Compound
The active ingredients in Janine include 2 mg of dienogest and 30 mcg of ethinyl estradiol .
In addition to these substances, the tablets contain lactose in the form of monohydrate, potato starch, magnesium stearate, talc, and gelatin. The shell is made using sucrose, dextrose, macrogol 35000, calcium carbonate , polyvidone, titanium dioxide, carnauba wax.
Pharmacodynamics and pharmacokinetics
The action of the pill is aimed at inhibiting the secretion of gonadotropic pituitary hormones, inhibiting the maturation of follicles and suppressing ovulation . With the use of the drug, the viscosity of the mucus filling the cervical canal increases, which creates obstacles for the penetration of sperm into the uterine cavity.
Taking Janine pills is accompanied by normalization of the menstrual cycle , a decrease in the pain of menstruation and the intensity of discharge, thereby significantly reducing the risk of developing anemia .
Dienogest , the progestin component of the drug, is a derivative of nortestosterone . Clinical studies of the use of this substance in patients with acne have established its pronounced antiandrogenic activity. In addition, dienogest is characterized by the ability to increase the concentration of high-density drugs in the blood.
After p/os administration of 1 tablet, dienogest is quickly and completely absorbed from the gastrointestinal tract . Plasma concentration reaches a maximum after 2 hours 30 minutes. The absolute bioavailability indicator is at the level of 96% (and this value remains the same in combination with the estrogenic component of Janine tablets).
About 1/10 of the administered dose of dienogest remains in the plasma in free form, the remaining 90% is nonspecifically bound to albumin . The substance does not bind to specific transport proteins. And this is precisely the reason that it does not displace cortisol and testosterone from their connections with CSG and SHBG, respectively.
The effect of the first passage is negligible. The resulting metabolic products are inactive.
The half-life of dienogest with a single dose is about 9 hours, with multiple doses - about 10. After oral administration, a little more than 85% of the dose is eliminated within 6 days (about half during the first day).
After p/os administration, ethinyl estradiol is quickly and completely absorbed from the digestive tract; the maximum plasma concentration is achieved in the next 1.5-4 hours after taking one tablet.
The substance is subject to the first passage effect, which accounts for its low bioavailability (this figure usually does not exceed 44%).
In free form, about 1.5% ethinyl estradiol , approximately 98.5% of the substance is bound to albumin . Ethinyl estradiol enhances the biosynthesis of CSG and SHBG without binding to these transport proteins .
After taking one tablet, the half-life of ethinyl estradiol is 10 hours. After three cycles of using the drug, it increases to 15 hours.
From 30 to 50% of metabolic products are excreted in the urine, about 30-40% are excreted in the intestinal contents.
Instructions for use JEANINE
– Dienogest
Absorption.
When taken orally, dienogest is rapidly and completely absorbed, its Cmax in the blood serum of 51 ng/ml is achieved after approximately 2.5 hours. Bioavailability is approximately 96%.
Distribution.
Dienogest binds to serum albumin and does not bind to sex steroid binding globulin (SGBS) and corticoid binding globulin (CBG). About 10% of the total concentration in the blood serum is found in free form; about 90% are not specifically associated with serum albumin. Induction of SHPS synthesis by ethinyl estradiol does not affect the binding of dienogest to serum protein.
Metabolism.
Dienogest is almost completely metabolized. Serum clearance after a single dose is approximately 3.6 L/h.
Excretion.
T1/2 is about 8.5-10.8 hours. A small amount in unchanged form is excreted by the kidneys in the form of metabolites (T1/2 - 14.4 hours), which are excreted by the kidneys and through the gastrointestinal tract in a ratio of approximately 3:
- 1.
Equilibrium concentration.
The pharmacokinetics of dienogest is not affected by the level of SHPS in the blood serum. As a result of daily administration of the drug, the level of the substance in the serum increases approximately 1.5 times.
– Ethinyl estradiol
Absorption.
After oral administration, ethinyl estradiol is rapidly and completely absorbed. Cmax in serum, equal to approximately 67 pg/ml, is achieved in 1.5-4 hours. During absorption and first passage through the liver, ethinyl estradiol is metabolized, resulting in its bioavailability when taken orally averaging about 44%.
Distribution.
Ethinyl estradiol is almost completely (approximately 98%), although nonspecifically, bound by albumin. Ethinyl estradiol induces the synthesis of SHBG. The apparent Vd of ethinyl estradiol is 2.8-8.6 l/kg.
Metabolism.
Ethinyl estradiol undergoes presystemic conjugation, both in the mucosa of the small intestine and in the liver. The main route of metabolism is aromatic hydroxylation. The clearance rate from blood plasma is 2.3-7 ml/min/kg.
Excretion.
The decrease in the concentration of ethinyl estradiol in the blood serum is biphasic; the first phase is characterized by a half-life of about 1 hour, the second - 10-20 hours. It is not excreted from the body unchanged. Ethinyl estradiol metabolites are excreted in urine and bile in a ratio of 4:
- 6 with T1/2 about 24 hours.
Equilibrium concentration.
Equilibrium concentration is achieved during the second half of the treatment cycle.
– Dienogest
Absorption.
When taken orally, dienogest is rapidly and completely absorbed, its Cmax in the blood serum of 51 ng/ml is achieved after approximately 2.5 hours. Bioavailability is approximately 96%.
Distribution.
Dienogest binds to serum albumin and does not bind to sex steroid binding globulin (SGBS) and corticoid binding globulin (CBG). About 10% of the total concentration in the blood serum is found in free form; about 90% are not specifically associated with serum albumin. Induction of SHPS synthesis by ethinyl estradiol does not affect the binding of dienogest to serum protein.
Metabolism.
Dienogest is almost completely metabolized. Serum clearance after a single dose is approximately 3.6 L/h.
Removal
. T1/2 is about 8.5-10.8 hours. A small amount in unchanged form is excreted by the kidneys in the form of metabolites (half-life - 14.4 hours), which are excreted by the kidneys and through the gastrointestinal tract in a ratio of approximately 3:
- 1.
Equilibrium concentration.
The pharmacokinetics of dienogest is not affected by the level of SHPS in the blood serum. As a result of daily administration of the drug, the level of the substance in the serum increases approximately 1.5 times.
– Ethinyl estradiol
Absorption.
After oral administration, ethinyl estradiol is rapidly and completely absorbed. Cmax in serum, equal to approximately 67 pg/ml, is achieved in 1.5-4 hours. During absorption and first passage through the liver, ethinyl estradiol is metabolized, resulting in its bioavailability when taken orally averaging about 44%.
Distribution.
Ethinyl estradiol is almost completely (approximately 98%), although nonspecifically, bound by albumin. Ethinyl estradiol induces the synthesis of SHBG. The apparent Vd of ethinyl estradiol is 2.8-8.6 l/kg.
Metabolism.
Ethinyl estradiol undergoes presystemic conjugation, both in the mucosa of the small intestine and in the liver. The main route of metabolism is aromatic hydroxylation. The clearance rate from blood plasma is 2.3-7 ml/min/kg.
Excretion.
The decrease in the concentration of ethinyl estradiol in the blood serum is biphasic; the first phase is characterized by T1/2 of about 1 hour, the second - 10-20 hours. It is not excreted unchanged from the body. Ethinyl estradiol metabolites are excreted in urine and bile in a ratio of 4:
- 6 with T1/2 about 24 hours.
Equilibrium concentration.
Equilibrium concentration is achieved during the second half of the treatment cycle.
Contraindications
Contraindications to the use of birth control pills are:
- thrombosis of veins and arteries (including a history; including PE, DVT, myocardial infarction , cerebrovascular disorders );
- conditions preceding thrombosis (including a history; for example, angina pectoris attacks of focal or cerebral disorders associated with cerebral circulation
- diabetes mellitus occurring with vascular complications ;
- severe and/or multiple factors that increase the risk of thrombosis of veins or arteries ;
- severe forms of liver disease (including a history; taking the drug is allowed only if the liver test results are normal);
- liver tumors;
- malignant diseases of the mammary glands or reproductive organs (as well as suspicion of them) caused by an imbalance of hormones
- vaginal bleeding of unspecified etiology;
- established or suspected pregnancy ;
- hypersensitivity to substances contained in the tablets.
