How I got asthma
My story is probably no different from thousands of others, how I acquired asthma as a child. My grandfather on my father’s side and my mother are asthmatics, so it is not surprising that an “asthmatic” child appeared in our family. Against the backdrop of frequent bronchitis and the low availability of good drugs in Soviet and post-Soviet times, they did their job, and she took root firmly in my life. Back then they were still treating with euffilin, who knows, he will remember all the horror of the side effects of this terrible drug. The doctors at the hospital managed to inject it into my butt in the form of injections. It was just pure hell - pain, despair and fear.
How did you treat asthma?
Time passed, in adolescence and later I tried to find out from doctors how to cure this damn disease? To which all my pulmonologists loudly repeated: “This cannot be cured! It’s like a disability for life!” Evil doctors!!! Therefore, having tried several drugs that caused me a lot of side effects, I completely gave up on treating my illness, I just walked around with a Ventalin or Salbutamol balloon all the time. During acute attacks of suffocation, which began with any cold or acute respiratory viral infection, I called an ambulance, they relieved the attack.
How I found out about the drug Relvar Ellipta
And then a general practitioner accidentally appeared in my life, who had a different opinion about my illness. I came to him with an attack of suffocation, he prescribed me therapy in the form of a bunch of tablets and inhalations, which are aimed specifically at relieving acute bronchospasm. And at the same time, he prescribed the drug Relvar Ellipta, which had to be sprayed once a day.
Medicine Relvar Ellipta
After the acute condition was relieved, I started using this drug, and, lo and behold! I forgot what a Salbutamol and Ventalin balloon was! Out of habit, of course, I carried it with me; the fear of bronchospasm haunted me for some time. Finding myself far from home and seeing that I don’t have a sprinkler with me - this fear has been sitting in me since childhood! At the doctor’s appointment, I asked: “Vitaly Veniaminovich, will I ever be able to cure my illness?” He replied: “Yes, but a number of conditions must be met!” There is hope for a better life here!
Relvar Ellipta powder for inhalation dosed 22 mcg + 92 mcg/dose N30
Registration Certificate Holder
GlaxoSmithKline Trading (Russia)
Dosage form
Medicine - Relvar Ellipta
Description
Powder for inhalation dosed
22 mcg + 92 mcg/dose,
white.
Strip with vilanterol (30 cells)
1 dose
* vilanterol triphenatate micronized 40 mcg, incl. the nominal amount of vilanterol is 25 mcg**, which corresponds to a delivered dose of vilanterol of 22 mcg
Excipients
: magnesium stearate - 125 mcg, lactose monohydrate - up to 12.5 mg.
Strip with fluticasone furoate (30 cells)
1 dose
* micronized fluticasone furoate 100 mcg**, which corresponds to the delivered dose of fluticasone furoate 92 mcg
Excipients
: lactose monohydrate - up to 12.5 mg.
30 doses - plastic inhalers with a dose counter (1) with two laminated aluminum strips (each with 30 cells) - multilayer containers made of aluminum foil (1) with an easy-to-open lid - cardboard packs.
* during the production of the finished drug, mixtures of active and auxiliary substances can be added to the final product in excess of up to 8% to compensate for losses when filling the cells. ** indicates the nominal amount of active substance added during the production process.
Indications
- bronchial asthma (as maintenance therapy);
- COPD (as maintenance therapy for airway obstruction in patients with COPD, including chronic bronchitis and/or emphysema). The use of Relvar Ellipta can reduce the number of exacerbations of COPD in patients with a history of repeated exacerbations.
Contraindications for use
- patients with a history of severe allergic reactions to milk protein or hypersensitivity to the active substances or any other component of the drug;
- children under 12 years of age for the treatment of bronchial asthma;
- Relvar Ellipta at a dose of 22 mcg + 184 mcg/dose is not indicated for the treatment of COPD.
With caution:
when taking sympathomimetics, including the drug Relvar Ellipta, adverse events such as arrhythmia (for example, supraventricular tachycardia and extrasystole) may be observed in the cardiovascular system. In this regard, patients suffering from severe forms of cardiovascular disease should be prescribed Relvar Ellipta with caution.
Like other medicinal products that contain corticosteroids, Relvar Ellipta should be prescribed with caution to patients with pulmonary tuberculosis, as well as to patients with chronic or untreated infections.
pharmachologic effect
Mechanism of action
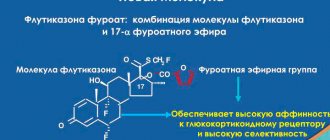
Vilanterol and fluticasone furoate belong to two different classes of drugs - a synthetic glucocorticoid and a selective long-acting beta2-agonist.
Pharmacodynamic effects
Vilanterol triphenatate
belongs to the class of selective long-acting beta2-adrenergic agonists (LABAs).
The pharmacological effects of β2-adrenergic agonists, including vilanterol triphenatate, are at least in part due to stimulation of intracellular adenylate cyclase, an enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic 3′,5′-adenosine monophosphate (cyclic AMP).
An increase in the level of cyclic AMP leads to relaxation of bronchial smooth muscles and inhibition of the release of mediators of immediate hypersensitivity reactions from cells (primarily from mast cells). Fluticasone furoate
is a synthetic trifluoride glucocorticoid with a pronounced anti-inflammatory effect. The exact mechanism of action to relieve the symptoms of bronchial asthma and chronic obstructive pulmonary disease (COPD) is unknown. Glucocorticoids have demonstrated a broad spectrum of action on various cell types (eg, eosinophils, macrophages, lymphocytes) and mediators (eg, cytokines and chemokines involved in inflammation).
Molecular interactions occur between glucocorticoids and LABAs, as a result of which steroid hormones activate the β2-adrenergic receptor gene, increasing the number of receptive adrenergic receptors. LABAs bind to the glucocorticoid receptor, providing its steroid-dependent activation and stimulating translocation into the cell nucleus. These synergistic interactions lead to enhanced anti-inflammatory activity, which has been demonstrated in in vitro and in vivo experiments with various inflammatory cells involved in the pathophysiological processes of asthma and COPD. The results of clinical studies using respiratory tract biopsies have also demonstrated the synergy of glucocorticoids and LABAs that occurs when these drugs are prescribed to patients with COPD at therapeutic doses.
Drug interactions
When the drug is prescribed in therapeutic doses, a clinically significant drug interaction with vilanterol or fluticasone furoate is considered unlikely due to the low plasma concentrations of the latter when administered by inhalation.
Beta-blockers may weaken or antagonize the effect of beta2-agonists. The simultaneous use of non-selective and selective beta-blockers should be avoided, unless their use is strictly necessary.
Vilanterol and fluticasone furoate undergo rapid primary metabolism in the liver via the cytochrome CYP3A4 isoenzyme. When prescribing the drug simultaneously with strong inhibitors of the cytochrome CYP3A4 isoenzyme (for example, ketoconazole, ritonavir), caution should be exercised, since it is possible to increase the systemic exposure of vilanterol and fluticasone furoate, which in turn may lead to an increased risk of adverse reactions.
