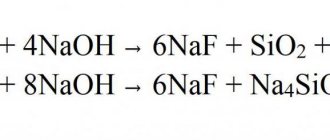Item Description
In the liquid form of ammonia, the molecules are connected by hydrogen bonds. The temperature, viscosity and density of ammonia are significantly lower compared to water. The boiling process starts at 33 degrees, and the burning or melting process starts at 77 degrees Celsius. The conductivity and dielectric constant of ammonia are low. Consequently, the bond strength in the liquid state is low.
Similar to water, ammonia associates in its liquid state due to the presence of hydrogen bonding. The transition of the chemical composition to the state of a colorless liquid with a density of 681 kilograms per cubic meter is rapid. There is practically no current conductivity in this state.
Content
- 1 Chemical properties
- 2 History
- 3 Origin of the name
- 4 Liquid ammonia
- 5 Complexation
- 6 Biological role
- 7 Physiological action
- 8 Application
- 9 Receipt 9.1 Consumption rates per ton of ammonia
Chemical properties
According to its properties, ammonia is an excellent solvent for a variety of organic and inorganic compounds. In the solid state, it is crystals that have no color. Can interact with oxygen, chloride, sulfuric acid, and also react to aqueous and saline solutions.
Ammonia has the following chemical properties:
- Plays the role of a nucleophile or complexing agent in chemical reactions. Upon addition of a proton, ammonium is formed: NH3 + H+ → NH4+.
- A weak alkaline reaction occurs in a liquid solution due to the ongoing process: NH 3 + H2O → NH4+ + OH-.
- When exposed to acids, an ammonium salt is obtained, as demonstrated by the equation: NH3 + HNO3 → NH4NO3.
- In combination with metals, due to its acidic properties, it forms amides: 2NH3 +2К →2КNH2 +Н2.
The amide, imide and metal nitride composition is formed by reaction with ammonia in the liquid state. Nitride is produced by heating the metal in a nitrogen atmosphere.
Amides have identical properties to hydroxides (due to non-electronic ions OH, NH2 and water molecules). The amide base is stronger than the hydroxide, so it is subject to hydrolysis, which is irreversible.
Solutions of ammonia-based amides conduct current and are subject to dissociation: MNH2 → M+ + NH2.
Phenolphthalein in the solution acquires a red tint, but after adding acids, the process of neutralization begins.
Ammonia in the liquid state has the ionizing functions of a solvent that can dissolve alkali metals and alkaline earth metals. At the same time, it acquires a blue color. The concentrated solution has a metallic shine. During evaporation, metals from alkali are obtained in their entirety, and alkaline earth metals form complexes with ammonia with the conductivity properties of metals.
As a result, metal atoms decompose into ions that are positively charged and electrons, sulfated, surrounded by NH 3 molecules. Solutions with the presence of free electrons have the properties of the strongest reducing agents.
Due to the electron-donating property, ammonia particles can be present in complex compounds in the form of ligands. To form amino complexes, an excess amount of ammonia is introduced into the metal saline solution.
A chemical reaction causes the reactants to change color. Compounds containing chromium and cobalt, the oxidation state of which is +3, have strong bonds of the complex.
Biological role
Ammonia is a substance formed in the organisms of living beings during metabolism, which is a product of nitrogen metabolism in them. It plays an important role in animal physiology, but it is highly toxic to organisms and is almost never found in them in its pure form. Most of it is processed by the liver into a harmless substance - urea or, as it is also called, urea.
It also helps neutralize acids entering the body with food, maintaining the acid-base balance of the blood.
Ammonia is an important source of nitrogen for plants. They mainly absorb it from the soil, but this is a very labor-intensive and inefficient process. Some plants are able to accumulate nitrogen contained in the atmosphere with the help of special enzymes - nitrogenases. After which they process nitrogen into useful compounds, such as proteins and amino acids.
Physical impact
According to its physical properties, ammonia is a substance with asphyxiating properties and an effect on the nervous system. If it enters the respiratory tract, it can infect the lungs with toxins and cause swelling with damage to various systems of the living body. Depending on the type of action, it can be local or resorptive.