Janine, 63 pcs., 0.03 mg+2 mg, film-coated tablets
If any of the conditions, diseases or risk factors listed below currently exist, the potential risks and expected benefits of using Janine should be carefully weighed in each individual case and discussed with the woman before she decides to start taking drug. If any of these conditions, diseases or risk factors worsen, intensify or manifest for the first time, a woman should consult her doctor, who may decide whether to discontinue the drug.
— Diseases of the cardiovascular system
There is epidemiological evidence of an increased incidence of venous and arterial thrombosis and thromboembolism (such as deep vein thrombosis, pulmonary embolism, myocardial infarction, stroke) when taking COCs. These diseases are rarely observed.
The risk of developing venous thromboembolism (VTE) is greatest in the first year of taking such drugs. An increased risk is present after initial use of a COC or resumption of use of the same or a different COC (after a dosing interval of 4 weeks or more). Data from a large prospective study involving 3 groups of patients indicate that this increased risk is predominantly present during the first 3 months.
The overall risk of VTE in women taking low-dose COCs (<0.05 mg ethinyl estradiol) is two to three times higher than in non-pregnant patients not taking COCs, although this risk remains lower than the risk of VTE during pregnancy and childbirth.
VTE can be life-threatening or lead to death (in 1-2% of cases).
VTE, manifested as deep vein thrombosis or pulmonary embolism, can occur with the use of all COCs.
It is extremely rare when using COCs that thrombosis of other blood vessels occurs, for example, hepatic, mesenteric, renal, cerebral veins and arteries or retinal vessels.
Symptoms of deep vein thrombosis: unilateral swelling of the lower extremity or swelling along a vein in the lower extremity, pain or discomfort in the lower extremity only in an upright position or when walking, local increase in temperature in the affected lower extremity, redness or discoloration of the skin of the lower extremity.
Symptoms of pulmonary embolism: difficulty or rapid breathing; sudden cough, including with hemoptysis; sharp pain in the chest, which may intensify with deep inspiration; sense of anxiety; severe dizziness; fast or irregular heartbeat. Some of these symptoms (eg, shortness of breath, cough) are nonspecific and may be misinterpreted as signs of other more common and less severe conditions (eg, respiratory tract infection).
Arterial thromboembolism can lead to stroke, vascular occlusion, or myocardial infarction. Symptoms of a stroke include: sudden weakness or loss of sensation in the face or limbs, especially on one side of the body, sudden confusion, problems with speech and comprehension; sudden unilateral or bilateral vision loss; sudden disturbance in gait, dizziness, loss of balance or coordination; sudden, severe or prolonged headache for no apparent reason; loss of consciousness or fainting with or without an epileptic seizure. Other signs of vascular occlusion: sudden pain, swelling and slight blue discoloration of the limbs, “acute” abdomen.
Symptoms of myocardial infarction: pain, discomfort, pressure, heaviness, a feeling of compression or fullness in the chest or behind the sternum, radiating to the back, jaw, left upper limb, epigastric region; cold sweat, nausea, vomiting or dizziness, severe weakness, anxiety or shortness of breath; fast or irregular heartbeat. Arterial thromboembolism can be life-threatening or fatal.
In women with a combination of several risk factors or high severity of one of them, the possibility of their mutual reinforcement should be considered. In such cases, the degree of increase in risk may be higher than with a simple summation of factors. In this case, taking the drug Zhanin® is contraindicated (see section “Contraindications”).
The risk of developing thrombosis (venous and/or arterial) and thromboembolism or cerebrovascular disorders increases:
- with age;
- in smokers (with an increase in the number of cigarettes or an increase in age, the risk increases, especially in women over 35 years old);
in the presence of:
— obesity (body mass index more than 30 kg/m2);
- family history (for example, venous or arterial thromboembolism ever in close relatives or parents at a relatively young age). In the case of a hereditary or acquired predisposition, the woman should be examined by an appropriate specialist to decide on the possibility of taking the drug Zhanine®;
- prolonged immobilization, extensive surgery, any operation on the lower extremities or major trauma. In these cases, taking the drug Zhanin® must be stopped (in the case of a planned operation, at least four weeks before it) and not resumed for two weeks after the end of immobilization. Temporary immobilization (eg, air travel lasting more than 4 hours) may also be a risk factor for the development of venous thromboembolism, especially in the presence of other risk factors;
- dislipoproteinemia;
- arterial hypertension;
- migraine;
— diseases of the heart valves;
- atrial fibrillation.
The possible role of varicose veins and superficial thrombophlebitis in the development of VGE remains controversial.
The increased risk of thromboembolism in the postpartum period should be taken into account.
Peripheral circulatory disorders may also occur in diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn's disease or ulcerative colitis) and sickle cell anemia.
An increase in the frequency and severity of migraine during use of the drug Zhanin® (which may precede cerebrovascular disorders) is grounds for immediate discontinuation of this drug.
Biochemical indicators indicating a hereditary or acquired predisposition to venous or arterial thrombosis include the following: resistance to activated protein C, hyperhomocysteinemia, antithrombin III deficiency. protein C deficiency, protein S deficiency. antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant).
When assessing the risk-benefit ratio, it should be taken into account that adequate treatment of the relevant condition may reduce the associated risk of thrombosis. It should also be taken into account that the risk of thrombosis and thromboembolism during pregnancy is higher than when taking low-dose COCs (<0.05 mg ethinyl estradiol).
— Tumors
The most significant risk factor for developing cervical cancer is persistent human papillomavirus infection. There are reports of a slight increase in the risk of developing cervical cancer with long-term use of COCs. However, the connection with taking COCs has not been proven. Controversy remains regarding the extent to which these findings are related to screening for cervical pathology or to sexual behavior (lower use of barrier methods of contraception).
A meta-analysis of 54 epidemiological studies showed that there is a slightly increased relative risk of developing breast cancer diagnosed in women currently taking COCs (relative risk 1.24). The increased risk gradually disappears within 10 years of stopping these drugs. Because breast cancer is rare in women under 40 years of age, the increase in breast cancer diagnoses in current or recent COC users is small relative to the overall risk of breast cancer. Its connection with COC use has not been proven. The observed increased risk may also be a consequence of earlier diagnosis of breast cancer in women using COCs. In women who have ever used COCs. earlier stages of breast cancer are detected than in women who have never used them.
In rare cases, during the use of COCs, the development of benign, and in extremely rare cases, malignant liver tumors, which in some cases led to life-threatening intra-abdominal bleeding, was observed. If severe abdominal pain, liver enlargement, or signs of intra-abdominal bleeding occur, this should be taken into account when making a differential diagnosis.
— Other states
Women with hypertriglyceridemia (or a family history of this condition) may have an increased risk of developing pancreatitis while taking COCs.
Although slight increases in blood pressure have been described in many women taking COCs, clinically significant increases have rarely been reported. However, if a persistent clinically significant increase in blood pressure develops while taking COCs, these drugs should be discontinued and treatment of arterial hypertension should be initiated. The drug can be continued if normal blood pressure values are achieved with antihypertensive therapy.
The following conditions have been reported to develop or worsen both during pregnancy and while taking COCs, but their relationship with COC use has not been proven: jaundice and/or pruritus associated with cholestasis; formation of gallstones; porphyria; systemic lupus erythematosus; hemolytic-uremic syndrome; chorea; herpes during pregnancy; hearing loss associated with otosclerosis. Cases of worsening the course of endogenous depression, epilepsy, Crohn's disease and ulcerative colitis during the use of COCs have also been described.
In women with hereditary forms of angioedema, exogenous estrogens may cause or worsen symptoms of angioedema.