Vilanterol and fluticasone furoate are P-gp substrates. According to the results of a clinical and pharmacological study involving healthy volunteers who were simultaneously prescribed vilanterol and a strong inhibitor of P-gp and a moderate inhibitor of the cytochrome CYP3A4 isoenzyme verapamil, no significant effect on the pharmacokinetics of vilanterol was detected. Clinical and pharmacological studies of the co-administration of a specific P-gp inhibitor and fluticasone furoate have not been conducted.
Dosage regimen
Relvar Ellipta is intended for inhalation use only.
Relvar Ellipta should be used once a day at the same time, morning or evening.
After inhalation, rinse your mouth with water without swallowing.
Bronchial asthma
The patient should be informed about the need for regular use of the drug Relvar Ellipta, even in the case of an asymptomatic course of the disease.
If symptoms of the disease occur between doses of the drug, inhaled forms of short-acting beta2-agonists should be used as emergency therapy.
The physician should regularly assess the patient's condition to ensure that the optimal dose of Relvar Ellipta is prescribed in a timely manner. The dose can be changed only on the recommendation of a doctor.
Adults and teenagers 12 years and older
Recommended dose of Relvar Ellipta:
- 1 inhalation 22 mcg+92 mcg 1 time/day
or
- 1 inhalation 22 mcg+184 mcg 1 time/day.
The starting dose of Relvar Ellipta 22 mcg + 92 mcg is prescribed to patients who require low or moderate doses of inhaled glucocorticoids used in combination with long-acting beta2-agonists.
Relvar Ellipta at a dosage of 22 mcg + 184 mcg should be prescribed to patients who require a higher dose of inhaled corticosteroids used in combination with long-acting beta2-agonists.
If the drug Relvar Ellipta at a dosage of 22 mcg + 92 mcg does not provide adequate control of the disease, the issue of increasing the dose to 22 mcg + 184 mcg is considered, which may improve the level of control over the course of bronchial asthma.
Children
The safety and effectiveness of Relvar Ellipta in children under 12 years of age has not been established.
COPD
Adults
The recommended dose of Relvar Ellipta is 1 inhalation 22 mcg + 92 mcg 1 time / day.
Relvar Ellipta at a dosage of 22 mcg + 184 mcg is not indicated for the treatment of patients with COPD.
Children
The drug is not used for COPD indications in children.
Special patient groups
Elderly patients (over 65 years old)
no dose adjustment of the drug is required.
Patients with impaired renal function
no dose adjustment of the drug is required.
According to a clinical and pharmacological study in patients with mild, moderate and severe liver dysfunction
There is a threefold increase in the degree of systemic exposure of fluticasone furoate (AUC). The drug should be prescribed with caution to patients with impaired liver function, in whom the risk of developing systemic adverse reactions caused by taking corticosteroids is higher. For patients with moderate to severe liver dysfunction, the maximum dose is 22 mcg + 92 mcg.
Recommendations for use
When using the Ellipta inhaler for the first time, there is no need to check its correct operation or special preparation of the inhaler for use. You just need to consistently follow the recommendations for use.
The Ellipta inhaler is packaged in a container. Do not open the container until you are ready to inhale the medication. When you are ready to use the inhaler, remove the cap from the container. The container contains a sachet of desiccant to reduce humidity. Do not open the sachet; it is not intended for food or inhalation and should be thrown away.
When you remove the inhaler from the container, the cap is in the closed position. Do not open it until you are ready to take the drug.
In the designated “Use By” field on the inhaler label, write the date that corresponds to the date of opening plus 6 weeks. The inhaler should not be used after this date.
Below are step-by-step instructions for using the Ellipta inhaler:
I. Read the following information before use
If you open and close the lid of the Ellipta inhaler without taking the medicine, one dose will be lost. This dose remains sealed inside the inhaler but will not be available for administration. It is impossible to accidentally receive a large dose or a double dose in one inhalation.
One dose of the drug is ready for inhalation after each opening of the lid.
The dose counter shows how many doses of the drug are left in the inhaler.
Before you start using the inhaler, the dose counter shows the number 30.
Each time the lid is opened, the number of doses decreases by 1.
When there are less than 10 doses left, half of the meter turns red.
After the last dose of the drug has been used up, half of the counter is highlighted in red and the counter shows the number 0. This means that the inhaler is empty.
If you open the lid after this, the dose counter will turn completely red.
II. Dose preparation
Do not open the cap until you are ready to take the drug. Do not shake the inhaler.
1. Lower the cover down until it clicks.
2. The dose of the drug is ready for inhalation, and to confirm this, the dose counter decreases the number of doses by one.
3. If the dose counter does not decrease the number of doses after you hear the click, then the inhaler is not ready to deliver a dose of medication. In this case, you should contact the phone number or address indicated in the section “Contact for additional information.”
4. Never shake the inhaler.
III. Inhalation of a drug
1. Hold the inhaler at some distance from your mouth. exhale as deeply as possible. Do not exhale into the inhaler.
2. Place the mouthpiece between your lips and wrap your lips tightly around it. Do not block the ventilation hole with your fingers.
3. Take one deep, long, even breath through your mouth. Hold your breath as much as possible (at least 3-4 seconds).
4. Remove the inhaler from your mouth.
5. Exhale slowly and calmly.
You may not taste or feel the medicine being delivered even if you use your inhaler correctly.
IV. Closing the inhaler and rinsing the mouth
If you want to wipe the mouthpiece, use a dry cloth before closing the lid.
1. Lift the lid all the way until the mouthpiece is completely closed.
2. After inhalation, rinse your mouth with water. This will reduce the likelihood of developing side effects such as sore throat and mouth.
If stored in the refrigerator, the inhaler should be kept at room temperature for at least one hour before use.
Overdose
Symptoms
During clinical studies, there was no evidence of overdose with the combination of vilanterol and fluticasone furoate.
An overdose of Relvar Ellipta can cause the development of symptoms and signs caused by the action of individual components of the drug and characteristic of an overdose of beta2-agonists and inhaled corticosteroids (see section "Special instructions").
Treatment
There is no specific treatment for overdose with the combination of vilanterol and fluticasone furoate. In case of overdose, symptomatic therapy is prescribed and, if necessary, appropriate monitoring of the patient is provided.
The use of cardioselective beta-blockers should be considered only in cases of severe effects of vilanterol overdose, which are clinically manifested by refractoriness to maintenance therapy. Cardioselective beta-blockers should be prescribed with caution to patients who have a history of episodes of bronchospasm.
Side effect
To determine the incidence of adverse reactions associated with taking Relvar Ellipta, data from large clinical trials among patients with COPD and bronchial asthma were used. The clinical development program for a drug for the treatment of bronchial asthma included 7034 patients who underwent a comprehensive assessment of adverse reactions. The clinical development program for a drug for the treatment of COPD included 6,237 patients who underwent a comprehensive assessment of adverse reactions.
Excluding pneumonia and fractures, the safety profiles of the drug in patients with COPD and bronchial asthma were similar. According to clinical studies, pneumonia and fractures were more often observed in patients suffering from COPD.
