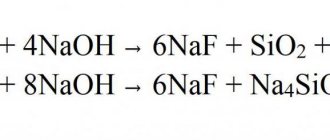Pharmacodynamics and pharmacokinetics
Sucralfate, under the influence of acids in the stomach (if the pH value is below 4), breaks down into sucrose sulfate and aluminum . Aluminum denatures mucus proteins so that sucrose sulfate can combine with them and fixate on dead ulcer tissue. Thus, a protective film is formed on the surface of the stomach, which acts as a barrier against the enzyme pepsin, bile and hydrochloric acid . The product adsorbs bile and has an anti-inflammatory effect.
The gel lines the entire surface of the affected tissues in the duodenum and stomach. The effect of the drug lasts about 6 hours. The medicine does not interact with the normal gastric mucosa.
The biological availability of the substance is low. of sucrose sulfate and less than one hundredth of a percent of aluminum are absorbed in the gastrointestinal tract . The drug is excreted mainly in feces, and a small amount through the kidneys.
Indications for use
Application of Sucralfate:
- for heartburn , medicinal ulcers ;
- with exacerbation of stomach and duodenal ulcers ;
- for the treatment of stress ulcers in the gastrointestinal tract;
- with the development of erosive and ulcerative lesions in the gastrointestinal tract associated with the use of non-steroidal anti-inflammatory drugs ;
- for reflux esophagitis, reflux gastritis, hyperacid gastritis, gastroduodenitis ;
- as part of the complex treatment of hyperphosphatemia , in persons suffering from uremia who periodically undergo hemodialysis .
Analogs
Analogues of this medicine are such drugs as: Ancrusan, Ventrisol, Flax Seeds, Alsukrsal, De-Nol, Ventr, Venter, Novobismol, Sukrat, Vis-Nol, Ulgastran, Sucrafil, Vikalin, Gastro-Norm, Ampilop, Gaviscon, Gastrotilin, Omez D, Domstal, Caleflon, Ribiril, Maalox, Alyugastrin, Gastrofarm, Dalargin, Bismofalk, Kalmagin, Vicair, Gastrotsepin, Alyugastrin, Liquiriton, Anacid, Rutacid.
Important! Only a specialist should select these or other drugs.
- If you have any symptoms of the disease, you should immediately consult a doctor. You can view a list of gastroenterology clinics on our website
- You will be interested! The article describes symptoms that make it possible to suspect the presence of liver disease in the early stages
- You will also be interested in learning more about the treatment of various diseases of the gastrointestinal tract https://gastrocure.net/bolezni.html
Side effects
Sucralfate may cause:
- indigestion , diarrhea or constipation, increased gas production ;
- headache, epigastric , back pain;
- drowsiness;
- nausea, dizziness;
- dryness of the oral mucosa;
- allergic rashes, itching , urticaria .
Of these effects, constipation .
Sucralfate, instructions for use (Method and dosage)
The dosage and duration of treatment depends on the disease and the dynamics of the disease.
Adult patients are usually prescribed 0.5 to 1 gram of Sucralfate per day, in 4 doses.
You can also take 1 gram in the morning and 1 more in the evening.
There is a treatment regimen in which they drink 2 grams of the drug, 2 times a day, 60 minutes before the first meal and at night.
The maximum amount of active ingredient that can be taken per day is 12 grams.
The average course of treatment lasts about 4-6 weeks, with a maximum of 3 months.
For children, the average daily dosage is 2 grams, divided into 4 doses.
On the recommendation of a doctor, the daily dosage can be reduced. For example, with hyperphosphatemia , if the concentration of phosphates in the plasma is reduced.
Storage
The medicine must be kept in a room where the temperature ranges from 15 to 25 degrees. In addition, the place should be dry and also dark. Under no circumstances should small children be allowed near the medicine.
If all recommendations are strictly followed, the medicine can be used for no more than three years. An expired product is not allowed for further use, because At best, such a medicine will have no effect, and at worst, it will only make things worse.
Interaction
The drug reduces the effectiveness of levothyroxine, tetracycline, sulpiride, phenytoin.
The drug is not recommended to be combined with digoxin ; this may lead to a decrease in the effectiveness of both drugs.
When taken simultaneously, the absorption capacity of amitriptyline and its effectiveness decreases.
