A runny nose creates discomfort and brings a lot of trouble to both children and adults. It is especially dangerous for children, as it can cause hypoxia (insufficient oxygen saturation of organs and tissues) as a result of difficulty breathing. For adults, a runny nose causes no less problems and can take a chronic form, which leads to sinusitis, which is treated with unpleasant procedures.
Rinofluimucil - spray for a runny nose
The question often arises: how to rinse your nose with a runny nose and how to stop snot from your nose? The answer is on the surface - it is not necessary to rinse your nose, sometimes it is enough to spray the spray into the nasal cavity. Rinofluimucil helps fight a runny nose at the first manifestation.
Rinofluimucil has the following indications:
- Acute and subacute inflammation of the nasal mucosa, accompanied by the release of difficult-to-discharge purulent secretion;
- Swelling in the nasal cavity;
- Chronic runny nose with the formation of crusts on the nasal mucosa;
- Nasal congestion and profuse runny nose, not associated with any allergen or infection;
- Inflammation of the mucous membrane of one or more paranasal sinuses (sinusitis).
The drug helps eliminate swelling in the nose, relieves a runny nose, restores breathing and relieves pain and burning in the sinuses.
Pharmacological action of the drug Rinofluimucil:
- Anti-inflammatory. The active substance N-acetylcysteine instantly breaks the bonds of mucous and purulent-mucosal elements. This leads to liquefaction of the masses and relief of nasal congestion. This substance also eliminates the accumulation of leukocytes, which leads to the relief of inflammation in the sinuses.
- Decongestant. The substances tuaminoheptane sulfate and sympathomimetic amine contained in the drug have a vasoconstrictor effect, which, in turn, relieves swelling in the nasal cavity.
- Mucolytic. The drug helps to liquefy sputum, which makes it easier to remove from the body.
Rinofluimucil spray is available in a single form. These are bottles made of tinted glass, equipped with a spray device. The volume of the bottles is 10 ml.
Can children use Rinofluimucil? Children under six years of age are not recommended to use the drug. At this age, there is a risk of damage to the mucous membrane by the components of the drug.
The following dosage is recommended for children over three years of age:
- 1 spray into each nostril;
- No more than 3 times a day;
- No more than 4 days in a row.
Helps with nasal congestion, inflammation of the mucous membrane, profuse runny nose and swelling of the child's nasal cavity.
There is no need to use the drug for more than 1 week, as it can lead to disruption of the capillary network and cause addiction.
The dosage for adults is as follows:
- 2 sprays into each nostril;
- No more than 4 times a day;
- No more than a week of continuous use.
Rinofluimucil
Before use, you need to assemble the bottle with other components, which are supplied separately from each other in one package. For the drug Rinofluimucil, the instructions for use are as follows:
- Remove the top cap from the bottle;
- Remove the protective cap from the bottle;
- Screw the aerosol sprayer with a dispenser onto the top of the bottle;
- Open the sprayer cap;
- Activate spraying by pressing the aerosol nozzle.
The use of a medicinal product creates a risk of miscarriage. Despite the fact that it is used topically, some of the substances included in the composition enter the circulatory system and can harm the fetus. The drug can be used by pregnant women only in case of urgent need and under the strict supervision of a doctor.
Breastfeeding women are also not recommended to use Rinofluimucil. In case of emergency, it can be used, but during this period the child’s feeding should be interrupted.
Treatment with this drug should only be carried out under the strict supervision of a physician and with extreme caution in the following cases:
- When prescribing the medicine to children under 6 years of age;
- People with bronchial asthma;
- Patients with arterial hypertension;
- People diagnosed with grade 3-4 angina and other heart pathologies.
With prolonged use of the drug, the normal function of the mucous membrane of the nasal and paranasal sinuses may change. Also, using the medication for a week can cause addiction. Without prior consultation with your doctor, it is highly recommended not to exceed the recommended dose and duration of treatment.
Rinofluimucil does not have any effect on the driving of motor vehicles, so after using it you can drive a car.
Rinofluimucil can only be used by instillation into the nose.