The adverse reactions presented below are listed according to the damage to organs and organ systems and the frequency of occurrence. The frequency of occurrence, according to the WHO classification, is determined as follows: very often (≥1/10), often (≥1/100 and <1/10), infrequently (≥1/1000 and <1/100), rarely (≥1/100) 10,000 and <1/1000), very rare (<1/10,000, including isolated cases).
Frequency of occurrence of adverse reactions
Infectious and parasitic diseases:
often - pneumonia, upper respiratory tract infections, bronchitis, influenza, candidiasis of the oral cavity and pharynx.
From the nervous system:
very often - headache.
From the cardiovascular system:
infrequently - extrasystole.
From the respiratory system:
very often - nasopharyngitis; often - oropharyngeal pain, sinusitis, pharyngitis, rhinitis, cough, dysphonia.
From the digestive system:
often - abdominal pain.
From the musculoskeletal system:
often - arthralgia, back pain, fractures.
General disorders and disorders at the injection site:
often - fever.
Post-marketing surveillance data
From the immune system:
rarely - hypersensitivity reactions, incl. anaphylaxis, angioedema, rash, urticaria.
Mental disorders:
rarely - anxiety.
From the nervous system:
rarely - tremor.
From the cardiovascular system:
rarely - rapid heartbeat, tachycardia.
From the respiratory system:
rarely - paradoxical bronchospasm.
From the musculoskeletal system:
often - muscle spasm.
special instructions
Exacerbations
The drug Relvar Ellipta is not intended for the relief of acute symptoms of bronchial asthma or exacerbation of COPD; in such cases, the prescription of short-acting bronchodilators is required. An increase in the frequency of taking short-acting bronchodilators to relieve symptoms indicates a deterioration in disease control and the need to consult a doctor.
Patients with bronchial asthma or COPD should not stop treatment with Relvar Ellipta without medical supervision, because discontinuation of therapy may lead to resumption of symptoms.
During treatment with Relvar Ellipta, adverse events associated with the course of bronchial asthma or exacerbation of the disease may develop. Patients should be advised to continue treatment. If the disease is not controlled or the condition worsens after starting therapy with Relvar Ellipta, consultation with a doctor is necessary.
Paradoxical bronchospasm
As with other types of inhalation therapy, paradoxical bronchospasm may develop after taking the drug, accompanied by a rapid increase in wheezing. In this case, emergency prescription of a short-acting inhaled bronchodilator and immediate discontinuation of the drug Relvar Ellipta are indicated. The patient should be examined by a physician and, if necessary, alternative therapy may be prescribed.
Liver dysfunction
Patients with moderate to severe liver dysfunction should be prescribed a dose of 22 mcg + 92 mcg, and such patients should be under medical supervision to monitor systemic adverse reactions associated with the use of GCS.
Systemic effects of GCS
When using inhaled corticosteroids (especially with long-term use in high doses), systemic adverse reactions may develop. Such adverse reactions develop much less frequently than with oral administration of GCS. Manifestations of possible adverse systemic effects include: suppression of the function of the hypothalamic-pituitary-adrenal axis, decreased bone mineral density, slowed growth rate in children and adolescents, cataracts and glaucoma.
Pneumonia
In patients with COPD receiving the drug Relvar Ellipta, there was an increase in the incidence of pneumonia, as well as the incidence of severe forms of pneumonia requiring hospitalization of the patient. In some cases, clinical episodes of pneumonia were fatal. Physicians should be aware of the possibility of pneumonia in patients with COPD, not forgetting that the clinical signs of such an infectious disease are masked by symptoms of exacerbation of COPD. The following groups of patients with COPD have a higher risk of developing pneumonia while taking Relvar Ellipta: smoking patients, patients who have previously had pneumonia, patients with a BMI <25 kg/m2 and patients with a forced expiratory volume (FEV1) <50% of predicted value . When prescribing therapy with Relvar Ellipta, the above factors should be taken into account; if pneumonia occurs, treatment should be reconsidered.
In patients with bronchial asthma, cases of pneumonia were observed infrequently. Patients with asthma who received Relvar Ellipta 22 mcg+184 mcg/dose may have had a higher risk of developing pneumonia compared with patients who received the lower dose of Relvar Ellipta (22 mcg+92 mcg/dose), or with the placebo group. Risk factors have not been established.
During clinical trials in patients suffering from COPD, a low incidence of bone fractures was revealed in all treatment groups, but in all groups receiving the combination of vilanterol and fluticasone furoate, it was slightly higher (2%) than in the group receiving the combination of vilanterol and fluticasone furoate. receiving vilanterol 22 mcg monotherapy (<1%).
Effect on the ability to drive vehicles and operate machinery.
Studies have not been conducted to study the effect of the drug Relvar Ellipta on the ability to drive vehicles and operate machinery. Based on the pharmacology of vilanterol or fluticasone furoate, an adverse effect of the drug on these activities is not expected.
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 25°C.
Best before date
The shelf life of an unopened aluminum container is 2 years; opened aluminum container - 6 weeks.
Do not use after the expiration date stated on the package.
Use during pregnancy and breastfeeding
Restrictions during pregnancy - With caution. Restrictions when breastfeeding - Contraindicated.
Fertility
There are no data on the effect on human fertility. Preclinical studies did not reveal any effects of vilanterol and fluticasone furoate on fertility.
Pregnancy
Data on the use of the drug during pregnancy are limited.
The use of Relvar Ellipta in pregnant women is permissible only if the potential benefit to the mother outweighs the possible risk to the fetus.
Breastfeeding period
There is insufficient data on the excretion of vilanterol or fluticasone furoate or their metabolites into human breast milk. However, other glucocorticoids and beta2-agonists are detected in breast milk. The risk of the drug entering the body of a newborn or child along with milk cannot be excluded.
Taking into account the ratio of the benefits of therapy for the mother and breastfeeding for the child, it is necessary to decide whether to discontinue the drug or stop breastfeeding.
Use for renal impairment
Restrictions for impaired renal function - No restrictions. Patients with impaired renal function do not require individual selection of the dose of the drug.
Use for liver dysfunction
Limitations for impaired liver function - With caution. The drug should be prescribed with caution to patients with impaired liver function, who are at higher risk of developing systemic adverse reactions caused by taking glucocorticosteroids.
Use in elderly patients
Restrictions for elderly patients - No restrictions.
Patients over 65 years of age do not require individual selection of the dose of the drug.
Use in children
Restrictions for children - With caution.
The use of the drug in children under 12 years of age is contraindicated.
Terms of sale
The drug is available with a prescription.
Contacts for inquiries
GlaxoSmithKline Trading JSC (Russia)
125167 Moscow Leningradsky Prospekt, 37a, bldg. 4 BC "Arcus III" Tel. Fax
Pros of using Relvar Ellipta
I have been using the drug Relvar Ellipta for two years at the time of writing this article. During this period, the attack occurred only once. It was a stupid coincidence and my carelessness. My husband and I moved to live in Anapa , but later, a year later, in late autumn, we returned to Kuzbass and stayed for the winter. The change in climate from warm to cold, plus smog from factories, plus FLU, brought me down, I even had to increase the dosage of the drug. But at the moment, I use the drug even every other day, it’s enough for me. By the way, when we lived in Anapa, I used Relvar Ellipta once every five days, while jogging and running 5-7 kilometers!