Combining the drug with indirect anticoagulants may lead to a decrease in the effectiveness of anticoagulants ( Warfarin ).
It is assumed that the combination of Sucralfate with amphotericin B, tobramycin, colistin sulfate may lead to the formation of chelate complexes and a decrease in the effectiveness of both drugs.
The medicine reduces the effectiveness of antimicrobial agents - fluoroquinolones .
When taken simultaneously with ketoconazole and fluconazole , the drugs are less absorbed.
The combination of a substance with theophylline leads to a change in the pharmacokinetics of the latter. It is also assumed that the absorption of theophylline from lek is significantly reduced. time-release forms.
of quinidine when combined with this substance has been reported The drug should be combined with caution with roxatidine, cimetidine and ranitidine .
If there is a need to take medication and antacids , then this should be done with an interval of 30 minutes.
It is not recommended to combine the drug with other products containing aluminum .
Price
Important! For more information, please contact your nearest pharmacy. The article presents average prices across the country.
The cost of a medicine is based on a number of factors, which include the individual markup on the product made by each pharmacy, the purchase price, and the costs incurred by the company for transporting the product. Depending on the country, the price will vary significantly. This is due to the fact that countries have their own currencies, which have exchange rates.
Russia
In Russia, you will have to pay an average of 295 rubles for this medicine.
Ukraine
In Ukraine, you need to pay 145.89 hryvnia for the same drug.
Video on the topic: Nutrition for gastritis
special instructions
If a patient develops convulsions and drowsiness , this may be a sign of aluminum toxicity .
phosphate during treatment .
Sucralfate is not intended for intravascular injection . This can lead to cerebral or pulmonary .
If the patient has a predisposition to the formation of bezoars , then the drug increases the likelihood of their occurrence.
ALPHA D3
Compound:
Active ingredient: alfacalcidol;
1 capsule contains alfacalcidol 0.25 mcg or 0.5 mcg, or 1 mcg;
Excipients: citric acid, propyl galate, vitamin E (DL-alpha-tocopherol), ethanol, peanut butter, gelatin, glycerin 85%, sorbitol (E 420), sorbitan anhydrides, mannitol (E 421), higher polyols, purified water;
Iron oxide red (E172) for a dose of 0.25 mcg
Titanium dioxide (E 171), iron oxide red (E172) for a dose of 0.5 mcg
Titanium dioxide (E 171), iron oxide yellow (E172) for a dose of 1 mcg.
Dosage form. Soft capsules.
Pharmacological group. Vitamins. Vitamin D preparations and its analogues. Alfacalcidol. ATS code A11C C03.
Clinical characteristics.
Indications.
Postmenopausal osteoporosis.
Osteoporosis associated with glucocorticoid treatment.
Softening of bones in old age (osteomalacia) as a result of insufficient absorption, for example in the case of malabsorption and post-gastrectomy syndrome.
To significantly reduce the incidence of falls in older people.
For hypoparathyroidism or hypophosphatemic (vitamin D-resistant) rickets/osteomalacia, additional therapy with Alpha D3-Teva may be indicated if plasma calcium levels are less than 2.2 mmol/l.
Diseases accompanied by impaired 1-alpha-hydroxylation in the kidneys, which in turn cause disturbances in the metabolism of vitamin D (for example, renal osteodystrophy with decreased calcium absorption and plasma calcium levels less than 2.2 mmol/l (less than 8.8 mg/100 ml ), which can occur as a consequence of impaired renal function without or with dialysis, as well as at the beginning of the condition after kidney transplantation).
Contraindications.
- hypersensitivity to alfacalcidol, peanuts, soy or any other components of the drug;
- hypersensitivity to vitamin D and manifestations of vitamin D intoxication;
- plasma calcium level is higher than 2.6 mmol/l, the product of plasma calcium × phosphate concentrations is greater than 3.7 (mmol/l)2;
- alkalosis with a venous blood pH level of more than 7.44 (lactate-alkalosis syndrome, Burnett syndrome);
- metastatic calcification;
- Hypersensitivity to vitamin D analogues.
Method of administration and dose.
The drug is taken orally. The capsule should be swallowed whole with plenty of water. The dose and duration of therapy is determined by the doctor individually and depends on the nature of the disease and the effectiveness of therapy. In some cases, the drug should be used throughout life.