No laboratory studies have been conducted on the interaction of the drug Rinofluimucil with alcohol.
It is necessary to use the drug after opening the bottle within 20 days. You may have a question: why can’t Rinofluimucil be used 20 days after opening? The fact is that after this period, the effectiveness of the components of the drug decreases.
The drug is incompatible with monoamine oxidase inhibitors used in the treatment of parkinsonism and narcolepsy. These drugs include Phenelzine, Nialamid, Metralindo, Befol and other similar drugs.
It is also not recommended to combine Rinofluimucil with drugs intended to lower blood pressure, as the effectiveness of the latter may decrease.
The drug Rinofluimucil has the following contraindications:
- Immunity or sensitivity of the body to a certain component that is part of the drug;
- Diseases related to the cardiovascular system;
- High blood pressure;
- Malignant tumor of adrenal tissue (pheochromocytoma);
- Pathologies of the lacrimal glands;
- Brain surgeries;
- Treatment with monoamine oxidase inhibitors and 14 days after its cessation;
- Simultaneous use with similar drugs intended for nasal use.
In answer to the question, at what age can Rinofluimucil be taken - from the age of six.
Rinofluimucil spray bottle
If the recommended doses of the drug are exceeded, the following side effects are possible:
- Cardiopalmus;
- Trembling of limbs;
- Unreasonable anxiety;
- High blood pressure;
- Getting used to the medicine.
In case of overdose in children, there is a risk of disruption of the central nervous system and a sharp increase in blood pressure. The reactions are caused by a vasoconstrictor effect and require immediate medical attention.
You can often hear a mistake when they say that Rinofluimucil is an antibiotic. But this is fundamentally wrong. It is incorrect to believe that the substance acetylcysteine contained in the drug has a bactericidal effect. This component thins mucus and makes it easier to remove from the body. Let's look at some analogues of this drug and compare their effect and composition with it.
The pharyngeal tonsil is one of the most significant elements of the lymphadenoid pharyngeal ring in childhood. Its hypertrophy and chronic inflammation, causing various clinical manifestations, including associated and concomitant diseases, are noted in approximately half of children of preschool and primary school age, and among frequently ill children, the frequency of this pathology increases to 70% [1-3]. Unlike acute adenoiditis, the main cause of which is usually respiratory viral infections, chronic adenoiditis is a polyetiological inflammatory disease, which is based on a violation of the physiological immune processes of the pharyngeal tonsil, often occurring against the background of its hyperplasia [4, 5]. The main etiological factors leading to the development of chronic adenoiditis are viral (adenoviruses, cytomegaloviruses, herpes viruses, etc.), bacterial infection, allergies, immunodeficiency states, acid-dependent pathology of the gastrointestinal tract, etc. [6-8]. Despite the fact that the main etiological factor in the development of chronic adenoiditis is difficult to identify, the most significant are frequent respiratory viral infections and chronic persistence of additional (coagulose-negative staphylococci ( S. epidermidis, S. Saprophyticus
), some types of moderately pathogenic α-hemolytic streptococci (
Str. bovis, Str. oralis, Str. sanguis, Str. suis, Str. mutans
), corynebacteria (
C. pseudodiphteriticum, C. xerosis, C. ulcerans
), hemophila (
H. influenzae, H. parainfluenzae, H. Aphrophilus
), yeast-like fungi of the genus
Candida
) and transient microorganisms, mainly cocci (
S. aureus, Str. pneumoniae, Str. pyogenes,
microorganisms of the family Enterobacteriaceae (
K. pneumoniae, K. oxytoca, E . coli
),
Moraxella
(
M. catarrhalis
),
Pseudomonas, Bacillus, Micrococcus,
etc.), with simultaneous inhibition of indigenous microflora (α-hemolytic streptococci (
Str. salivarius, Str. mitis, Str. vestibularis, Str. faecium, Str. uberis
), Neisseria (
N. sicca, N. mucosa, N. lactamica, N. flava, N. subflava
), etc.). Associations of microbes are sown more often than monoflora, and the degree of bacterial contamination reaches 104-105 CFU/ml [4, 6]. This may explain the persistent nature of the course of chronic adenoiditis and the difficulty of its treatment.