Relvar Ellipta
Suction
The absolute bioavailability of vilanterol and fluticasone furoate after inhalation administration of the combination of vilanterol and fluticasone furoate averaged 27.3% and 15.2%, respectively. The oral bioavailability of vilanterol and fluticasone furoate was low, averaging <2% and 1.26%, respectively. Taking into account the low oral bioavailability, the systemic effect of vilanterol and fluticasone furoate after inhalation is primarily due to the absorption of the portion of the inhalation dose that reaches the lungs.
Distribution
After intravenous administration, vilanterol and fluticasone furoate are actively distributed in the body, with average volumes of distribution at steady state being 165 L and 661 L, respectively.
Both substances - vilanterol and fluticasone furoate - have a low ability to bind to red blood cells. in vitro studies
The binding of vilanterol and fluticasone furoate to human plasma proteins was high and reached an average of 93.9% and > 99.6%, respectively.
The degree of binding to plasma proteins in vitro
was not reduced in patients with impaired liver or kidney function.
Although vilanterol and fluticasone furoate are P-glycoprotein (P-gp) substrates, when a combination of vilanterol and fluticasone furoate is administered concomitantly with P-gp inhibitors, changes in the systemic exposure of vilanterol or fluticasone furoate are considered unlikely, since both substances are well absorbed ability.
Metabolism
Based on these in vitro experiments, it can be concluded that the main metabolic pathways of vilanterol and fluticasone furoate in the human body are primarily mediated by the CYP3A4 isoenzyme.
Vilanterol is predominantly metabolized by O-dealkylation to form a number of metabolites with significantly lower beta1- and beta2-adrenomimetic activity.
Fluticasone furoate is predominantly metabolized by hydrolysis of the S-fluoromethylcarbothioate group to form metabolites with significantly lower glucocorticosteroid activity.
A clinical study was conducted to evaluate drug interactions with the CYP3A4 isoenzyme during repeated administration of a combination of vilanterol and fluticasone furoate (22 mcg + 184 mcg/dose) and a strong CYP3A4 isoenzyme inhibitor, ketoconazole (400 mg) in healthy volunteers. Co-administration of vilanterol with fluticasone furoate and ketoconazole resulted in an increase in the mean area under the pharmacokinetic curve (AUC(0-24)) and mean maximum concentration (Cmax) of fluticasone furoate by 36% and 33%, respectively. Increased exposure to fluticasone furoate was associated with a 27% decrease in mean serum cortisol concentrations measured over the 0-24 hour period.
Co-administration of vilanterol and fluticasone furoate with ketoconazole resulted in an increase in the mean AUC(0-t) and Cmax values of vilanterol by 65% and 22%, respectively. Increased exposure to vilanterol did not result in increased systemic effects of beta-agonists on heart rate, blood potassium, or corrected QT interval (QTcF).
Removal
After oral administration of fluticasone furoate in humans, it was metabolized primarily to form metabolites that were predominantly excreted through the gastrointestinal tract, with the exception of <1% radioactive dose excreted by the kidneys. The plasma half-life for fluticasone furoate after inhalation administration of the drug averaged 24 hours.
After oral administration, vilanterol in humans was mainly metabolized to form metabolites that were excreted by the kidneys and intestines in a ratio of approximately 70% and 30% of the radioactive dose, respectively. The plasma half-life for vilanterol after inhalation of the combination of vilanterol and fluticasone furoate averaged 2.5 hours.
Special patient groups
During the third phase of clinical trials, a population-based meta-analysis was conducted on the pharmacokinetics of vilanterol and fluticasone furoate in patients with bronchial asthma or COPD. A population pharmacokinetic analysis assessed the effect of demographic covariates (age, sex, weight, body mass index (BMI), race and ethnicity) on the pharmacokinetics of vilanterol and fluticasone furoate.
Race
Patients with asthma or COPD of East Asian, Japanese and Southeast Asian races (12-14% of patients) had on average higher AUC(0-24) scores (no more than 53% higher) compared with Caucasian patients. However, these populations did not show evidence of higher systemic exposure associated with greater effects on renal cortisol excretion over a 24-hour period. In patients suffering from COPD, the influence of race on the pharmacokinetic parameters of vilanterol was not detected.
On average, vilanterol Cmax was 220-287% higher and AUC(0-24) was comparable in Asian patients compared to other racial groups. However, higher Cmaxvilanterol did not have a clinically significant effect on heart rate.
Children
For adolescents (12 years of age or older), there are no recommendations for changing the dosage regimen.
The pharmacokinetics of the combination of vilanterol and fluticasone furoate in patients under 12 years of age have not been studied. The safety and effectiveness of the combination of vilanterol and fluticasone furoate in children under 12 years of age have not yet been established.
Elderly patients
The effect of age on the pharmacokinetics of vilanterol and fluticasone furoate was studied in phase 3 clinical studies that included patients with COPD and bronchial asthma.
In patients with bronchial asthma, no evidence of the influence of age (12-84 years) on the pharmacokinetic profile of fluticasone furoate and vilanterol was found.
Despite an increase (37%) in the AUC(0-24) of vilanterol in patients with COPD in the age range from 41 to 84 years, there was no evidence of an effect of patient age on the pharmacokinetic profile of fluticasone furoate. In an elderly patient (aged 84 years) with a low body weight (35 kg), the AUC(0-24) of vilanterol is predicted to be 35% higher than that calculated for the population (average COPD patient aged 60 years and weighing body 70 kg), while Cmax of vilanterol will remain unchanged. It is unlikely that these differences are clinically relevant.
Patients with impaired renal function
According to a clinical pharmacological study for vilanterol and fluticasone furoate, severe renal impairment (creatinine clearance < 30 ml/min) does not lead to a significant increase in the systemic exposure of vilanterol or fluticasone furoate or to the development of more pronounced systemic effects of glucocorticosteroids or beta2-agonists compared with healthy volunteers. No dose adjustment is required for patients with impaired renal function.
The effect of hemodialysis has not been studied.
Patients with liver dysfunction
After repeated dosing of the combination of vilanterol and fluticasone furoate for 7 days in patients with impaired liver function (according to the Child-Pugh classification of cirrhosis stages A, B or C), an increase in systemic exposure to fluticasone furoate was observed (measured by AUC (0-24 ) up to three times) compared to healthy volunteers. An increase in systemic exposure to fluticasone furoate (when prescribing a combination of vilanterol and fluticasone furoate at a dosage of 22 mcg + 184 mcg/dose) in patients with moderate liver dysfunction (Child-Pugh stage B) was accompanied by a decrease in serum cortisol concentrations by an average of 34 % compared to healthy volunteers.
In patients with severe hepatic impairment (Child-Pugh stage C) receiving the lower dose of 11 mcg + 92 mcg, no decrease in serum cortisol concentrations was observed. For patients with moderate to severe liver dysfunction, the maximum dose is 22 mcg + 92 mcg (see section "Dosage and Administration").