Unless otherwise prescribed by a physician, the starting dose is 1 mcg of alfacalcidol (4 capsules of 0.25 mcg or 2 capsules of 0.5 mcg or 1 capsule of 1 mcg) daily.
For patients with more severe bone disease, give high doses of 1-3 mcg alfacalcidol (4-12 capsules of 0.25 mcg, 2-6 capsules of 0.5 mcg, 1-3 capsules of 1 mcg) daily.
Children over 6 years old weighing over 20 kg who are able to swallow a capsule - 1 mcg/day (except in cases of renal osteodystrophy).
For patients with hypoparathyroidism, the dose should be reduced once normal blood calcium levels are achieved (2.2-2.6 mmol/L 8.8-10.4 mg/100 ml) or when the product of plasma calcium x phosphate concentrations is 3 .5-3.7 (mmol/l)2.
Alpha D3-Teva 0.25 mcg
If the daily dose is 0.5 mcg of alfacalcidol, take 1 capsule morning and evening daily. If the daily dose is 1 mcg of alfacalcidol, take 2 capsules morning and evening daily.
Alpha D3-Teva 0.5 mcg
If the daily dose is 0.5 mcg, take 1 capsule in the evening daily. If the daily dose is 1-3 mcg of alfacalcidol (2-6 capsules of 0.5 mcg), it should be divided in half and taken one half in the morning and the other in the evening.
Alpha D3-Teva 1 mcg
If the daily dose is 1 mcg of alfacalcidol, take 1 capsule in the evening daily. If the daily dose is 3 mcg of alfacalcidol, take 1 capsule in the morning and 2 capsules of Alpha D3-Teva in the evening.
Adverse reactions.
If the dose of alfacalcidol is not adjusted, there may be an increase in calcium levels in the blood, which disappears when the dose is reduced or the drug is temporarily stopped. Signs of a possible increase in the level of calcium in the blood are fatigue, gastrointestinal disorders (vomiting, heartburn, abdominal pain, nausea, discomfort in the epigastric region, constipation, diarrhea), anorexia, dry mouth, moderate pain in muscles, bones, joints , weight loss, polyuria, increased sweating, headache, thirst, vertigo, increased levels of calcium and phosphate in the urine, nephrocalcinosis, hypersensitivity reactions, including itching, rash, urticaria.
During therapy with alfacalcidol, adverse reactions may occur, which are determined by frequency: very often (≥1/10), often (≥1/100 to <1/10), infrequently (≥1/1000 to <1/100), rarely (≥1/10000 to <1/1000), very rare (<1/10000), unknown (cannot be determined from available data).
Common: hypercalcemia*, hypercalciuria.
Rarely (> 1/10000, <1/1000): hyperphosphatemia, to prevent which the patient can be prescribed phosphate absorption inhibitors (such as aluminum compounds) tachycardia, weakness, headache, dizziness, drowsiness.
Very rare (<1/10,000), including isolated cases: heterotopic calcification (cornea and blood vessels), which disappears after discontinuation of the drug, slight increase in high-density lipoproteins in the blood plasma. In patients with severe renal impairment, hyperphosphatemia may develop; allergic skin reactions (itching) and anaphylactic shock, the latter can be caused by peanut oil, which is part of the drug.
Unknown: hypersensitivity.
* Hypercalcemia is also associated with reactions such as hyperkaliuria, ectopic calcification, kidney and heart damage.
Overdose.
With a single overdose (25-30 mcg) of alfacalcidol, no harm to health is observed. The following cases of alfacalcidol overdose can cause hypercalcemia.
Symptoms of overdose: anorexia, weakness, lethargy, dizziness, headache, nausea, dry mouth, constipation, diarrhea, heartburn, vomiting, abdominal pain, bone pain, increased sweating, drowsiness, itching, tachycardia. Polyuria, polydipsia, nocturia, thirst, weight loss, proteinuria may occur if renal function is impaired.
Treatment of overdose: stop taking the drug. Depending on the severity of hypercalcemia, calcium-free or low-calcium diets, fluid intake, dialysis, loop diuretics, glucocorticosteroids, and calcitonin may be used.