Ammonia vapor irritates the mucous membrane of the eye and skin. The process proceeds with a strong unpleasant odor. Due to exposure to steam, profuse release of tears and pain in the facial area occurs . The result is a burn to the cornea of the eye. A person's vision deteriorates or is completely lost. This is followed by a coughing attack, a change in skin color with severe irritation.
When the solution gets on the skin, the affected surface burns and blisters and ulcers form. Due to its properties, in a compressed state it absorbs heat during the evaporation process. Once on the skin, the chemical element can cause frostbite of varying degrees. The presence of odor is felt at a concentration of 37 milligrams per cubic meter.
The level of ammonia concentration in the workplace should not exceed 20 milligrams per cubic meter. It is prohibited to work under more severe conditions without personal protective equipment.
Irritation of the eyes appears at 490 milligrams of concentration in the air, and of the throat - 280. Toxic poisoning and pulmonary edema occurs at a concentration of the composition of 1.5 grams per cubic meter (if you are in the area of the vapor cloud for two hours).
Aggregate states
Ammonia can be in different states of aggregation:
- It is present as a colorless gas with an unpleasant, pungent odor under normal conditions.
- It can also dissolve very well in water, so it can be stored in the form of an aqueous solution with a certain concentration. It liquefies and becomes a liquid as a result of pressure and extreme cooling.
- Ammonia has a solid state in which it appears as colorless cubic crystals.
Application and Use
Ammonia is found in a large number of substances in industry. Production volumes of this chemical element reach 150 million tons. Most often produced:
- nitrogen fertilizers (ammonium and urea);
- explosive substances;
- nitric acids.
It is possible to use ammonia as a solvent. In the refrigeration industry it is found in the form of a refrigerant (R 717).
In the field of medicine, ammonia or ammonia (the more common name is ammonia) brings a person out of a state of fainting and stimulates the gag reflex. For external use, it is used as a disinfectant for insect bites and when treating the hands of doctors. If used carelessly, it is possible to burn the digestive organs and stop the functioning of the lungs.
Use for dermatitis, skin diseases, as well as skin damage due to injury is strictly prohibited. Topical use is allowed on healthy skin areas . If the solution is used carelessly and the solution gets on the mucous part of the eyeball, the affected area should be treated with water or boric acid. Treatment must be repeated every 10 minutes.
Oil-based substances and various ointments should not be used. If it gets on the nasal area, wash it with citric acid or juice from natural fruits. If it gets into the mouth, it is recommended to drink plenty of water and fruit juice.
Ammonia poisoning
As mentioned above, ammonia is an extremely toxic and poisonous substance. It is classified as hazard class four.
Poisoning with this gas is accompanied by disruption of many body processes:
- First, the nervous system is affected and the absorption of oxygen by nerve cells is reduced.
- When penetrating into the pharynx, then the trachea and bronchi, ammonia settles on the mucous membranes, dissolves, forming an alkali, which begins to have a detrimental effect on the body, causing internal burns, destroying tissues and cells.
- This substance also has a destructive effect on fatty components, which in one form or another are part of all human organs.
- The cardiovascular and endocrine systems are affected and their work is disrupted.
After contact with ammonia, almost the entire human body, its internal tissues and organs suffer, and life processes deteriorate.
Most often, cases of poisoning with this gas occur in chemical plants as a result of its leakage, but you can also be poisoned by it at home, for example, if the container containing ammonia is not tightly closed and its vapors accumulate in the room.
Poisoning can occur even when a swab soaked in ammonia is brought to the nose during a fainting state. If the victim is allowed to smell it for more than five seconds, then the risk of intoxication is high, so ammonia should always be handled with extreme caution.
Methods of obtaining
Ammonia is produced by the interaction of hydrogen and nitrogen molecules in industry. The production technique is called the Haber process. The entire reaction proceeds with the release of heat and a decrease in volume. Thus, the reaction is carried out at reduced ambient temperature and elevated pressure. The balance shifts to the right. Under these conditions, the reaction rate is low, and as the temperature readings increase, the rate begins to increase. To carry out the reaction safely, special equipment is required to maintain elevated pressure.