After repeated administration of the combination of vilanterol and fluticasone furoate for 7 days in patients with mild, moderate or severe hepatic impairment (Child-Pugh stages A, B and C), there was no significant increase in the systemic exposure of vilanterol (Cmax and AUC).
Compared with healthy volunteers, patients with mild to moderate hepatic impairment (taking vilanterol 22 mcg) or severe hepatic impairment (taking vilanterol 11 mcg) did not experience clinically significant beta-adrenergic systemic effects (changes in heart rate or concentration). serum potassium) caused by taking a combination of vilanterol and fluticasone furoate.
Gender, body weight, body mass index (BMI)
According to the results of a population pharmacokinetic analysis of data from the third phase of clinical trials, which included 1213 patients with bronchial asthma (712 women) and 1225 patients with COPD (392 women), no evidence of influence of gender, body weight or BMI on the pharmacokinetic profile of fluticasone furoate was found.
According to a population pharmacokinetic analysis involving 856 patients with bronchial asthma (500 women) and 1091 patients with COPD (340 women), no evidence of the influence of gender, body weight or BMI on the pharmacokinetic profile of vilanterol was found.
No dose adjustment is required based on gender, weight or BMI.
What is the dosage of Relvar Ellipta?
This drug has two dosages 22mcg+92mcg and 22mcg+184mcg . This means that the active substance Vilanterol contains the same amount in both cases - 22 µg, and the substance Fluticasone furoate is contained in one case 92 µg, and in the second - 184 µg. Which is very important to know, because one dosage of 22+184 is not equal to a dosage of 22+94!!!
Relvar Ellipta large dosage 22mcg+184mcg
Why do you need an increased dosage of Relvar? In my case, my doctor prescribed a low dosage, but warned me that in the fall, winter or spring, I may need to take a higher dosage to alleviate my asthma. It is also necessary during acute respiratory diseases. Or when your favorite factories began releasing a lot of caustic exhaust into the atmosphere. In short, I take higher dosages under unfavorable circumstances that may cause uncontrolled bronchospasm. Consult your doctor, maybe he will prescribe a regimen for you so that you can breathe normally, deeply, in any situation.
Relvar Ellipta por d/ing 22 mcg+184 mcg/dose 30 doses No. 1 (inhaled)
Suction
The absolute bioavailability of vilanterol and fluticasone furoate after inhalation administration of the combination of vilanterol and fluticasone furoate averaged 15.2 and 27.3%, respectively. The oral bioavailability of both substances was low and averaged 1.26 and
Distribution
After IV administration, vilanterol and fluticasone furoate are actively distributed in the body, with average Vss being 165 and 661 L, respectively. Both substances have a low ability to bind to red blood cells. In in vitro studies, the binding of vilanterol and fluticasone furoate to human plasma proteins was high and averaged >93.9 and 99.6%, respectively. The degree of binding to plasma proteins in vitro was not reduced in patients with impaired liver or kidney function. Although vilanterol and fluticasone furoate are P-glycoprotein (P-gp) substrates, when co-administering a combination of vilanterol and fluticasone furoate with P-gp inhibitors, a change in the systemic exposure of vilanterol or fluticasone furoate is considered unlikely, because both substances have good absorption capacity.
Metabolism
Based on in vitro experiments, it can be concluded that the key metabolic pathways of vilanterol and fluticasone furoate in the human body are primarily mediated through the cytochrome CYP3A4 isoenzyme.
Vilanterol is predominantly metabolized by O-dealkylation to form a number of metabolites with significantly lower beta1- and beta2-adrenomimetic activity.
Fluticasone furoate is predominantly metabolized by hydrolysis of the S-fluoromethylcarbothioate group to form metabolites with significantly lower GCS activity.
A clinical study of drug interactions with the cytochrome CYP3A4 isoenzyme was conducted with long-term administration of a combination of vilanterol and fluticasone furoate (22 + 184 mcg/dose) and a strong inhibitor of the cytochrome CYP3A4 isoenzyme - ketoconazole (400 mg) - using healthy volunteers as an example. Coadministration of the drugs resulted in an increase in the mean AUC0-24 and mean Cmax of fluticasone furoate by 36 and 33%, respectively. Increased exposure to fluticasone furoate was associated with a 27% decrease in mean serum cortisol concentrations measured over a period of 0–24 hours. Coadministration of the combination of vilanterol and fluticasone furoate and ketoconazole resulted in an increase in mean vilanterol AUC0-t and Cmax by 65 and 22%, respectively. Increased exposure to vilanterol did not lead to an increase in the systemic effects characteristic of beta-agonists - effects on heart rate, blood potassium levels, or corrected QT interval (QTcF).
Removal
After oral administration of fluticasone furoate in the human body was mainly metabolized to form metabolites, which were predominantly excreted through the gastrointestinal tract, with the exception of the dose of radioactive substance
Following oral administration, vilanterol was primarily metabolized in humans to form metabolites that were excreted in urine and feces at approximately 70% and 30% of the radioactive dose, respectively. T1/2 of vilanterol plasma after inhalation of the drug averaged 2.5 hours.
Special patient groups
During the third phase of clinical trials, a population-based meta-analysis of the pharmacokinetics of vilanterol and fluticasone furoate in patients with bronchial asthma and COPD was conducted. This analysis assessed the effect of demographic covariates (age, sex, weight, body mass index (BMI), race and ethnicity) on the pharmacokinetics of vilanterol and fluticasone furoate.
Race.
The AUC0–24 of fluticasone furoate was assessed in elderly patients with asthma or COPD. According to the data obtained, patients of East Asian, Japanese and South Asian races (12-14% of patients) had on average higher AUC0-24 scores (no more than 53%) compared to Caucasian patients. However, in these populations there was no evidence of higher systemic exposure manifested by a greater effect on urinary cortisol excretion over a 24-hour period. In patients suffering from COPD, the influence of race on the pharmacokinetic parameters of vilanterol was not detected. On average, vilanterol Cmax was 220% to 287% higher and AUC0-24 was comparable in Asian patients to other racial groups. However, the higher Cmax of vilanterol did not have a clinically significant effect on heart rate.
Children.
For adolescents (12 years of age or older), there are no recommendations for changing the dosage regimen. The pharmacokinetics of the combination of vilanterol and fluticasone furoate in patients under 12 years of age have not been studied. The safety and effectiveness of the combination of vilanterol and fluticasone furoate in children under 12 years of age have not yet been established.
Elderly patients.
The effect of age on the pharmacokinetics of vilanterol and fluticasone furoate was studied in phase 3 clinical studies that included patients with COPD and bronchial asthma. In patients with bronchial asthma, there was no evidence of an effect of age (12–84 years) on the pharmacokinetic profile of fluticasone furoate and vilanterol. Despite an increase (37%) in the AUC0-24 of vilanterol in patients with COPD throughout the observed age range from 41 to 84 years, there was no evidence of an effect of patient age on the pharmacokinetic profile of fluticasone furoate. In an elderly patient (aged 84 years) with a low body weight (35 kg), the AUC0-24 of vilanterol would be 35% higher than that calculated for the population (average COPD patient aged 60 years and weighing 70 kg), in while the Cmax of vilanterol will remain unchanged. It is unlikely that these differences are clinically relevant.