Acute or chronic liver dysfunction may require discontinuation of COCs until liver function tests return to normal. Recurrence of cholestatic jaundice, which developed for the first time during a previous pregnancy or previous use of sex hormones, requires discontinuation of COC use.
Although COCs may have an effect on insulin resistance and glucose tolerance, there is usually no need to adjust the dose of hypoglycemic drugs in patients with diabetes mellitus using low-dose COCs (<0.05 mg ethinyl estradiol). However, women with diabetes mellitus should be carefully monitored while taking COCs.
Chloasma can sometimes develop, especially in women with a history of pregnancy chloasma. Women with a tendency to chloasma while taking Zhanine® should avoid prolonged exposure to the sun and ultraviolet radiation.
Preclinical data on safety
Preclinical data from routine repeated-dose toxicity, genotoxicity, carcinogenicity and reproductive toxicity studies do not indicate a particular risk to humans. However, it should be remembered that sex hormones can promote the growth of certain hormone-dependent tissues and tumors.
Laboratory tests
Taking the drug Zhanin® may affect the results of some laboratory tests, including indicators of liver, kidney, thyroid, adrenal function, the concentration of transport proteins in plasma, indicators of carbohydrate metabolism, parameters of blood coagulation and fibrinolysis. Changes usually do not go beyond normal values.
Reduced efficiency
The effectiveness of Zhanine® may be reduced in the following cases: missed pills, gastrointestinal disorders or as a result of drug interactions.
Frequency and severity of menstrual-like bleeding
While taking the drug Zhanine®, irregular bleeding may occur (“spotting” spotting and/or “breakthrough” uterine bleeding), especially during the first months of use. Therefore, any irregular bleeding should be assessed only after an adaptation period of approximately three cycles.
If irregular bleeding recurs or develops after previous regular cycles, careful evaluation should be performed to rule out malignancy or pregnancy.
No regular menstrual bleeding
Some women may not develop bleeding during a break in taking the pills. “ooContraindications” and “With caution”;
— Local compaction in the mammary gland;
- Concomitant use of other medications (see also “Interaction with other medications”);
- If prolonged immobility is expected (for example, a cast is applied to the lower limb), hospitalization or surgery is planned (at least four weeks before the proposed operation);
- Unusually heavy bleeding from the vagina;
- Missed a pill in the first week of taking the package and had sexual intercourse seven days or less before;
— Absence of regular menstrual-like bleeding two times in a row or suspicion of pregnancy (you should not start taking pills from the next package before consulting your doctor).
You should stop taking the pills and consult your doctor immediately if there are possible signs of thrombosis, myocardial infarction or stroke: unusual cough; unusually severe pain behind the sternum, radiating to the left arm; unexpected shortness of breath, unusual, severe and prolonged headache or migraine attack; partial or complete loss of vision or double vision; slurred speech; sudden changes in hearing, smell, or taste; dizziness or fainting; weakness or loss of sensation in any part of the body; severe abdominal pain; severe pain in the lower limb or sudden swelling of any of the lower limbs.
Side effects of Janine
The most common side effects of taking estrogen-progestin oral contraceptives are:
- an increase in the size and tension of the mammary glands, their soreness, as well as the appearance of discharge from them;
- bloody discharge from the genital tract of varying intensity (can be spotting or have the character of breakthrough bleeding);
- headaches (migraine attacks are also possible);
- mood lability;
- change in libido ;
- deterioration of tolerance to contact lenses;
- visual impairment;
- abdominal pain;
- nausea;
- skin rashes;
- vomit;
- changes in the nature of vaginal discharge;
- nodular (nodous) or erythema multiforme ;
- cholestatic jaundice;
- generalized itching;
- weight fluctuations;
- fluid retention;
- allergic reactions.
Sometimes side effects of Zhanine are expressed in the form of increased plasma concentrations of triglycerides , diarrhea , increased fatigue, decreased tolerance to carbohydrates, chloasma (the risk of focal hyperpigmentation is especially high in women who chloasma during pregnancy).
Like other combined hormonal contraceptives for oral use, Janine can cause thrombosis or thromboembolism .
Janine
A contraceptive based on estrogen (ethinyl estradiol) and progestogen (dienogest). One package contains one blister with 21 tablets, 3 blisters of 21 tablets each.
Zhanine belongs to the new generation of contraceptives and is a low-dose monophasic drug. The Janine effect is achieved through a complex effect on the body:
- suppression of ovulation;
- impact on the properties of cervical mucus - it becomes denser and impermeable to sperm;
- impact on the structure of the endometrium, as a result of which the fertilized cell cannot attach to it.
Like many modern contraceptives, Janine is characterized by a low hormone content and has minimal side effects. However, there are a number of situations in which taking Janine is either completely contraindicated or should be carried out with extreme caution, especially in the first months.
Contraindications are:
- thrombosis (venous and arterial) and thromboembolism, both currently diagnosed and present or in history (including deep vein thrombosis, pulmonary embolism, myocardial infarction);
- conditions preceding thrombosis, for example, transient ischemic attacks, angina pectoris;
- migraine;
- diabetes mellitus with vascular complications;
- diseases of the cardiovascular system, such as: damage to the valvular apparatus of the heart, heart rhythm disturbances, diseases of the cerebral vessels or coronary arteries of the heart, high blood pressure;
- pancreatitis with severe hypertriglyceridemia;
- liver failure and severe liver disease; liver tumors;
- diagnosed hormone-dependent malignant diseases or suspicion of them;
- vaginal bleeding of unknown origin.
Janine should not be taken during pregnancy, if pregnancy is suspected, or during lactation. Dosage should be canceled (or the start of dosage delayed) in situations involving prolonged limitation in physical activity, planned or emergency surgery, or serious injuries.
Janine should be used with caution, listening to the body's reactions in the following cases:
- severe disorders of fat metabolism (obesity, hyperlipidemia);
- thrombophlebitis of superficial veins;
- otosclerosis with hearing loss;
- congenital hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndromes);
- diabetes;
- systemic lupus erythematosus;
- hemolytic uremic syndrome;
- Crohn's disease;
- sickle cell anemia;
- hypertension.
If pregnancy is detected while taking the drug Zhanine, it must be discontinued immediately. There is no need to panic and think that a contraceptive taken in the early stages of pregnancy can cause severe pregnancy or fetal pathologies. Numerous and large-scale studies clearly indicate that such consequences will not occur.
We will tell you below how quickly after giving birth you can start taking Janine. However, you should always remember that this depends primarily on whether the mother is breastfeeding or not. Remember: hormonal contraceptives affect the composition and quantity of breast milk; their use during lactation is unacceptable!
Reception scheme
The tablets are taken every day, preferably at the same time. The order in which tablets are selected is determined by the arrows on the blister. It is recommended to take the tablets with drinking water. Duration of treatment: 21 days. This is followed by a week-long break, during which menstrual-like bleeding usually occurs (in most cases it begins on the second or third day after taking the last tablet).
Start of taking Janine
The choice of the first day of admission depends on many factors. In particular, it depends on what contraceptives the woman used previously.
If hormonal contraceptives were not used in the previous month (in other words, in the previous menstrual cycle), then taking Zhanine should be started on the first day of the menstrual cycle, that is, on the day when menstrual bleeding began. It is also possible to start taking it on the second to fifth day of the cycle, but in this case you should additionally use barrier contraceptives for a week (until seven tablets from the package are taken).
If you took another oral contraceptive in the previous month, then it is better to start taking Zhanine the next day after taking the last tablet of the previous drug. It does not matter whether this drug was biphasic or monophasic (that is, it contained 21 or 28 tablets in the package). Thus, there is no one-week break between two cycles of taking contraceptives.
It is possible to start taking Janine with a break between cycles, but you should not allow the break to be more than seven days.
If in the previous month you took an oral contraceptive containing only gestagens (“mini-pills”), then you can switch to taking Janine any day. A break between the two drugs is not required.
If a contraceptive injection was used in the previous month, then the switch to Janine is carried out on the day when the next injection was supposed to be given.