To accelerate the achievement of an equilibrium state, catalyst materials are used - iron with a porous composition and a certain percentage of additives.
In all respects, the process of producing ammonia occurs at a temperature of 500 degrees Celsius and in the presence of high pressure, reaching 350 atmospheres. The percentage of production if these factors are met will be 30 percent. In industry, the process is circular. The composition is cooled and ammonia is removed, and unreacted nitrogen and hydrogen are returned for re-synthesis. This method of ammonia extraction in industry is considered the most economical.
In laboratory conditions, ammonia is produced through the action of alkalis. The chemical element is obtained by heating ammonium with lime. To dry ammonia, use lime and soda.
Due to the physical and chemical properties of ammonia, it is successfully used in industry, manufacturing, medicine, chemistry and many other areas of human activity.
Story
Ammonia was first isolated in its pure form by J. Priestley in 1774, who called it “alkaline air.” Eleven years later, in 1785, C. Berthollet established the exact chemical composition of ammonia. Since that time, research has begun around the world on producing ammonia from nitrogen and hydrogen. Ammonia was very necessary for the synthesis of nitrogen compounds, since their production from Chilean saltpeter was limited by the gradual depletion of the latter's reserves. The problem of decreasing nitrate reserves became more acute towards the end of the 19th century. Only at the beginning of the 20th century was it possible to invent a process for the synthesis of ammonia suitable for industry. This was carried out by F. Haber, who began working on this problem in 1904 and by 1909 had created a small contact apparatus in which he used increased pressure (in accordance with Le Chatelier’s principle) and an osmium catalyst. On July 2, 1909, Haber tested the apparatus in the presence of K. Bosch and A. Mittasch, both from the Baden Aniline and Soda Works (BASF), and obtained ammonia. By 1911, K. Bosch had created a large-scale version of the apparatus for BASF, and then the world's first ammonia synthesis plant was built and put into operation on September 9, 1913, which was located in Oppau (now a district within the city of Ludwigshafen am Rhein) and belonged to BASF. In 1918, F. Haber won the Nobel Prize in Chemistry “for the synthesis of ammonia from its constituent elements.” In Russia and the USSR, the first batch of synthetic ammonia was produced in 1928 at the Chernorechensky Chemical Plant.
Prevention in case of poisoning
First aid in this case consists of several simple steps. First, you need to take the victim out into the fresh air and wash his face and eyes with running water. Even those who were not very good at chemistry know from school: alkali is neutralized by acid, so the mouth and nose must be rinsed with water with the addition of lemon juice or vinegar.
If the poisoned person has lost consciousness, you should lay him on his side in case of vomiting, and if his pulse and breathing stop, give him a cardiac massage and artificial respiration.
Symptoms of poisoning
Below are a number of signs of ammonia poisoning:
- Severe cough, difficulty breathing.
- Burning in the eyes, tearing, painful reaction to bright light.
- Burning in the mouth and nasopharynx.
- Dizziness, headache.
- Abdominal pain, vomiting.
- Reduced hearing threshold.
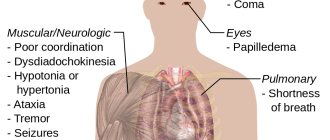
- With more serious poisoning, the following are possible: loss of consciousness, convulsions, respiratory arrest, acute heart failure. The combination of violations can lead the victim to a coma.
Consequences of poisoning
After ammonia intoxication, a person can face very serious irreversible consequences. First of all, the central nervous system suffers, which entails a number of complications:
- The brain ceases to fully perform its functions and begins to malfunction, because of this, intelligence decreases, mental illness, amnesia, and nervous tics appear.
- The sensitivity of some parts of the body decreases.
- The functioning of the vestibular apparatus is disrupted. Because of this, a person feels constant dizziness.
- The hearing organs begin to lose their functionality, which leads to deafness.
- When the eye covers are damaged, vision and its acuity are reduced; in the worst case, the victim will experience blindness.
- The onset of death. It depends on how high the gas concentration in the air was and how much ammonia vapor entered the body.
Knowing and following the prescribed safety measures means protecting yourself from the risk of a threat to your own life or a worse fate - disability, loss of hearing or vision.