Existing treatment methods are divided into surgical and conservative. When choosing a treatment method, it is necessary to take into account that the pharyngeal tonsil is a secondary peripheral organ of the immune system and has a unique structural organization that allows it not only to function as an immune barrier, but also to carry out lymphopoiesis, providing the mucous membrane of the upper and lower respiratory tract with immune-competent cells. The active participation of the pharyngeal tonsil in the ontogenetic development of immunity determines the need for careful treatment of the organ. The absolute indication for adenotomy is only grade II-III hypertrophy of the pharyngeal tonsil, accompanied by obstructive sleep apnea syndrome or chronic purulent otitis media. Exudative otitis media, recurrent otitis media, recurrent rhinosinusitis, concomitant diseases of the nervous system are an indication only in the absence or short-term effect of conservative therapy. In this regard, in case of chronic adenoiditis, even accompanied by persistent impairment of nasal breathing, it is advisable to carry out complex conservative therapy, including irrigation methods of treatment, antibacterial drugs, topical glucocorticoids, mucolytics, immunomodulators, herbal remedies, complex homeopathic remedies, physiotherapy, etc. [6].
Despite the wide range of groups of drugs used to treat adenoiditis, a prerequisite for complex therapy is the use of irrigation therapy and antibacterial agents, which are usually prescribed empirically. In the vast majority of observations, the drugs of choice are topical antibacterial drugs, which must meet the following requirements: a wide spectrum of action on pathogenic bacterial flora, low toxicity, no inhibition of mucociliary clearance, optimal form of delivery (dosed-dose spray), good applicative properties on the nasal mucosa, Possibility of use in young children.
Antibacterial agents for topical intranasal use include the drug isofra (manufactured in France). The basis of the nasal spray is the aminoglycoside antibiotic framycetin, the content of which in 1 ml of the drug is 8000 units. Framycetin has a bactericidal effect on most gram-negative and gram-positive microorganisms that cause infections in the upper respiratory tract. It has low systemic absorption and can be used in children of any age, including the first year of life. The only contraindication to its use is hypersensitivity to framycetin and other antibiotics from the aminoglycoside group. The product is available in the form of a spray, which makes it convenient to use.
The purpose of the work is to study the effectiveness of framycetin in the treatment of chronic adenoiditis in children.
Patients and methods
The study included 67 children (35 boys and 32 girls) aged from 2.5 to 13 years (mean age 6.9±2.7 years). The criteria for inclusion in the study were clinical and anamnestic data of chronic adenoiditis: the presence of characteristic complaints (nasal congestion, nasal discharge, a feeling of mucus running down the back of the throat, night and morning cough); adenoid vegetations of I-III degrees, detected by endoscopy; smoothness and hyperemia of the mucous membrane of the pharyngeal tonsil; the presence of mucopurulent exudate on the adenoids; drainage of mucopurulent discharge down the back wall of the pharynx or its “hanging” due to the soft palate; duration of the disease is at least 1 month. To examine the nasal cavity and nasopharynx, pediatric fibrorhinolaryngoscopes “Pentax” FNL-7RP3, with a diameter of 2.5 mm, were used (the examination was carried out at the initial visit and at the final visit).
Exclusion criteria were the need for systemic antibacterial therapy at initial treatment or during the study; self-cessation of treatment or failure to appear for a follow-up examination. The need for a planned adenotomy (obstructive sleep apnea syndrome, grade III adenoid vegetations, etc.) was not a criterion for excluding a child from the study, since some children received therapy as preoperative preparation.
All patients were combined into two groups. Group 1 included 35 children (average age 6.7±2.9 years) who received topical antibacterial therapy with isofra, 1 injection into each nostril
3 times a day for 10 days; Children of group 2 (32 children, average age 7.1±2.5 years) were prescribed endonasal administration of a 2% solution of silver proteinate, 2-3 drops in each nostril 3 times a day for 10 days. Additionally, all patients were prescribed irrigation therapy with saline solution of 3-5 ml in each half of the nose before each administration of the antibacterial drug. In addition, all patients were prescribed for 2 weeks, in an age-specific dosage, a combination herbal preparation, Sinupret (Bionorica, Germany), which has secretolytic, secretomotor, anti-inflammatory and immunostimulating effects.