If an intrauterine contraceptive or implant was used, then the transition to Janine is carried out on the day of removal of the contraceptive or implant.
Please note: in all cases of switching from progestin drugs or agents, it is recommended to additionally use barrier methods of contraception for a week in the first week of taking Zhanine.
Starting after an abortion or childbirth
After an abortion in the first trimester of pregnancy, you can start taking Zhanine immediately, on the same day. Additional contraception is not required in this case.
After premature birth or abortion in the second trimester of pregnancy, taking Zhanine should begin on days 21-28. If the day of taking the first pill is later, then barrier contraceptives should also be used during the week. Please note: if sexual contact has taken place during the time that has passed since childbirth or abortion, before taking Zhanine you need to make sure that there is no pregnancy. As an option, wait until your first menstruation after childbirth or abortion and start taking the drug according to the regimen indicated above.
What to do if you forget to take your pill?
If the delay is less than 12 hours, the contraceptive effect is not reduced. The drug still has its effects on the body. Therefore, in such a situation, you need to take the pill as soon as possible. No other measures are required. The subsequent tablet is taken according to the usual schedule.
If the delay is more than 12 hours, the contraceptive effect is reduced and conception becomes more likely. Actions in such a situation depend on which week of admission it occurred. You also need to always remember two rules:
- Reception of Janine should not be interrupted for a period exceeding seven days.
- In order for the maximum contraceptive effect to be achieved, it is necessary to take the drug for at least seven days.
Actions when the interval between two tablets exceeds 36 hours (delay in taking more than 12 hours):
First week of taking the drug
You need to take the missed pill as soon as possible - if the delay is approaching 24 hours, then you need to take two pills at the same time. Then the intake continues according to the usual schedule, but barrier methods of protection are also used during the week. It is necessary to take into account that if there was a sexual contract during the week before missing the pill, there is a possibility of pregnancy. Remember: the more pills you miss, and the closer they are to the week break, the greater the chance of pregnancy. In other words, a missed pill in the third week of use entails a greater likelihood of pregnancy than a missed pill in the first week.
Second week of taking the drug
Take the missed pill as soon as possible, then proceed according to your usual schedule. If a woman is confident that she adhered to her dosage schedule during the week before missing the pill, no additional precautions are required. If the previous appointment occurred with serious deviations from the schedule, then it makes sense to additionally use barrier methods.
Third week of taking the drug
If you miss taking a pill in the third week, the risk of a decrease in the contraceptive effect, as well as the risk of possible pregnancy, is inevitable. You can act in such a situation according to two schemes.
First scheme
- Take the missed pill as soon as possible, then take the drug according to your usual schedule.
- When all the pills from the current package are drunk, the next package is moved on - that is, without a seven-day break.
With this regimen, the onset of menstrual-like bleeding in the current cycle is unlikely; scanty spotting and breakthrough bleeding may occur while taking the second package.
Second scheme
- We consider the current packaging to be complete. We don’t take the remaining pills in it - we take a week’s break, the first day of which is considered the day you missed taking a pill.
- After the break, we begin taking tablets from the next package.
If bleeding occurs during the break, pregnancy must be ruled out.
Cases where vomiting or diarrhea occurs within 4 hours after taking a pill should be regarded as missing a pill. And act according to the recommendations presented above.
Possible side effects:
- painful sensations, enlargement and tension of the mammary glands, discharge from the mammary glands;
- spotting and bleeding on days of taking pills, abdominal pain;
- headaches, migraine, nausea, vomiting, diarrhea;
- change in libido;
- mood changes, irritability, fatigue;
- poor tolerance to contact lenses, blurred vision;
- skin rash, itching, allergic reactions;
- fluid retention in the body, swelling;
- changes in body weight, leg pain, cramps.
Sometimes taking Janine can cause chloasma, especially in women with a history of chloasma during pregnancy. In such cases, prolonged exposure to the sun should be avoided.
A few more points that are important to know
- If you are undergoing elective surgery, you should stop taking Janine four weeks in advance. After surgery, you can start taking it no earlier than two weeks later.
- If you are taking drugs that affect microsomal enzymes, you must additionally use a barrier method of contraception during this period, as well as for 28 days after stopping taking these drugs.
- While taking antibiotics (such as ampicillins and tetracyclines), as well as for a week after their discontinuation, you should additionally use a barrier method of contraception.
- You should stop taking the drug and consult a doctor in the following cases:
- pain in the legs, swelling of the legs;
- sudden severe pain in the chest or abdomen;
- sudden shortness of breath, weakness, dizziness;
- coughing attacks without a cold;
- any unusual, severe, long-lasting headache;
- problems with vision and speech.
- Irregular light bleeding or breakthrough bleeding may occur while taking the tablets, especially during the first months of use. If such bleeding appears after three months of regular use of Janine, you should consult a doctor. Consultation is also required in situations where irregular bleeding occurs after several regular cycles.
- Before starting to use Zhanine, it is recommended to undergo a thorough general medical and gynecological examination (including examination of the mammary glands and cytological examination of cervical mucus) and exclude pregnancy. In addition, disorders of the blood coagulation system should be excluded.
- With long-term use of the drug, it is necessary to conduct control examinations every six months.
Contraceptive pills Janine, instructions for use (Method and dosage)
Zhanine tablets are intended for regular use; violation of the standard regimen of use provokes intermenstrual bleeding , and also reduces the contraceptive and therapeutic effectiveness of the drug.
According to the instructions for use, Janine is taken daily with water in the order indicated on the package. One cycle consists of 21 days of taking the pills and 7 days off, during which (usually on the 2nd or 3rd day) the woman begins menstrual-like bleeding . Sometimes withdrawal bleeding does not stop until you start taking pills from a new package.
How to take Janine tablets for the first time?
If in the previous month a woman did not protect herself with hormonal drugs , then they begin to drink pills from the 1st day of the menstrual cycle (on the first day of bleeding). barrier contraceptives should be used for a week after taking the first pill .
How to take the drug correctly when switching from other contraceptives?
When switching from other combined hormonal drugs, pills should be taken the next day after the last tablet with the active substances of the previous drug is taken.
The appointment must begin no later than:
- the next day after the standard one-week break (if the woman used a drug containing 21 tablets);
- the next day after taking the last pill - “placebo” (if package No. 28 is used).
When switching from a progestin drug (implant, mini-pill , injectable contraceptives), tablets begin to be taken without interruption:
- on any day, if the transition is made from a mini-pill;
- from the day when the next injection was planned, if the transition is made from contraceptives in injections;
- on the day of implant removal.
barrier contraceptives should be used in the first week of taking Janine tablets .
Rules for admission after childbirth or abortion
After termination of pregnancy in the first 13 weeks, the drug can be started immediately. Additional contraception is not needed.
After termination of pregnancy between 14 and 27 weeks, and also if the pregnancy ends in childbirth, taking the pills begins for 21-28 days. If the first pill is taken later, barrier contraceptives .
If sexual relations took place between taking the drug and childbirth/abortion, before taking Zhanine you should exclude the possibility of pregnancy or wait until your first period.
How to take pills if you miss them?
The missed pill should be taken as soon as possible, the next one from the package should be taken at the usual time. A delay of less than 12 hours does not reduce the contraceptive effect of the drug.
The breaks between taking tablets should not be more than 7 days, since it is within 7 days of continuous use of the drug that adequate suppression of the functional activity of the hypothalamic-pituitary-ovarian .
If the delay exceeds 12 hours in the first 14 days of taking the drug, the next pill is taken immediately when remembered (even if this involves taking 2 pills at the same time). Barrier contraceptives should be used for the next 7 days .
The greater the number of tablets missed and the closer the missed period is to the standard weekly break, the higher the woman’s risk of becoming pregnant.
If a delay of more than 12 hours occurred from days 15 to 21 of taking the drug, the next pill must be taken immediately when remembered (even if this involves taking 2 pills at the same time).
