroxypropyl methylcellulose (hypromellose) 1.2 mg, lactose monohydrate 1.6 mg, macrogol-4000 0.4 mg, titanium dioxide 0.8 mg).
10 pieces. — cellular contour packages (3) — cardboard packs. 15 pcs. — contour cell packaging (2) — cardboard packs.
Description of the active components of the drug "Domperidone"
pharmachologic effect
Antiemetic. It has an antiemetic effect, soothes hiccups and eliminates nausea in some cases. The action is due to the blockade of central dopamine receptors. Thanks to this, the inhibitory effect of dopamine on the motor function of the gastrointestinal tract is eliminated and the evacuation and motor activity of the stomach increases.
Indications
Nausea, vomiting, hiccups of various origins (with toxemia, radiation therapy, diet disorders, taking certain medications (morphine), endoscopic and radiopaque studies, in the postoperative period). Postoperative hypotension and atony of the stomach and intestines, biliary dyskinesia, flatulence, reflux esophagitis, cholecystitis, cholangitis, various types of dyspepsia. Nausea and vomiting caused by dopaminomimetics.
Dosage regimen
Adults and children over 5 years old - 10 mg 3 times a day 15-30 minutes before meals, and if necessary, an additional 10 mg before bedtime. In acute cases of nausea and vomiting, severe dyspepsia - 20 mg 3-4 times a day. In case of renal failure, the frequency of use should be reduced.
Side effect
From the side of the central nervous system:
rarely - increased excitability and/or extrapyramidal disorders, headache.
From the digestive system:
spasms of smooth muscles of the gastrointestinal tract, dry mouth, thirst.
Allergic reactions:
itching, rash, urticaria.
Other:
increased plasma prolactin levels, galactorrhea, gynecomastia.
Contraindications
Gastrointestinal bleeding, mechanical intestinal obstruction, perforation of the stomach or intestines, prolactinoma, children under 1 year of age (up to 5 years and children weighing up to 20 kg - for tablets), hypersensitivity to domperidone, pregnancy, lactation (breastfeeding) ).
Pregnancy and lactation
Domperidone is contraindicated for use during pregnancy and lactation.
There are no adequate and strictly controlled studies of the safety of domperidone during pregnancy.
It is not known whether domperidone is excreted in breast milk. If use is necessary during lactation, breastfeeding should be discontinued.
Use for liver dysfunction
Use with caution in case of liver dysfunction.
Use for renal impairment
Use with caution in case of impaired renal function. In case of renal failure, the frequency of use should be reduced.
Application for children
Contraindicated in children under 1 year of age (under 5 years and children weighing up to 20 kg - for tablets).
special instructions
Use with caution in case of impaired liver and kidney function.
Drug interactions
When used simultaneously with antacids, antisecretory drugs (including cimetidine, sodium bicarbonate), the bioavailability of domperidone decreases.
When used simultaneously with anticholinergic drugs, the effect of domperidone is inhibited.
Since domperidone is metabolized mainly with the participation of the CYP3A4 isoenzyme, it is believed that with the simultaneous use of domperidone and inhibitors of this isoenzyme (including antifungals of the azole group, macrolide antibiotics, HIV protease inhibitors, nefazodone), an increase in the level of domperidone in the blood plasma is possible .
Drug interactions
When used simultaneously with antacids, antisecretory drugs (including cimetidine, sodium bicarbonate), the bioavailability of domperidone decreases.
When used simultaneously with anticholinergic drugs, the effect of domperidone is inhibited.
Since domperidone is metabolized mainly with the participation of the CYP3A4 isoenzyme, it is believed that with the simultaneous use of domperidone and inhibitors of this isoenzyme (including antifungals of the azole group, macrolide antibiotics, HIV protease inhibitors, nefazodone), an increase in the level of domperidone in the blood plasma is possible .
In what cases is the drug required?
Motilium. Release form: suspension
When the following symptoms develop, the use of motilium relieves their pronounced manifestation by eliminating the immediate cause of their formation:
- Nausea and vomiting while taking dopamine agonists when diagnosing Parkinson's pathology.
- Digestive disorders caused by systemic reflux.
- The length of time food remains in the gastric cavity.
- Feelings of bloating and excessive gas.
- Belching and heartburn.
- Pain in the epigastric region.
- Dyspeptic phenomena against the background of organic, infectious or functional origin.
- Vomiting syndrome and a feeling of nausea associated with dietary restrictions, medicinal chemistry and radiation therapy.