Renal dysfunction.
According to a clinical and pharmacological study, severe renal impairment (Cl creatinine
Liver dysfunction.
After continuous administration of the combination of vilanterol and fluticasone furoate for 7 days, patients with hepatic impairment experienced up to a threefold increase in systemic exposure to fluticasone furoate (as measured by AUC0-24) compared with healthy volunteers (according to the Child-Pugh classification of liver cirrhosis: stages cirrhosis A, B or C). An increase in systemic exposure to fluticasone furoate (when prescribing a combination of vilanterol and fluticasone furoate at a dosage of 22 + 184 mcg / dose) in patients with moderate hepatic impairment (Child-Pugh stage B) was associated with a decrease in serum cortisol concentrations by an average of 34 % compared to healthy volunteers. Dose-normalized systemic exposure to fluticasone furoate was similar in patients with moderate to severe hepatic impairment (Child-Pugh stages B and C). Therefore, although patients with impaired liver function do not require individual dosing, caution should be exercised when prescribing this drug to them. After continuous administration of the combination of vilanterol and fluticasone furoate for 7 days in patients with mild, moderate or severe hepatic impairment (Child-Pugh stages A, B and C), there was no significant increase in the systemic exposure of vilanterol (as measured by Cmax and AUC0- 24). Compared with healthy volunteers, patients with mild to moderate hepatic impairment (vilanterol 22 mcg) or severe hepatic impairment (vilanterol 11 mcg) did not experience clinically significant beta-adrenergic systemic effects (changes in heart rate or serum potassium concentrations). ) caused by taking a combination of vilanterol and fluticasone furoate.
Gender, body weight, BMI.
According to the third phase of population pharmacokinetic analysis, which included 1213 patients with bronchial asthma (712 women) and 1225 patients with COPD (392 women), there was no evidence of the influence of gender, body weight or BMI on the pharmacokinetic profile of fluticasone furoate. According to a population pharmacokinetic analysis involving 856 patients with bronchial asthma (500 women) and 1091 patients with COPD (340 women), there was no evidence of an effect of gender, body weight or BMI on the pharmacokinetic profile of vilanterol. Individual dose selection is not required based on gender, body weight or BMI.
What does Relvar Ellipta contain?
I am not a pharmacist, but I know that this is a glucocorticosteroid drug, it has anti-inflammatory and bronchodilator effects. There are two dosages. The tablet lists the active and excipients.
| 1 dose contains | |
| Vilanterol | 22 mcg |
| Fluticasone furoate | 92 mcg or 184 mcg |
| Magnesium stearate | 125 mcg |
| Lactose monohydrate | up to 12.5 mg |
composition of the drug
Guide to using Relvar Ellipta
Instructions for use RELVAR™ ELLIPTA™ (RELVAR™ ELLIPTA™)
Mechanism of action
Fluticasone furoate and vilanterol are two classes of drugs (synthetic corticosteroid and long-acting selective β2-receptor agonist).
Pharmacodynamic action
Fluticasone furoate
Fluticasone furoate is a synthetic trifluorinated corticosteroid with pronounced anti-inflammatory effects. The exact mechanism of action of fluticasone furoate on symptoms of asthma and chronic obstructive pulmonary disease (COPD) is unknown. Corticosteroids are known to have broad effects on many cell types (eg, eosinophils, macrophages, lymphocytes) and mediators (eg, cytokines and chemokines involved in the inflammatory process).
Vilanterol triphenatate
Vilanterol triphenatate is a selective, long-acting beta2-agonist (LABA).
The pharmacological action of β2-adrenergic agonists, including vilanterol triphenatate, is at least in part due to stimulation of intracellular adenyl cyclase, an enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic 3',5'-adenosine monophosphate (cyclic AMP). Elevated levels of AMP cause relaxation of bronchial smooth muscle and inhibition of the release of immediate hypersensitivity mediators from cells, especially mast cells.
A molecular interaction occurs between corticosteroids and LABAs, causing the steroids to activate the β2 receptor gene, thereby increasing the number and sensitivity of the receptors. LABAs prepare the glucocorticoid receptor for steroid-dependent activation and enhance nuclear translocation in cells. These synergistic interactions are reflected in the enhanced anti-inflammatory effects observed in vitro and in vivo in a number of inflammatory cells relevant to the pathophysiology of both asthma and COPD. Fluticasone furoate and vilanterol airway biopsy studies also provided evidence of synergy between corticosteroids and LABAs at clinical doses in patients with COPD.
Clinical efficacy and safety
Asthma
Three randomized, double-blind, phase III studies (HZA106827, HZA106829, and HZA106837) of varying duration assessed the safety and efficacy of fluticasone furoate/vilanterol in adults and adolescents with persistent asthma. All patients had been taking an ICS (inhaled corticosteroid) with or without a LABA for at least 12 weeks prior to Visit 1. In Study HZA106837, all patients had at least one exacerbation in the year preceding Visit 1 that required oral corticosteroid therapy. The duration of the HZA106827 study was 12 weeks. The study assessed the effectiveness of the combination of fluticasone furoate/vilanterol 92/22 mcg [n=201] and fluticasone furoate 92 mcg [n=205]) compared with placebo [n=203]. All drugs were taken 1 time/day. The duration of the HZA106829 study was 24 weeks. The study assessed the effectiveness of the combination of fluticasone furoate/vilanterol 184/22 mcg [n=197] and fluticasone furoate 184 mcg [n=194]) when used once a day compared with fluticasone propionate 500 mcg twice a day [n= 195].
In studies HZA106827/HZA106829, the combined primary efficacy endpoints were change from baseline in trough FEV1 at the clinical visit (pre-bronchodilator and pre-dosing) at the end of the treatment period in all patients and the weighted mean of serial FEV1 at 0 -24 hours post-dose, calculated for the subset of patients at the end of the treatment period. Change in percentage of 24-hour rescue drug-free periods from baseline was the secondary endpoint. Results for the primary and key secondary endpoints of these studies are shown in Table 1.