In the future, the reception is continued as usual and at the same time. In the next 7 days after missing, you should use barrier contraceptives. In addition, you will need to start taking pills from a new package immediately when the previous one ends, i.e. without taking a seven-day break.
Typically, withdrawal bleeding in this case does not begin until the second pack is completed. However, the possibility of spotting and even breakthrough bleeding cannot be ruled out.
The absence of withdrawal bleeding after missing pills during the seven-day period free from taking Janine is a reason to assume pregnancy.
Vomiting within three to four hours after taking the pills reduces the absorption of the active substances of the drug. In this case, you must follow the recommendations when skipping pills.
If a woman does not plan to change her usual dosage regimen, it is recommended to take additional pills from the next package if necessary.
To delay the onset of cyclic bleeding, the drug is continued to be taken continuously using a new package. You can take pills from a new pack for as long as the woman wants (until the pack runs out). At this time, spotting and breakthrough bleeding are possible.
Reception from the next pack begins after a week's break.
How long can I take Janine? If the drug is well tolerated, it can be used for as long as the need for contraception remains.
Janine and endometriosis
The exact cause of endometriosis has not been established; it is only known that hormonal imbalance . The effectiveness of the drug for endometriosis is determined by its mechanism of action.
In the second phase of the cycle after ovulation, there is intense preparation of the organs of the reproductive system for pregnancy, one of the manifestations of which is the growth of the uterine mucosa.
Janine prevents the release of the egg from the ovary (that is, ovulation), and, therefore, reduces the severity of post-ovulation changes in the endometrium of the uterus .
Why are tablets prescribed for endometriosis?
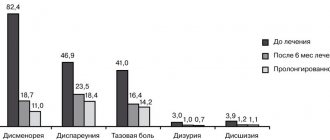
In accordance with the instructions, the effect of the drug Janine is aimed at relieving pain, normalizing the disrupted cycle, reducing the intensity of bleeding, that is, eliminating the symptoms that are the main (although not the only) ones for endometriosis .
The release of blood during menstruation is normally due to the fact that the mucous membrane formed in the second half of the cycle is rejected, and the blood vessels are damaged. Since Janine suppresses ovulation, the endometrium does not grow so actively, and accordingly, the surrounding tissues do not become inflamed and do not compress the nerve trunks.
In addition, like any hormonal drug , the drug allows you to normalize the cycle, making it strictly periodic.
How to take for endometriosis?
Janine is effective for both genital and extragenital (outside the genital organs) endometriosis . The components of the drug exhibit high activity in the body, which allows them to be used in the lowest possible dose.
Several regimens for the use of the drug Janine for endometriosis . At the same time, the doctor must select treatment individually for each woman.
In some cases, it is considered advisable to drink pills in accordance with the contraceptive regimen: one per day, from the first day of the cycle. After three cycles of “ 21 days of admission + 7 days of break, ” the patient should undergo tests for blood clotting, monitor the condition of endometriosis , and also evaluate the functional state of the liver using a biochemical blood test.
It is also possible to take the drug according to a prolonged regimen, according to which the pills should be taken continuously for 63 or 84 days in a row, and then take a week's break.
In addition to the direct effect of the active components of the drug on tissues and organs, with this treatment regimen the drug reduces the number of menstrual bleedings (instead of 3-4, one usually passes), which also has a beneficial effect on the patient’s condition.
Reviews from doctors about Janine for endometriosis indicate the high effectiveness of the drug. Their statements are also confirmed by the results of numerous studies and practical experience: according to statistics, the use of the drug can achieve positive results in approximately 85% of cases.
In addition, experts note that Janine is well absorbed in the body (its bioavailability is 90%) and rarely provokes side effects.
Janine dragee 21 pcs ➤ instructions for use
The pills should be taken orally in the order indicated on the package, every day at approximately the same time, with a small amount of water. Janine® should be taken 1 tablet/day continuously for 21 days. Each subsequent package begins after a 7-day break, during which withdrawal bleeding (menstrual-like bleeding) is observed. It usually begins on the 2-3rd day after taking the last pill and may not end until you start taking a new package. Starting taking Zhanine If you have not taken any hormonal contraceptives in the previous month, taking Zhanine starts on the 1st day of the menstrual cycle (i.e. on the 1st day of menstrual bleeding). It is possible to start taking it on the 2-5th day of the menstrual cycle, but in this case it is recommended to use a barrier method of contraception during the first 7 days of taking the tablets from the first package. When switching from combined oral contraceptives, a vaginal ring, or a transdermal patch, taking Zhanine should begin the day after taking the last active pill from the previous package, but in no case later than the next day after the usual 7-day break in taking (for drugs containing 21 tablets) or after taking the last inactive tablet (for drugs containing 28 tablets per package). When switching from a vaginal ring or transdermal patch, it is preferable to start taking Janine on the day the ring or patch is removed, but no later than the day when a new ring is to be inserted or a new patch is applied. When switching from contraceptives containing only gestagens (“mini-pills”, injectable forms, implant) or from a gestagen-releasing intrauterine contraceptive (Mirena), a woman can switch from taking the “mini-pill” to Zhanine® on any day (without a break), from an implant or intrauterine contraceptive with gestagen - on the day of its removal, from an injectable contraceptive - on the day when the next injection is due. In all cases, it is necessary to use an additional barrier method of contraception during the first 7 days of taking the pill. After an abortion in the first trimester of pregnancy, a woman can start taking the drug immediately. In this case, the woman does not need additional methods of contraception. After childbirth or abortion in the second trimester of pregnancy, it is recommended to start taking the drug on the 21-28th day after childbirth or abortion in the second trimester of pregnancy. If use is started later, it is necessary to use an additional barrier method of contraception during the first 7 days of taking the pill. However, if a woman has already been sexually active, pregnancy should be excluded before taking Zhanine or she must wait until her first menstruation. Taking missed pills If the delay in taking the pill is less than 12 hours, contraceptive protection is not reduced. A woman should take the missed pill as soon as possible, and the next pill should be taken at the usual time. If the delay in taking the pill is more than 12 hours, contraceptive protection may be reduced. In this case, you can be guided by the following two basic rules: - taking the drug should never be interrupted for more than 7 days; — to achieve adequate suppression of the hypothalamic-pituitary-ovarian system, 7 days of continuous use of the pill are required. Accordingly, if the delay in taking active pills was more than 12 hours (the interval from the moment of taking the last active pill is more than 36 hours), the following can be recommended: The first week of taking the drug It is necessary to take the last missed pill as soon as possible, as soon as the woman remembers it (even , if this requires taking two pills at the same time). The next pill is taken at the usual time. Additionally, a barrier method of contraception (for example, a condom) should be used for the next 7 days. If sexual intercourse took place within a week before missing the pills, the possibility of pregnancy must be taken into account. The more tablets are missed, and the closer they are to a break in taking active substances, the greater the likelihood of pregnancy. Second week of taking the drug It is necessary to take the last missed tablet as soon as possible, as soon as the woman remembers it (even if this requires taking two tablets at the same time). The next pill is taken at the usual time. Provided that the woman took the pill correctly during the 7 days preceding the first missed pill, there is no need to use additional contraceptive measures. Otherwise, as well as if you miss two or more pills, you must additionally use barrier methods of contraception (for example, a condom) for 7 days. Third week of taking the drug The risk of pregnancy increases due to the upcoming break in taking the pill. A woman must strictly adhere to one of the following two options. Moreover, if during the 7 days preceding the first missed pill, all pills were taken correctly, there is no need to use additional contraceptive methods. 1. It is necessary to take the last missed pill as soon as possible, as soon as the woman remembers it (even if this requires taking two pills at the same time). The next pill is taken at the usual time, until the pills from the current package run out. The next pack should be started immediately without interruption. Withdrawal bleeding is unlikely until the second pack is finished, but spotting and breakthrough bleeding may occur while taking the pill. 2. A woman can also stop taking pills from the current package. She should then take a break for 7 days, including the day she missed the pills, and then start taking a new pack. If a woman misses taking a pill and then does not have withdrawal bleeding during a break in taking it, pregnancy must be ruled out. Recommendations in case of vomiting and diarrhea If a woman has vomiting or diarrhea within 4 hours of taking active tablets, absorption may not be complete and additional contraceptive measures should be taken. In these cases, you should follow the recommendations when skipping pills. Changing the day of the start of the menstrual cycle To delay the onset of menstruation, a woman should continue taking pills from the new package of Janine immediately after taking all the pills from the previous one, without interruption in taking. The pills from this new package can be taken for as long as the woman wishes (until the package runs out). While taking the drug from the second package, a woman may experience spotting or breakthrough uterine bleeding. You should resume taking Janine from a new package after the usual 7-day break. To move the start of menstruation to another day of the week, a woman should shorten the next break in taking the pills by as many days as she wants. The shorter the interval, the higher the risk that she will not have withdrawal bleeding and will continue to have spotting and breakthrough bleeding while taking the second package (the same as in the case when she would like to delay the onset of menstruation). Additional information for special categories of patients For children and adolescents, Zhanine® is indicated only after menarche. After menopause, Zhanine® is not indicated. Zhanine® is contraindicated in women with severe liver disease until liver function tests have returned to normal. The drug Zhanin® has not been specifically studied in patients with impaired renal function. Available data do not suggest changes in treatment in these patients.