A follow-up examination was carried out 14 days after the start of treatment. The criteria for the effectiveness of treatment were: subjective assessment (degree of nasal congestion; the presence, nature and amount of discharge, assessed by the patient or his parents using a visual analogue scale (VAS), where 0 points is the absence of a symptom, and 5 is its maximum severity), objective assessment, which was made on the basis of nasopharyngoscopy and endoscopy of the nose and nasopharynx; the presence or absence of relapse of adenoiditis. Additionally, at the final visit, the patients’ parents assessed treatment compliance using a 3-point VAS, where a score of “3 points” corresponded to complete satisfaction with the prescribed therapy.
Results and discussion
At the time of inclusion in the study, the severity of complaints and clinical symptoms in the groups were statistically comparable (p>0.05). In particular, parents of all children complained of nasal congestion of varying severity; the severity of the symptom according to VAS was 3.5±1.27 points in group 1 and 3.4±1.48 points in group 2. Mucous and mucopurulent discharge from the nose or drainage of mucus along the back wall of the pharynx during the initial examination was noted in 25 (71.4%) patients in group 1 and in 22 (68.7%) in group 2; Parents rated the severity of symptoms according to VAS as 3.1±1.69 and 3.3±1.51 points, respectively. Night and morning cough was observed in 21 (60%) patients in the 1st group (assessment of symptom severity on the VAS scale 3.3±1.05 points) and in 23 (71.8%) in the 2nd group (assessment VAS 3.1±1.32 points). During endoscopy of the nasopharynx, grade I adenoid vegetations were detected in 6 (17.1%) patients in group 1 and in 4 (12.5%) in group 2; grade II adenoids - in 17 (48.6%) and 15 (46.8%), and grade III hypertrophy was diagnosed in 12 (34.3%) and 13 (40.6%) children, respectively.
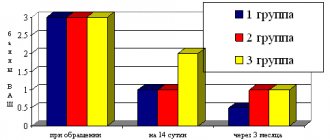
By the 14th day, 5 (14.3%) patients of group 1 and 7 (21.9%) patients of group 2 dropped out of the study. In both groups, there was a statistically significant (p <0.05) positive dynamics compared to the initial examination. Although nasal congestion persisted in 18 (60%) patients of the 1st group and 17 (68%) of the 2nd group, the severity of the symptom in patients of the 1st group was statistically significantly less (p <0.05) compared with the same indicator in children of the 2nd group, amounting to 1.4±1.35 and 1.8±1.52 VAS points, respectively. Similar results were obtained for the remaining analyzed parameters: the severity of the VAS assessment of rhinorrhea decreased to 1.1±0.95 and 1.9±1.64 points; night and morning cough - up to 1.4±0.95 and 1.9±1.32 points, respectively (see figure).
Figure 1. Dynamics of adenoiditis symptoms during treatment (according to the visual analogue scale). The assessment of therapy compliance by parents of children in group 1 was also statistically significantly higher (p<0.05): 93.3% (28 patients) in group 1 and 80% (20 patients) in group 2 gave good and satisfactory assessments. th group. During control endoscopy of the nasopharynx, grade I hypertrophy of the pharyngeal tonsil was diagnosed in 7 (23.3%) patients in group 1 and in 4 (16%) in group 2; grade II hypertrophy in 15 (50%) and 13 (48%); grade III adenoids - 8 (26.7% and 32%) patients, respectively, in each group (the difference between the groups is statistically insignificant, p>0.05). No adverse events were observed during therapy in any case.
Thus, the use of the drug isofra in the complex treatment of adenoiditis, including in children referred for adenotomy, is undoubtedly advisable, as it increases the effectiveness and compliance of treatment compared to traditional antibacterial topical drugs.