In case of acute overdose, a positive effect can be observed with gastric lavage and/or administration of mineral oil (which helps to reduce absorption and increase excretion of the drug in feces).
There are no specific antidotes for alfacalcidol.
Use during pregnancy or breastfeeding.
Although there has been no evidence of harmful effects on the fetus or infant, Alpha D3-Teva should not be used during pregnancy or lactation.
Due to the possible risk of developing persistent hypercalcemia, which can cause physical and mental retardation, supravalvular aortic stenosis, retinopathy in infants, overdose of vitamin D analogues during pregnancy should be avoided.
The use of Alpha D3-Teva during breastfeeding may increase the level of calcitriol in breast milk. Considering this, the drug should not be used during breastfeeding.
Children.
Use in children over 6 years of age weighing 20 kg or more who can swallow the capsule.
Features of application.
Alfacalcidol should not be prescribed to patients with hypercalcemia.
Prescribe the drug with caution:
- patients prone to hypercalcemia, especially patients with urolithiasis;
- patients who are simultaneously using cardiac glycosides or digitalis preparations since hypercalcemia can lead to arrhythmia in such patients.
During the period of use of the drug, it is necessary to regularly (at least once every 3 months) monitor the level of calcium in the blood plasma and urine, monitor the development of the therapeutic effect and, if necessary, adjust the dose of alfacalcidol to avoid the development of hypercalcemia and hypercalciuria. If there are biochemical signs of normalization of bone structure (normalization of alkaline phosphatase in the blood plasma), it is necessary to reduce the dose of Alpha D3-Teva, which avoids the development of hypercalcemia. Hypercalcemia or hypercalciuria can be eliminated by discontinuing the drug and reducing calcium intake until its plasma concentration normalizes. Typically this period is 1 week. Therapy can then be continued, starting with half the last dose that was used.
Plasma phosphate levels should be monitored during alfacalcidol therapy to reduce the risk of ectopic calcification.
Supplementing the diet with vitamin D may be harmful for individuals who already obtain adequate amounts from food and sunlight, since the difference between therapeutic and toxic concentrations is very small.
Because alfacalcidol is the most potent form of vitamin D, it is expected to have the greatest risk of toxicity; however, its effects are rapidly reversible if supplementation is discontinued.
Alfacalcidol increases the absorption of calcium and phosphate in the intestine, so their plasma concentrations should be monitored in patients with renal failure.
Clinical manifestations of hypo- or hypercalcemia in elderly patients should be monitored, especially in the presence of concomitant renal or cardiac pathology.
Early symptoms of hypercalcemia include:
- polyuria;
- polydipsia;
- weakness, headache, nausea, constipation;
- dry mouth;
- muscle and bone pain;
- metallic taste in the mouth.
The medicine contains soybean oil. If you have an allergy to peanuts or soy, do not take this medication.
The ability to influence the reaction rate when driving a vehicle or working with other mechanisms.
No effect on the ability to drive vehicles or operate complex machinery has been identified, but the possibility of adverse reactions such as drowsiness and dizziness should be taken into account.
Interaction with other drugs and other types of interactions.
In the treatment of osteoporosis, Alpha D3-Teva can be prescribed in combination with antiresorptive drugs of different groups and estrogens. The effect of alfacalcidol is enhanced by the simultaneous use of estrogens in premenopausal and postmenopausal women.
Vitamin D and its derivatives should not be used concomitantly with Alpha D3-Teva due to the possibility of additive interactions and an increased risk of hypercalcemia.
With the simultaneous use of Alpha D3-Teva with digitalis preparations, the risk of developing arrhythmia increases.
When used concomitantly with barbiturates, anticonvulsants (for example, carbamazepine, phenobarbital, phenytoin and primidone) and other drugs that activate microsomal oxidation enzymes in the liver, it is necessary to take a higher dose of Alpha D3-Teva.
GCS may counteract the effect of vitamin D.
The absorption of alfacalcidol is reduced when used with cholestyramine, colestipol, sucralfate, and antacids with a high aluminum content.
Alpha D3-Teva and aluminum-based antacids cannot be used simultaneously; the interval between doses should be 2 hours.
When used simultaneously with Alpha D3-Teva, magnesium-based antacids or laxative dialysis increase the risk of developing hypermagnesemia.