Interaction
The simultaneous use of Zhanine birth control pills with drugs that induce microsomal enzymes of liver cells (including barbiturates , hydantoins , Rifampicin , Carbamazepine , Primidon and, probably, Topiramate , Griseofulvin , Felbamate ), provokes an increase in the clearance of dienogest and ethinyl estradiol , which may cause a decrease in contraceptive effect.
As a rule, the maximum activity of liver enzymes is observed 2-3 weeks after the start of treatment with these drugs, however, it can be observed over the next 4 weeks after completion of the course.
When Zhanine is used in combination with ampicillin and tetracycline of ethinyl estradiol decreases .
It should be remembered that women who take any of the above-mentioned drugs for a short course should additionally use barrier contraceptives throughout the entire period of treatment and for 7 days after its completion.
If a woman is undergoing treatment with Rifampicin , the need to use additional contraceptive measures continues for a full 4 weeks after its completion. If concomitant therapy is started at the end of taking a package of hormonal tablets, the next one should be started without taking the usual break.
Janine n21 dragee
- in the absence of taking any hormonal contraceptives in the previous month. Taking Janine® begins on the first day of the menstrual cycle. It is possible to start taking it on the 2nd–5th day of the menstrual cycle, but in this case it is recommended to additionally use a barrier method of contraception during the first 7 days; - when switching from other combined oral contraceptives (from a vaginal ring, transdermal patch). It is preferable to start taking Zhanine® the day after taking the last tablet from the previous package, but in no case later than the next day after the usual 7-day break (for preparations containing 21 tablets) or after taking the last inactive tablet (for preparations containing containing 28 tablets per package). When switching from a vaginal ring or transdermal patch, it is preferable to start taking Janine® on the day the ring or patch is removed, but no later than the day when a new ring is to be inserted or a new patch is applied; - when switching from contraceptives containing only gestagens (mini-pills, injectable forms, implant), or a gestagen-releasing intrauterine contraceptive. You can switch from a mini-pill to Janine® on any day (without a break), from an implant or intrauterine contraceptive with gestagen - on the day of its removal, from an injection form - from the day when the next injection would have been given. In all cases, it is necessary to use an additional barrier method of contraception during the first 7 days; - after an abortion in the first trimester of pregnancy. You can start taking Janine immediately; - after childbirth or abortion in the second trimester of pregnancy. It is recommended to start taking Zhanine on the 21st–28th day after childbirth or abortion in the second trimester of pregnancy. If taking Zhanine is started later, it is necessary to use an additional barrier method of contraception during the first 7 days of use. If a woman has already been sexually active, pregnancy should be excluded before starting to take Zhanine® or she must wait until her first menstruation. Taking missed pills. If the delay in taking Zhanine is less than 12 hours, contraceptive protection is not reduced. The woman should take Janine as soon as possible, the following should be taken at the usual time. If the delay in taking Zhanine is more than 12 hours, contraceptive protection may be reduced. In this case, you can be guided by two rules: - taking Janine should never be interrupted for more than 7 days; - 7 days of continuous use of Janine are required to achieve adequate suppression of hypothalamic-pituitary-ovarian regulation. If the delay in taking Zhanine is more than 12 hours: First week of taking Zhanine The woman should take the last missed pill as soon as possible. The next pill is taken at the usual time. Additionally, a barrier method of contraception must be used for the next 7 days. If sexual intercourse took place during the week before Janine's omission, the possibility of pregnancy must be taken into account. Second week of taking Janine The woman should take the last missed pill as soon as possible. The next pill is taken at the usual time. Provided that the woman took Janine correctly during the 7 days preceding the first missed pill, there is no need to use additional contraceptive measures. Otherwise, as well as if you miss two or more tablets, you must additionally use barrier methods of contraception for 7 days. The third week of taking Zhanine The risk of decreased reliability is inevitable due to the upcoming break in taking Zhanine. The woman should strictly adhere to one of the following two options (if all tablets have been taken correctly in the 7 days preceding the first missed pill, there is no need to use additional contraceptive methods): 1. The woman should take the last missed pill as soon as possible. The next pill is taken at the usual time, until the pills from the current package run out. The next pack should be started immediately. 2. A woman can also stop taking Janine. She should then take a break for 7 days, including the day Janine missed, and then start taking a new pack. Changing the start day of the menstrual cycle In order to delay the onset of menstruation, a woman should continue taking pills from a new package of Zhanine® immediately after taking all the pills from the previous one, without interruption. Janine from this new package can be taken for as long as the woman wishes. While taking Zhanine from the second package, a woman may experience spotting or breakthrough uterine bleeding. You should resume taking Zhanine® from a new pack after the usual 7-day break. In order to postpone the start of menstruation to another day of the week, a woman should be advised to shorten the next break in taking Zhanine by as many days as she wants.special instructions
The use of the drug Zhanine is contraindicated before the onset of menarche and after the onset of menopause .
In some cases, the use of sex hormones can cause the development of tumors in the liver . liver size , severe abdominal pain, as well as signs of intraperitoneal bleeding must be taken into account when making a differential diagnosis.
Taking the drug Janine may be accompanied by irregular bleeding (both in the form of spotting and breakthrough bleeding), especially in the first months of therapy. In this regard, assessment of irregular bleeding should be carried out only after an adaptation period of approximately 3 cycles.
If such bleeding recurs or occurs after previous regular cycles, a non-hormonal cause should be sought. Diagnosis is carried out to exclude the presence of a malignant neoplasm or pregnancy . In some cases, diagnostic curettage may be required.
Janine does not protect against STDs and HIV infection.
Jeanine®
If any of the conditions, diseases or risk factors listed below currently exist, the potential risks and expected benefits of using Janine should be carefully weighed in each individual case and discussed with the woman before she decides to start taking drug. If any of these conditions, diseases or risk factors worsen, intensify or manifest for the first time, a woman should consult her doctor, who may decide whether to discontinue the drug.
— Diseases of the cardiovascular system
There is epidemiological evidence of an increased incidence of venous and arterial thrombosis and thromboembolism (such as deep vein thrombosis, pulmonary embolism, myocardial infarction, stroke) when taking COCs. These diseases are rarely observed.
The risk of developing VTE is greatest in the first year of taking such drugs. An increased risk is present after initial use of a COC or resumption of use of the same or a different COC (after a dosing interval of 4 weeks or more). Data from a large prospective study involving 3 groups of patients indicate that this increased risk is predominantly present during the first 3 months.
The overall risk of VTE in women taking low-dose COCs (<0.05 mg ethinyl estradiol) is two to three times higher than in non-pregnant patients not taking COCs, although this risk remains lower than the risk of VTE during pregnancy and childbirth.
VTE can be life-threatening or lead to death (in 1-2% of cases).