- Physiological disorders of motility of the digestive tract in children of early age.
Reception technique
For early childhood, it is recommended to take the suspension form of the drug. The age category over 5 years allows taking self-absorbing tablets by immersing them in the oral cavity on the surface of the tongue. In this case, the tablet completely dissolves in a short period of time and is swallowed without requiring additional volume of water.
When using self-absorbing tablets, you should take into account the subtleties of packaging the drug form in a blister and the fragility of the structure, which can lead to breakage when opening the package. To do this you need:
- Hold the blister by the edge and remove the surface coating from the foil.
- Press down on the convex part of the package and carefully remove the tablet.
Features of taking the suspension include the following necessary technical rules for the use of packaging material and the solution itself, which must be shaken before use due to the uneven distribution of layers of the dosage form of the drug:
- The bottle cap is protected from accidental falling into children's hands, so it must be pressed and turned with force in a counterclockwise direction.
- Remove the closing part of the bottle.
- When using a package of 100 ml or more, the kit includes a pipette, which must be removed from the outer shell.
- Move the upper clamp of the pipette to the marking corresponding to the weight of the child, holding the lower clamp in place while drawing the suspension inside.
- Remove the filled pipette from the bottle, holding the lower mark clamp.
- After use, rinse the pipette with water and put on the packaging case.
- Seal the bottle with a lid
Recommended dosage
Motilium. Release form - tablets
In cases of signs of nausea and vomiting in children over 5 years of age, the permissible daily dosage is 0.04 g per day, with a frequency of 3-4 doses, including the range before meals and before bedtime. The suspension form is calculated depending on the child’s weight, which is 5 ml per 10 kg of weight, with the maximum daily dose determined at 2.4 mg per kilogram.
The prolonged course of functional disorders of the digestive tract includes regular use of the drug in a dosage of 10 mg half an hour before the next meal three times a day. If necessary, an extraordinary dose before bedtime is allowed. The maximum daily dose is no more than 0.08 g per day. The suspension form of the drug is used based on the weight of the child, with the calculation of 2.5 ml per 10 kilogram of weight, which is 250 mg per 1 kilogram.
In case of disturbances in the activity of the excretory system, it is recommended to increase the interval between taking Motilium. Coordination of dosage is not required, since a small percentage of the active substance is utilized unchanged by the kidneys. When reusing the drug, it is necessary to reduce the frequency of administration to 1-2 times a day; also, depending on the individual course of the characteristics of the pathological disorder, adjustment of the dosage used may be necessary.
Instructions for use
Indications for use, contraindications and dosage regimen for Motizhekt are described in detail in the instructions. In separate paragraphs, the manufacturer indicates the specifics of using the medication for different categories of patients, the nuances of drug interactions of the antiemetic and possible adverse reactions if contraindications are ignored or overdose. The dosage regimen can be changed by the doctor if the patient has special instructions or if long-term therapy with Motizhekt is necessary.
pharmachologic effect
Motizhekt belongs to the group of prokinetics and is a centrally acting antiemetic drug. The medication contains domperidone. The substance acts on certain receptors that provoke the gag reflex. You can take the drug during attacks of nausea, hiccups and vomiting of various etiologies.
In some cases, this drug is prescribed before procedures during which a gag reflex may occur (including during surgery).
Pharmacological properties:
- improvement of gastric evacuation activity;
- normalization of motor and motor function of the digestive tract;
- increased levels of lactogenic hormone in the blood serum;
- blockade of central dopamine receptors.
Indications for use
The drug is intended to relieve nausea, eliminate vomiting and severe hiccups. Hypotension associated with surgery;- nausea;
- vomit;
- hiccups of various origins, arising for various reasons;
- intestinal atony;
- gastric atony;
- flatulence;
- reflux esophagitis;
- biliary dyskinesia;
- cholecystitis;
- dyspepsia;
- cholangitis.
Mode of application
The drug is taken before meals, 13 minutes (or half an hour before). The norm is 30 mg, divided into three times a day. If the feeling of nausea remains during the day or the problem occurs in the late afternoon, it is recommended to take 10 mg of the drug before bed. After taking, do not eat.
For dyspepsia, take 20 mg 3-4 times a day. In case of renal failure, intake is reduced to 10-20 mg per day. The drug is prescribed to children over 5 years of age, adults and people of retirement age. For the elderly, the dosage is not reduced unless there are special precautions.