Table 1. Results for primary and key secondary endpoints of studies HZA106827 and HZA106829
| Study No. | HZA106829 | HZA106827 | ||
| Dose of FF/VI* (mcg) | FF/VI 184/22 1 time/day and FF 184 1 time/day | FF/VI 184/22 1 time/day and FP 500 2 times/day | FF/VI 92/22 1 time/day and FF 92 1 time/day | FF/VI 92/22 1 time/day and placebo 1 time/day |
| Change in trough FEV1 with last observation carried forward (LOC) from baseline | ||||
| Differences between drugs P value (95% CI) | 193 ml p<0.001 (108, 277) | 210 ml p<0.001 (127, 294) | 36 ml p=0.405 (-48, 120) | 172 ml p<0.001 (87, 258) |
| Weighted mean of serial FEV1 0-24 hours after dosing | ||||
| Differences between drugs P value (95% CI) | 136 ml p=0.048 (1, 270) | 206 ml p=0.003 (73, 339) | 116 ml p=0.06 (-5, 236) | 302 ml p<0.001 (178, 426) |
| Change in percentage of 24-hour periods without rescue medications from baseline | ||||
| Differences between drugs P value (95% CI) | 11.7% p<0.001 (4.9, 18.4) | 6.3% p=0.067 (-0.4, 13.1) | 10.6% p<0.001 (4.3, 16.8) | 19.3% p<0.001 (13.0, 25.6) |
| Change in percentage of 24-hour symptom-free periods from baseline | ||||
| Differences between drugs | 8.4% | 4.9% | 12.1% | 18.0% |
| P value (95% CI) | p=0.010 (2.0, 14.8) | p=0.137 (-1.6, 11.3) | p<0.001 (6.2, 18.1) | p<0.001 (12.0, 23.9) |
| Change in morning peak expiratory flow from baseline | ||||
| Differences between drugs P value (95% CI) | 33.5 l/min p<0.001 (22.3, 41.7) | 32.9 l/min p<0.001 (24.8, 41.1) | 14.6 l/min p<0.001 (7.9, 21.3) | 33.3 l/min p<0.001 (26.5, 40.0) |
| Change in evening peak expiratory flow from baseline | ||||
| Differences between drugs P value (95% CI) | 30.7 l/min p<0.001 (22.5, 38.9) | 26.2 l/min p<0.001 (18.0, 34.3) | 12.3 l/min p<0.001 (5.8, 18.8) | 28.2 l/min p<0.001 (21.7, 34.8) |
*FF/VI = fluticasone furoate/vilanterol combination
FP = fluticasone propionate
Study HZA106837 used varying treatment durations (minimum duration 24 weeks, maximum duration 76 weeks; most patients received a minimum duration of treatment of 52 weeks). In study HZA106837, patients were randomized to receive fluticasone furoate/vilanterol 92/22 mcg [n=1009] or FF 92 mcg [n=1010]. Both drugs were taken 1 time/day. In study HZA106837, the primary endpoint was time to first severe asthma exacerbation. Severe asthma exacerbation was defined as worsening asthma requiring use of systemic corticosteroids for at least 3 days, inpatient hospitalization, or emergency department visit due to asthma requiring use of systemic corticosteroids. The adjusted mean change from baseline in trough FEV1 was also assessed as a secondary endpoint.
In study HZA106837, the risk of severe asthma exacerbation in patients receiving fluticasone furoate/vilanterol 92/22 mcg was reduced by 20% compared with patients receiving FF 92 mcg alone (hazard ratio 0.795, p=0.036 95% CI 0.642 , 0.985). The rate of severe asthma exacerbation per patient per year was 0.19 in the FF 92 mcg group (approximately 1 in every 5 years) and 0.14 in the fluticasone furoate/vilanterol 92/22 mcg group (approximately 1 in every 7 years). The ratio of the incidence of exacerbations when taking the combination of fluticasone furoate/vilanterol 92/22 mcg and FF 92 mcg was 0.755 (95th CI 0.603, 0.945). It follows that the reduction in the incidence of severe asthma exacerbations in patients receiving the combination of fluticasone furoate/vilanterol 92/22 mcg was 25% compared to patients receiving FF 92 mcg (p = 0.014). The 24-hour bronchodilator effect of the fluticasone furoate/vilanterol combination was maintained throughout the 1-year treatment period. There were no signs of loss of effectiveness (tachyphylaxis was absent). When using the combination of fluticasone furoate/vilanterol 92/22 mcg, there was a sustained improvement in trough FEV1 from 83 ml to 95 ml at 12, 36 and 52 weeks, as well as at the end point compared with indicators when using FF 92 µg (p<0.001 95% CI 52, 126 ml at end point). In 44% of patients in the fluticasone furoate/vilanterol 92/22 mcg group, asthma was well controlled (ACQ7 ≤0.75) at the end of therapy. In patients in the FF 92 mcg group, this figure was 36% (p<0.001 95% CI 1.23, 1.82).
Comparative studies with salmeterol/fluticasone propionate combination
In a 24-week study (HZA113091) in adults and adolescents with persistent asthma, improvements in pulmonary function compared to baseline were observed with both fluticasone furoate/vilanterol 92/22 mcg once daily in the evening and with salmeterol. /FP 50/250 mcg 2 times/day. Adjusted mean increases in weighted mean FEV1 over the period 0-24 hours of 341 ml during therapy compared to baseline (fluticasone furoate/vilanterol combination) and 377 ml (salmeterol/FP combination) indicated an overall improvement in pulmonary function over the course of treatment. 24 hours when using both types of therapy. The adjusted mean difference by treatment between groups of 37 mL was not statistically significant (p=0.162). In terms of trough FEV1, patients in the fluticasone furoate/vilanterol group experienced a change in mean limit from baseline of 281 mL, and patients in the salmeterol/FP group experienced a change of 300 mL from baseline; (adjusted mean difference 19 ml (95% CI:
- -0.073, 0.034) was not statistically significant (p=0.485).
Comparative studies with salmeterol/FP or other ICS/LABA combinations have not been conducted to adequately compare the effects on asthma exacerbations.
Fluticasone furoate monotherapy
A randomized, double-blind, placebo-controlled, 24-week study (FFA112059) assessed the safety and efficacy of FF 92 mcg once daily [n=114] and FF 250 mcg twice daily [n=114] compared with placebo [n =115] in adult patients and adolescents with persistent asthma. All patients were required to have received a stable dose of ICS for at least 4 weeks prior to Visit 1 (screening visit). LABA use was not permitted for 4 weeks after Visit 1. The primary efficacy endpoint was the change from baseline in trough FEV1 at the clinical visit (pre-bronchodilator and pre-dosing) at the end of the treatment period. Change in percentage of 24-hour treatment-free periods from baseline over the 24-week treatment period was a secondary endpoint. At the 24 week time point, FF and AF increased trough FEV1 by 146 ml (95% CI 36, 257 ml, p=0.009) and 145 ml (95% CI 33, 257 ml, p=0.011), respectively. , compared to placebo. Both drugs (FF and FP) increased the percentage of 24-hour treatment-free periods by 14.8% (95% CI 6.9, 22.7, p<0.001) and 17.9% (95% CI 10.0, 25.7, p<0.001 ), respectively, compared to placebo.
Study of the reaction to a provocative test with an allergen
The bronchoprotective effects of the fluticasone furoate/vilanterol 92/22 mcg combination on early and late asthmatic responses to inhaled allergen were assessed in a placebo-controlled, four-period, multiple-dose crossover study (HZA113126) in patients with mild asthma. Patients were randomized to receive a combination of fluticasone furoate/vilanterol 92/22 mcg, FF 92 mcg, vilanterol 22 mcg, or placebo once daily for 21 days; 1 hour after the last dose, an allergen inhalation test was performed. The following allergens were used:
- house dust mite, cat dander or birch pollen. The selection was made on the basis of screening tests. Serial FEV1 measurements were compared with those obtained after saline inhalation before the allergen test (baseline). Overall, the greatest effect on early asthmatic response was observed with the fluticasone furoate/vilanterol 92/22 mcg combination compared with FF 92 mcg or vilanterol 22 mcg monotherapy. Both the combination of fluticasone furoate/vilanterol 92/22 μg and FF 92 μg virtually eliminated the late asthmatic response compared with vilanterol monotherapy. The use of the fluticasone furoate/vilanterol 92/22 mcg combination provided significantly greater protection against allergen-induced bronchial hyperreactivity than FF or vilanterol monotherapy. Assessment was carried out on Day 22 using a methacholine challenge.