VTE, manifested as deep vein thrombosis or pulmonary embolism, can occur with the use of all COCs.
It is extremely rare when using COCs that thrombosis of other blood vessels occurs, for example, hepatic, mesenteric, renal, cerebral veins and arteries or retinal vessels.
Symptoms of deep vein thrombosis: unilateral swelling of the lower extremity or swelling along a vein in the lower extremity, pain or discomfort in the lower extremity only in an upright position or when walking, local increase in temperature in the affected lower extremity, redness or discoloration of the skin of the lower extremity.
Symptoms of pulmonary embolism: difficulty or rapid breathing; sudden cough, including with hemoptysis; sharp pain in the chest, which may intensify with deep inspiration; sense of anxiety; severe dizziness; fast or irregular heartbeat. Some of these symptoms (eg, shortness of breath, cough) are nonspecific and may be misinterpreted as signs of other more common and less severe conditions (eg, respiratory tract infection).
Arterial thromboembolism can lead to stroke, vascular occlusion, or myocardial infarction. Symptoms of a stroke include: sudden weakness or loss of sensation in the face or limbs, especially on one side of the body, sudden confusion, problems with speech and comprehension; sudden unilateral or bilateral vision loss; sudden disturbance in gait, dizziness, loss of balance or coordination; sudden, severe or prolonged headache for no apparent reason; loss of consciousness or fainting with or without an epileptic seizure. Other signs of vascular occlusion: sudden pain, swelling and slight blue discoloration of the limbs, “acute” abdomen.
Symptoms of myocardial infarction: pain, discomfort, pressure, heaviness, a feeling of compression or fullness in the chest or behind the sternum, radiating to the back, jaw, left upper limb, epigastric region; cold sweat, nausea, vomiting or dizziness, severe weakness, anxiety or shortness of breath; fast or irregular heartbeat. Arterial thromboembolism can be life-threatening or fatal.
In women with a combination of several risk factors or high severity of one of them, the possibility of their mutual reinforcement should be considered. In such cases, the degree of increase in the risk of blood clots may be higher than with a simple summation of factors. In this case, taking the drug Zhanin® is contraindicated (see section “Contraindications”).
The risk of developing thrombosis (venous and/or arterial) and thromboembolism or cerebrovascular disorders increases:
- with age;
- in smokers (with an increase in the number of cigarettes or an increase in age, the risk increases, especially in women over 35 years old);
in the presence of:
— obesity (body mass index 30 kg/m2 or more);
- family history (for example, venous or arterial thromboembolism ever in close relatives or parents under the age of 50 years). In the case of a hereditary or acquired predisposition, the woman should be examined by an appropriate specialist to decide on the possibility of taking the drug Zhanine®;
- prolonged immobilization, extensive surgery, any operation on the lower extremities or major trauma. In these cases, taking the drug Zhanin® must be stopped (in the case of a planned operation, at least four weeks before it) and not resumed for two weeks after the end of immobilization. Temporary immobilization (eg, air travel lasting more than 4 hours) may also be a risk factor for the development of venous thromboembolism, especially in the presence of other risk factors;
- dislipoproteinemia;
- arterial hypertension;
- migraine;
— diseases of the heart valves;
- atrial fibrillation.
The possible role of varicose veins and superficial thrombophlebitis in the development of VTE remains controversial.
The increased risk of thromboembolism in the postpartum period should be taken into account.
Peripheral circulatory disorders may also occur in diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn's disease or ulcerative colitis) and sickle cell anemia.
An increase in the frequency and severity of migraine during use of the drug Zhanin® (which may precede cerebrovascular disorders) is grounds for immediate discontinuation of this drug.
Biochemical indicators indicating a hereditary or acquired predisposition to venous or arterial thrombosis include the following: resistance to activated protein C, hyperhomocysteinemia, antithrombin III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant).
When assessing the risk-benefit ratio, it should be taken into account that adequate treatment of the relevant condition may reduce the associated risk of thrombosis. It should also be taken into account that the risk of thrombosis and thromboembolism during pregnancy is higher than when taking low-dose COCs (<0.05 mg ethinyl estradiol).
— Tumors
The most significant risk factor for developing cervical cancer is persistent human papillomavirus infection. There are reports of a slight increase in the risk of developing cervical cancer with long-term use of COCs. However, the connection with taking COCs has not been proven. Controversy remains regarding the extent to which these findings are related to screening for cervical pathology or to sexual behavior (lower use of barrier methods of contraception).
A meta-analysis of 54 epidemiological studies showed that there is a slightly increased relative risk of developing breast cancer (BC) diagnosed in women currently taking COCs (relative risk 1.24). The increased risk gradually disappears within 10 years of stopping these drugs. Due to the fact that breast cancer is rare in women under 40 years of age, the increase in the number of breast cancer diagnoses in women who are currently or recently taking COCs is insignificant in relation to the overall risk of this disease. Its connection with COC use has not been proven. The observed increase in the risk of developing breast cancer may be due not only to earlier diagnosis of breast cancer, but also to the biological effect of sex hormones or a combination of these two factors. Women who have ever used COCs are diagnosed with earlier stages of breast cancer than women who have never used them.
In rare cases, during the use of COCs, the development of benign, and in extremely rare cases, malignant liver tumors, which in some cases led to life-threatening intra-abdominal bleeding, was observed. If severe abdominal pain, liver enlargement, or signs of intra-abdominal bleeding occur, this should be taken into account when making a differential diagnosis.
— Other states
Women with hypertriglyceridemia (or a family history of this condition) may have an increased risk of developing pancreatitis while taking COCs.
Although slight increases in blood pressure have been described in many women taking COCs, clinically significant increases have rarely been reported. However, if a persistent clinically significant increase in blood pressure develops while taking COCs, these drugs should be discontinued and treatment of arterial hypertension should be initiated. The drug can be continued if normal blood pressure values are achieved with the help of antihypertensive therapy.
The following conditions have been reported to develop or worsen both during pregnancy and while taking COCs, but their relationship with COC use has not been proven: jaundice and/or pruritus associated with cholestasis; formation of gallstones; porphyria; systemic lupus erythematosus; hemolytic-uremic syndrome; chorea; herpes during pregnancy; hearing loss associated with otosclerosis. Cases of worsening the course of endogenous depression, epilepsy, Crohn's disease and ulcerative colitis during the use of COCs have also been described.
In women with hereditary forms of angioedema, exogenous estrogens may cause or worsen symptoms of angioedema.
Acute or chronic liver dysfunction may require discontinuation of COCs until liver function tests return to normal. Recurrence of cholestatic jaundice, which developed for the first time during a previous pregnancy or previous use of sex hormones, requires discontinuation of COC use.
Although COCs may have an effect on insulin resistance and glucose tolerance, there is usually no need to adjust the dose of hypoglycemic drugs in diabetic patients using low-dose COCs (<0.05 mg ethinyl estradiol). However, women with diabetes mellitus should be carefully monitored while taking COCs.
Chloasma can sometimes develop, especially in women with a history of pregnancy chloasma. Women with a tendency to chloasma while taking Zhanine® should avoid prolonged exposure to the sun and exposure to ultraviolet radiation.
Preclinical safety data
Preclinical data from routine repeated-dose toxicity, genotoxicity, carcinogenicity and reproductive toxicity studies do not indicate a particular risk to humans. However, it should be remembered that sex hormones can promote the growth of certain hormone-dependent tissues and tumors.
Laboratory tests
Taking the drug Zhanin® may affect the results of some laboratory tests, including indicators of liver, kidney, thyroid, adrenal function, the concentration of transport proteins in plasma, indicators of carbohydrate metabolism, parameters of blood coagulation and fibrinolysis. Changes usually do not go beyond normal values.
Reduced efficiency
The effectiveness of Zhanine® may be reduced in the following cases: missed pills, gastrointestinal disorders or as a result of drug interactions.