Release form, composition
Release form: small, film-coated tablets. The color is light yellow or almost white.
Each tablet contains 10 mg of domperidone, 16.6 mg of potato starch, 79 mg of milk sugar, 5 mg of microcrystalline cellulose, 1.2 mg of aerosil, 1.4 mg of povidone, 1.2 mg of magnesium stearate, 1.5 mg of sodium lauryl sulfate.
The film shell contains 1.2 mg of hypromellose, 1.6 mg of lactose monohydrate, 0.4 mg of macrogol 4000, 0.8 mg of titanium dioxide. Sold in packs of 30 tablets in a cardboard package. There are two options: 10 tablets for 3 plates and 15 tablets for two plates.
Interaction with other drugs
Domperidone may have a lesser effect on the body if taken simultaneously with antacids, anticholinergics and antisecretory drugs.
It is also believed that domperidone increases in plasma concentration if the drug is taken simultaneously with HIV inhibitors, macrolides, and nefazodone. The same applies to drugs aimed at suppressing various types of fungi (most drugs).
The drug not only relieves symptoms, but also has a therapeutic effect on the walls of the stomach and stimulates the functioning of the digestive organs.
Conditions limiting intake
The drug is well tolerated, proving a high level of functional effect, but the drug has some limitations in administration:
- Gastrointestinal bleeding.
- Perforation of the walls of the digestive tract organs, since the drug, while increasing the motor abilities of tissues, can provoke a sharp deterioration of the condition.
- Acute intestinal obstruction.
- Tumor-like transformation in the tissues of the pituitary gland with impaired secretion of prolactin.
- Combined use with Ketoconazole.
- Individual intolerance to the components of the drug.
- Chronic or inborn errors of metabolism in liver tissue
In any case, before taking the drug, it is necessary to consult with a specialized specialist to exclude the development of possible negative consequences that worsen the current symptoms and to exclude the presence of pathological conditions unacceptable for use.
Side effects
Deviations from the required dosage and disturbances in the structure of administration, as well as individual characteristics of the body that reflect the use of the drug, can provoke the development of the following manifestations:
- Allergic reaction.
- Spasm of the muscle fibers of the digestive tract.
- Disorders of neurological origin, which are associated with a lack of stability of the blood-brain barrier in early childhood, with the disappearance of symptoms immediately after discontinuation of the drug.
- Deviations in the activity of the endocrine system in the form of gynecomastia, rarely galactorrhea
special instructions
Influence on the ability to drive vehicles and control mechanisms
Side symptoms when taking Motizhekt may include drowsiness, headache and increased excitability. If such conditions occur, it is necessary to exclude driving vehicles and operating dangerous mechanisms. If the dosage regimen is followed and contraindications are excluded, the risk of such manifestations is eliminated.
Pregnancy and lactation
Contraindicated for pregnant women and those who are breastfeeding. Motizhekt is not recommended for pregnant women. In most cases, the gag reflex during gestation is provoked by toxicosis. This condition requires taking special medications. During the lactation period, the use of Motizhekt is prohibited (the reaction of the body of a newborn baby can be unpredictable).
Use in childhood
Children from the age of five can take Motizhekt to relieve the gag reflex.
For impaired renal function
For renal pathologies, a special dosage regimen for Motizhekt is required. A dosage regimen must be drawn up by a doctor. In case of inflammatory processes in the kidneys, the antiemetic is used with caution.
For liver dysfunction
Some liver dysfunction is the basis for reducing the dosage of Motizhekt (in case of renal failure, the number of doses of the drug is halved). Consultation with a doctor is recommended in advance.
Conditions for dispensing from pharmacies
Presentation of a prescription is required.
Special Recommendations
Motilium is recognized as an effective remedy for children
Antisecretory or antacid agents should not be used together with Motilium, which are recommended, if necessary, to be transferred to a time interval after meals. Care should be taken when taking the drug against the background of functional disorders of the excretory system and liver, which indicates a high degree of excretion of domperidone by tissues. When prescribing a course of therapy for a long time, you should regularly see your doctor.
In pediatric practice, since in the first stages of a child’s life there is incomplete stability of the blood-brain barrier, the drug should be used with special attention and under the supervision of a specialist. The development of deviations in the sphere of nervous activity is allowed at the age of the first year of life, reflecting the permeability of the blood-brain barrier. In addition, exceeding the dosage contributes to the manifestation of pathological disorders in nervous activity.