With the simultaneous use of calcium supplements and thiazide diuretics, the risk of developing hypercalcemia increases.
The effect of alfacalcidol is enhanced by the simultaneous use of estrogens in premenopausal and postmenopausal women.
Rifampicin and isoniazid may reduce the effectiveness of vitamin D.
Pharmacological properties.
Pharmacodynamics.
Alpha D3-Teva is a highly effective active metabolite of vitamin D3, which regulates the metabolism of calcium and phosphorus. Alphacalcidol (1-alpha-hydrocholecalciferol) is very quickly converted to calcitriol (1,25-dihydrocholecalciferol, D-hormone) in the liver. Calcitriol belongs to the main metabolites of cholecalciferol (vitamin D3) in maintaining calcium and phosphorus homeostasis. Alpha D3-Teva is transformed into 1,25-dihydrocholecalciferol (D-hormone) and thus increases its level in the blood. This leads to an increase in the absorption of calcium and phosphorus in the intestines, an increase in their reabsorption in the kidneys, increased bone mineralization, and a decrease in the level of parathyroid hormone in the blood. In patients with impaired 1-alpha-hydroxylation in the kidneys, which occurs with age, taking Alpha D3-Teva promotes sufficient formation of calcitriol, which neutralizes the deficiency of D-hormone. Alpha D3-Teva restores a positive calcium balance, resulting in a decrease in the intensity of bone resorption, which helps reduce the incidence of fractures. Increases bone mineral density. With a course of use of the drug, there is a weakening of bone and muscle pain associated with impaired phosphorus-calcium metabolism, improved coordination of movements and maintenance of balance, increased muscle strength, as a result of which the frequency of falls decreases.
Pharmacokinetics.
After oral administration, Alpha D3-Teva is rapidly absorbed from the gastrointestinal tract. The time to reach the maximum concentration of the drug in the blood plasma ranges from 8 to 18 hours. The onset of action is after 6 hours, the duration of action is up to 48 hours.
In the liver, alfacalcidol is quickly transformed into calcitriol (1,25-dihydrocholecalciferol, D-hormone). A smaller part of the drug is transformed in bone tissue. Unlike natural vitamin D3, the biotransformation of the drug does not occur in the kidneys, which allows its use in patients with renal pathology.
Pharmaceutical characteristics.
Basic physical and chemical properties:
0.25 mcg Capsules: Oval, opaque, red-brown elastic soft gelatin capsules stamped in black ink "0.25" on one side, containing a pale yellow oil solution.
0.5 mcg Capsules: Oval, opaque, pale pink elastic soft gelatin capsules stamped with "0.5" in black ink on one side, containing a pale yellow oil solution.
1.0 mcg Capsules: Oval, opaque, cream to ivory elastic soft gelatin capsules stamped in black ink "1.0" on one side, containing a pale yellow oil solution.
Best before date. 3 years.
Storage conditions. Store in a tightly closed container at a temperature not exceeding 25 °C out of the reach of children.
Package. 30 or 60 capsules per container; 1 container per box.
Vacation category. On prescription.
Manufacturer. Teva Pharmaceutical Industries Ltd.
Location. St. Eli Hurwitz 18 Ind. zone, Kfar Saba /
St. Kiryat Hamad 20 Har Hozvim Prom. zone, Jerusalem, Israel.
Drugs containing (Sucralfate analogues)
Level 4 ATC code matches:
Ventrisol
Novobismol
Vis-Nol
De-Nol
Gastrocepin
Dalargin
Venter
Vikair
Omez DSR
Omez D
Gastrofarm
Gaviscon
Vikalin
Medicines that contain Sucralfate: Venter, Alsukral, Sucralfate, Ancrusal, Sukrat, Ulgastran .
Reviews of Sucralfate
Some reviews about the drug:
- “... My husband is a driver. Due to the nature of his profession, he has frequent stomach pains. The doctor advised me to take Venter. It's inexpensive and helps a lot. My husband says my stomach hurts less and less often”;
- “... An excellent remedy for those who have stomach ulcers, and also very economical. During exacerbations, after taking a few tablets, I already feel better”;
- “...I have gastritis. I tried a lot of medications, expensive and cheap. During an exacerbation of the disease, I immediately start taking these pills, relief occurs almost immediately. The only side effects are constipation, and even that is rare.”