Frequency and severity of menstrual-like bleeding
While taking the drug Zhanine®, irregular bleeding may occur (“spotting” spotting and/or “breakthrough” uterine bleeding), especially during the first months of use. Therefore, any irregular bleeding should be assessed only after an adaptation period of approximately three cycles.
If irregular bleeding recurs or develops after previous regular cycles, careful evaluation should be performed to rule out malignancy or pregnancy.
No regular menstrual bleeding
Some women may not develop bleeding during a break in taking pills. “Contraindications” and “With caution”;
— Local compaction in the mammary gland;
- Concomitant use of other medications (see also “Interaction with other medications”);
- If prolonged immobility is expected (for example, a cast is applied to the lower limb), hospitalization or surgery is planned (at least four weeks before the proposed operation);
- Unusually heavy bleeding from the vagina;
- Missed a pill in the first week of taking the package and had sexual intercourse seven days or less before;
— Absence of regular menstrual-like bleeding two times in a row or suspicion of pregnancy (you should not start taking pills from the next package before consulting your doctor).
You should stop taking the tablets and consult your doctor immediately if there are possible signs of thrombosis, myocardial infarction or stroke: unusual cough; unusually severe pain behind the sternum, radiating to the left arm; unexpected shortness of breath, unusual, severe and prolonged headache or migraine attack; partial or complete loss of vision or double vision; slurred speech; sudden changes in hearing, smell, or taste; dizziness or fainting; weakness or loss of sensation in any part of the body; severe abdominal pain; severe pain in the lower limb or sudden swelling of any of the lower limbs.
Analogs
Level 4 ATC code matches:
Ovidon
Rigevidon
Non-Ovlon
Mercilon
Yarina Plus
Yarina
Miniziston 20 fem
Novinet
Microgynon
Lindineth
Cyclo-Proginova
Regulon
Logest
Midiana
Belara
Femoden
Jess Plus
Jess
Zoely
Generics: Zhenetten , Diecyclen , Siluet , Bonade .
Analogues of Zhanin by mechanism of action: Belara , Yarina , Dailla , Midiana , Jess , Logest , Evra , Lindinet 30 , Mercilon , Marvelon , Egestrenol , Femoden , Oralcon , Dimia .
Janine or Silhouette - which is better?
The drugs Zhanine and Silhouette are structural analogues, that is, they contain identical substances in the same dosage as active components, they have the same mechanism of action, indications and contraindications.
The products are produced by different companies and have a significant difference in price; Silhouette is about half the price of its counterpart.
Which is better: Claira or Janine?
The basis of the drug Qlaira is dienogest and estradiol valerate of 17β-estradiol produced by the human body ). Each package contains 5 types of tablets, which differ in the composition of the active ingredients and their concentration.
The ethinyl estradiol contained in the Janine dragee exhibits greater metabolic stability in contrast to the estrogenic component of Qlaira , however, it also has a more pronounced effect on the liver .
The mechanism of action of Qlaira is due to the ability of its active components to suppress ovulation and change the properties of cervical mucus. In addition, the drug reduces pain and intensity of bleeding during menstruation , prevents the development of iron deficiency anemia , and reduces the risk of developing ovarian and endometrial cancer .
Which is better: Janine or Diana 35?
Diane-35 is a combination of cyproterone (2 mg) and ethinyl estradiol (35 mcg). If Zhanine is prescribed primarily to prevent pregnancy in women with endometriosis , then the use of Diane-35 is advisable for contraception in women with pronounced signs of androgenization .
Janine or Visanne - which is better?
The drug Visanne of micronized dienogest as an active component . The drug is intended for the treatment of endometriosis. To achieve a therapeutic effect, the tablets are taken for six months.
The main indication for use of the drug Zhanine is contraception (in particular contraception in women with endometriosis ).
According to doctors and patients who were treated with both drugs, treatment of endometriosis with Janine is not always as effective as treatment with Visanne. In addition, the latter is often better tolerated and causes fewer side effects.
When wondering which drug to choose, you should remember that each woman’s body is individual, in addition, in each specific case, the indications for use may differ. In this regard, a specialist must prescribe this or that remedy.
Use with alcohol
In the instructions for the drug, the manufacturer does not give any recommendations regarding the possibility of using Janine tablets with alcoholic beverages.
The recommended dose of alcohol for a woman taking oral contraceptives is 20 grams of ethanol (a glass of wine).
However, please remember that:
- the reaction of different organisms to the same dose of alcohol may differ;
- hormonal drugs are an additional burden for the liver , which is responsible for the breakdown of ethyl alcohol (i.e., with an overdose of alcohol, the consequences can be quite serious both for the liver and for the body as a whole);
- with increased activity of liver enzymes due to an overdose of alcohol, the breakdown and elimination of substances contained in the drug are significantly accelerated (i.e., the contraceptive effect may be reduced);
- An overdose of alcohol, accompanied by vomiting, leads to the fact that the active substances of the tablets do not have time to be absorbed from the gastrointestinal tract, and as a result, the effectiveness of the drug decreases.
According to doctors, you should wait at least 3 hours between taking pills and alcoholic drinks.
During pregnancy
In the course of epidemiological studies, it was found that Janine does not increase the risk of teratogenic effects in a child whose mother took birth control pills before pregnancy or, unknowingly, in the first weeks.
However, during pregnancy, taking the drug is contraindicated.
Since combined hormonal drugs are characterized by the ability to suppress lactation and affect the composition of breast milk, nursing women are advised to refrain from taking them.
If you are planning a child, you should stop using birth control pills. Doctors advise trying to get pregnant from the beginning of a new cycle. Pregnancy usually occurs fairly quickly after taking the pills.
Reviews about Janine
Reviews about Janine on the forums are quite varied. For the most part, they are positive, since the drug is well tolerated by women, is easy to use, and copes well with its main task.
In addition, patients and doctors in reviews of Janine note that the drug, in addition to contraception, has a number of other positive side effects: it helps with acne, normalizes the menstrual cycle, and reduces the intensity and pain of bleeding. In women who stop taking Zhanine tablets due to a desire to become pregnant, pregnancy after discontinuation of the drug often occurs within the first 2-3 cycles.
Due to the drug's ability to stabilize hormonal levels and suppress foci of pathological growth of the endometrium , it is often effective for fibroids and endometriosis (usually in situations where the disease is not too advanced).
However, there are also negative reviews about the drug, which are associated either with the lack of results in the treatment of endometriosis , or with side effects that significantly worsen the woman’s quality of life. Experts are inclined to believe that such phenomena can occur if the product was selected without taking into account contraindications for use, or is simply not suitable for a particular woman.
Analyzing doctors' reviews of Zhanine birth control pills, we can conclude that experts speak positively about this drug. Such a high rating is due to the fact that, being a low-dose drug, the latter has a pronounced contraceptive effect, helps restore hormonal imbalances and has a positive effect on the general condition of a woman.
Janina price, where to buy
The price of Janine birth control pills depends on which pharmacy sells them. How much a drug costs in pharmacies is influenced by the cost of transportation and the pricing features of a particular pharmacy chain. Considering that the pills are produced by only one pharmaceutical company, there is no difference between a more expensive and a cheaper drug.
The price of Zhanin in Ukrainian pharmacies is on average 215 UAH (for package No. 21).
The cost of a package containing 21 tablets in Russian pharmacies is approximately more than 870 rubles. You can buy a package of 63 dragees for approximately 2,200 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Janine dragee 21 pcs. Bayer Weimar GmbH and Co. KG
RUB 1,027 order - Janine dragee 63 pcs. Bayer Weimar/Bayer Pharma
RUB 2,553 order
Pharmacy Dialogue
- Janine (other No. 21) Bayer
RUB 1,038 order
- Janine (other No. 21x3) Bayer
RUB 2,498 order
show more
Pharmacy24
- Janine No. 21 tablets Bayer Weimar GmbH & Co.
KG, Nimechchyna 223 UAH. order
PaniPharmacy
- Janine tablets Janine etc. No. 21 Germany, Bayer Weimar
253 UAH order
show more