Nomides capsules 75 mg 10 pcs ➤ instructions for use
The drug oseltamivir is taken orally, regardless of meals or with meals. Treatment The drug should be started no later than 2 days after the development of symptoms of the disease. Adults and adolescents ≥ 12 years of age The recommended daily dose is 150 mg. The drug is prescribed in a dose of 75 mg (one capsule 75 mg or one capsule 30 mg + one capsule 45 mg) 2 times a day orally for 5 days. Children weighing more than 40 kg or aged ≥ 8 years Children who can swallow capsules can also be treated by taking 75 mg (one 75 mg capsule or one 30 mg capsule + one 45 mg capsule) twice daily within 5 days. Children aged ≥ 1 year The recommended dosage regimen for oseltamivir capsules is 30 and 45 mg. Body weight Recommended dose for 5 days ≤15 kg: 30 mg twice a day >15-23 kg: 45 mg twice a day >23-40 kg: 60 mg twice a day Prevention The drug should be started no later than 2 days after contact with patients. Adults and adolescents aged ≥ 12 years 75 mg (one 75 mg capsule or one 30 mg capsule + one 45 mg capsule) once daily orally for at least 10 days after contact with the patient. During a seasonal flu epidemic - 75 mg 1 time per day for 6 weeks. The preventive effect lasts as long as the drug is taken. Children weighing more than 40 kg or aged ≥ 8 years Children who can swallow capsules can also receive preventive therapy by taking 75 mg (one 75 mg capsule or one 30 mg capsule + one 45 mg capsule) once daily within 10 days. Children aged ≥ 1 year The recommended dosage regimen for oseltamivir capsules is 30 and 45 mg. The recommended dosage regimen for oseltamivir capsules is 30 and 45 mg. Body weight Recommended dose for 10 days ≤15 kg: 30 mg once daily >15-23 kg: 45 mg once daily >23-40 kg: 60 mg once daily Dosing in special cases Patients with impaired function kidneys: Treatment Patients with creatinine clearance more than 60 ml/min do not require dose adjustment. In patients with a creatinine clearance of 30 to 60 mL/min, the dose of oseltamivir should be reduced to 30 mg twice daily for 5 days. In patients with a creatinine clearance of 10 to 30 ml/min, the dose of oseltamivir should be reduced to 30 mg once daily for 5 days. For patients on chronic hemodialysis, oseltamivir at an initial dose of 30 mg can be taken before starting dialysis if flu-like symptoms appear within 48 hours between dialysis sessions. To maintain plasma concentrations at therapeutic levels, oseltamivir should be taken 30 mg after each dialysis session. For patients on peritoneal dialysis, oseltamivir should be taken at an initial dose of 30 mg before starting dialysis, then 30 mg every 5 days. The pharmacokinetics of oseltamivir in patients with end-stage chronic renal failure (with creatinine clearance ≤10 ml/min) not on dialysis have not been studied. In this regard, there are no dosage recommendations for this group of patients. Prevention Patients with creatinine clearance more than 60 ml/min do not require dose adjustment. In patients with a creatinine clearance of 30 to 60 ml/min, the dose of oseltamivir should be reduced to 30 mg once daily. In patients with creatinine clearance from 10 to 30 ml/min, it is recommended to reduce the dose of oseltamivir to 30 mg every other day. For patients on chronic hemodialysis, oseltamivir at an initial dose of 30 mg can be taken before starting dialysis. To maintain plasma concentrations at therapeutic levels, oseltamivir should be taken 30 mg after each subsequent odd-numbered dialysis session. For patients on peritoneal dialysis, oseltamivir should be taken at an initial dose of 30 mg before starting dialysis, then 30 mg every 7 days. The pharmacokinetics of oseltamivir in patients with end-stage chronic renal failure (with creatinine clearance less than 10 ml/min) not on dialysis have not been studied. In this regard, there are no dosage recommendations for this group of patients. Patients with impaired liver function No dose adjustment is required for the treatment and prevention of influenza in patients with mild to moderate hepatic impairment. The safety and pharmacokinetics of oseltamivir in patients with severe hepatic impairment have not been studied. Elderly and senile patients No dose adjustment is required for the prevention or treatment of influenza. Patients with weakened immune systems (after transplantation). For seasonal prophylaxis of influenza in immunocompromised patients aged ≥1 year - for 12 weeks, no dose adjustment is required.
Extemporaneous preparation of Nomides® suspension from capsules
In cases where adults, adolescents and children have difficulty swallowing capsules or there are signs of "aging" of the capsules, it is necessary to open the capsule and pour its contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food product (normal chocolate syrup). sugar or unsweetened, honey, light brown sugar or table sugar dissolved in water, sweet dessert, sweetened condensed milk, applesauce or yogurt) to cover up the bitter taste. The mixture must be mixed thoroughly and given to the patient as a whole. The mixture should be swallowed immediately after preparation.
Capsules 75 mg
If patients require a dose of 75 mg , the following instructions should be followed:
1. Holding one Nomides® 75 mg capsule over a small container, carefully open the capsule and pour the powder into the container.
2. Add a small amount (no more than 1 teaspoon) of a suitable sweetened food (to cover the bitter taste) and mix well.
3. Mix the mixture thoroughly and drink it immediately after preparation. If there is a small amount of mixture left in the container, you should rinse the container with a small amount of water and drink the remaining mixture.
If patients require doses of 30-60 mg , the following instructions should be followed for proper dosing:
1. Holding one Nomides® 75 mg capsule over a small container, carefully open the capsule and pour the powder into the container.
2. Add 5 ml of water to the powder using a syringe with marks indicating the amount of liquid collected. Mix thoroughly for 2 minutes.
3. Draw the required amount of mixture into the syringe from the container according to the following table.
Body weight / Recommended dose / Amount of Nomides® mixture per 1 dose
≤15 kg / 30 mg / 2 ml
>15-23 kg / 45 mg / 3 ml
>23-40 kg / 60 mg / 4 ml
There is no need to collect undissolved white powder as it is an inactive filler. By pressing the plunger of the syringe, inject all its contents into the second container. Any remaining unused mixture should be discarded.
4. In a second container, add a small amount (no more than 1 teaspoon) of a suitable sweetened food to cover the bitter taste and mix well.
5. Mix the mixture thoroughly and drink it immediately after preparation. If there is a small amount of mixture left in the container, you should rinse the container with a small amount of water and drink the remaining mixture.
This procedure should be repeated before each dose of the drug.
Actions in case of overdose
Exceeding the permissible dose of the drug may contribute to the development of the following symptoms:
- Loss of orientation
- Drowsiness
- Extrapyramidal reactions
If these signs appear while taking the drug, you should immediately stop using the subsequent dose; activated charcoal and observation by your doctor are recommended. The development of extrapyramidal reactions requires additional treatment with antihistamines and anticholinergics.
Reviews
The drug is often prescribed to patients when a feeling of nausea occurs, as well as vomiting of various origins. It was noticed that most patients were satisfied with the therapeutic effect that the product provides. 90% of patients emphasized that after taking the drug, the condition of the stomach improved within an hour, and 30 minutes after the first dose, the feeling of nausea subsided.
Many people taking the drug were also satisfied with the price-quality ratio, noting the fairly low cost of the product in pharmacies. However, the availability of Motizhekt turned out to be quite problematic - not every pharmacy could find it.
Combination with other drugs
The combined use of antisecretory and antacid drugs disrupts the absorption reaction of domperidone, which significantly reduces the effectiveness of Motilium. Also, its action inhibits the intake of sodium bicarbonate and the neutralizing ability of anticholinergic drugs.
Since the active substance has a stimulating effect on the tissues of the digestive tract, it is possible to increase the absorption capacity of other drugs when taken in combination with Motilium. In particular, this applies to tablet forms with an intestinal soluble coating or drugs with an extended absorption interval of the main components. A practical study, when taking Motilium in combination with Paracetamol or Digoxin, showed that the concentration level of the main active components in the blood plasma remained unchanged.
Experimental data suggest an increase in the concentration of domperidone in the blood plasma when taking drugs together that significantly inhibit the action of the enzyme. Such substances include:
- Antimycotics.
- Macrolides.
- Nefazodone.
- Drugs that inhibit HIV protease
The active component of the drug does not have a potentiating effect from the joint use of antipsychotics, which allows simultaneous use. Motilium eliminates side effects when taking dopaminergic receptor agonists, such as Levopod and Bromocriptine.
Storage conditions and periods
The drug should not be frozen or stored at temperatures above 25 degrees. Leave out of the reach of children.
Shelf life is two years from the date of production. The date of manufacture is indicated on the packaging.
- See our article “The best folk remedy for gastritis!” To get rid of it QUICKLY and FOREVER...
- How to get rid of gases in the intestines and why do they occur?
- Activated carbon cleaning - is it worth starting?