Chronic obstructive pulmonary disease
The following randomized controlled trials were included in the COPD clinical research program:
- one of 12 weeks (HZC113107), two of 6 months (HZC112206, HZC112207) and two of 1 year (HZC102970, HZC102871) in patients with a clinical diagnosis of COPD. These studies examined measures of lung function, dyspnea, and moderate to severe exacerbations of the disease.
6 month studies
HZC112206 and HZC112207 were randomized, double-blind, placebo-controlled, parallel group studies of 24 weeks duration comparing the combination with vilanterol and FF alone and placebo. The HZC112206 study assessed the effectiveness of the combination of fluticasone furoate/vilanterol 46/22 μg [n=206] and the combination of fluticasone furoate/vilanterol 92/22 μg [n=206] in comparison with the effectiveness of FF 92 μg [n=206], vilanterol 22 μg [n=205] and placebo [n=207], all drugs were taken 1 time/day. The HZC112207 study assessed the effectiveness of the combination of fluticasone furoate/vilanterol 92/22 μg [n=204] and the combination of fluticasone furoate/vilanterol 184/22 μg [n=205] in comparison with FF 92 μg [n=204], FF 184 μg [ n=203] and vilanterol 22 mcg [n=203] and placebo [n=205], all drugs were taken 1 time/day.
Patients participating in the studies had the following requirements:
- smoking history of at least 10 pack-years;
- the FEV1/FGE ratio after salbutamol use is less than or equal to 0.70;
- post-salbutamol FEV1 less than or equal to 70% predicted and dyspnea ≥2 (scale 0 to 4) on the modified British Medical Research Council (mMRC) questionnaire at screening. At screening, mean pre-bronchodilator FEV1 was 42.6% and 43.6% predicted, and mean reversibility was 15.9% and 12.0% in studies HZC112206 and HZC112207, respectively. The primary endpoints in both studies were weighted mean FEV1 values from 0 to 4 hours after the Day 168 dose and change from baseline in trough FEV1 to the Day 169 dose.
Based on the full analysis of both studies, it was concluded that the combination of fluticasone furoate/vilanterol 92/22 mcg resulted in a clinically significant improvement in lung function. At Day 169, the combination of fluticasone furoate/vilanterol 92/22 mcg and vilanterol resulted in an increase in adjusted mean trough FEV1 of 129 mL (95% CI: 91, 167 mL, p < 0.001) and 83 mL (95% CI: 91, 167 mL, p < 0.001) CI: 46, 121 ml, p <0.001), respectively, compared with placebo. The use of the combination of fluticasone furoate/vilanterol 92/22 mcg resulted in an increase in trough FEV1 by 46 ml compared with vilanterol (95% CI: 8.83 ml, p=0.017). On Day 168, the combination of fluticasone furoate/vilanterol 92/22 mcg and vilanterol resulted in an increase in adjusted weighted mean FEV1 at 0-4 hours by 193 mL (95% CI: 156, 230 mL, p <0.001) and 145 ml (95% CI: 108, 181 ml, p <0.001), respectively, compared with placebo. The use of the combination of fluticasone furoate/vilanterol 92/22 mcg resulted in an increase in the adjusted mean weighted mean FEV1 value over 0-4 hours by 148 ml compared with the use of FF alone (95% CI:
- 112, 184 ml, p <0.001).
12 month studies
Studies HZC102970 and HZC102871 were randomized, double-blind, parallel group studies of 52 weeks duration comparing fluticasone furoate/vilanterol 184/22 mcg, fluticasone furoate/vilanterol 92/22 mcg, fluticasone furoate/vilanterol 46/22 mcg. mcg and vilanterol 22 mcg (all drugs were used once a day) per annual rate of moderate/severe exacerbations of the disease in patients with COPD with a smoking history of at least 10 pack-years; the FEV1/FJE ratio after the use of salbutamol is less than or equal to 0.70; a post-salbutamol FEV1 of less than or equal to 70% predicted and a documented history of ≥1 exacerbation of COPD requiring antibiotic and/or oral corticosteroid therapy or hospitalization in the 12 months before visit 1. The primary endpoint was annual progression rate moderate and severe exacerbations of the disease. Moderate/severe exacerbations were defined as an increase in symptoms that required treatment with oral corticosteroids and/or antibiotics or hospitalization. Both studies had a 4-week run-in period during which all subjects received unmasked salmeterol/FP 50/250 twice daily to standardize COPD pharmacotherapy and stabilize disease prior to randomization to masked study drug for 52 weeks. Before the start of the run-in period, study participants stopped taking previously used medications for the treatment of COPD with the exception of short-acting bronchodilators. The use of concomitant long-acting inhaled bronchodilators (beta2-agonists and anticholinergics), combination drugs containing ipratropium/salbutamol, oral beta2-agonists and theophylline preparations was not permitted during the entire treatment period. The use of oral corticosteroids and antibiotics for the acute treatment of exacerbations of COPD was permitted according to specific guidelines. During the studies, participants used salbutamol as needed.
The results of both studies indicate that therapy with the combination of fluticasone furoate/vilanterol 92/22 mcg 1 time/day leads to a decrease in the annual rate of moderate/severe exacerbations of COPD compared to therapy with vilanterol (Table 2).
Table 2: Analysis of the incidence of exacerbations after 12 months of therapy
| End point | HZC102970 | HZC102871 | HZC102970 and HZC102871 (integrated analysis) | |||
| Vilanterol (n=409) | fluticasone furoate/vilanterol 92/22 (n=403) | Vilanterol (n=409) | fluticasone furoate/vilanterol 92/22 (n=403) | Vilanterol (n=818) | fluticasone furoate/vilanterol 92/22 (n=806) | |
| Moderate and severe exacerbations | ||||||
| Adjusted average annual level | 1.14 | 0.90 | 1.05 | 0.70 | 1.11 | 0.81 |
| Correlation with VI 95% CI p value % reduction (95% CI) | 0.79 (0.64, 0.97) 0.024 21 (3, 36) | 0.66 (0.54, 0.81) <0.001 34 (19, 46) | 0.73 (0.63, 0.84) <0.001 27 (16, 37) | |||
| Absolute difference in quantity per year compared with vilanterol (95% CI) | 0.24 (0.03, 0.41) | 0.36 (0.20, 0.48) | 0.30 (0.18, 0.41) | |||
| Time to first exacerbation: Risk ratio (95% CI) % risk reduction p value | 0.80 (0.66, 0.99) 20 0.036 | 0.72 (0.59, 0.89) 28 0.002 | 0.76 (0.66, 0.88) 24 p<0.001 | |||
In an integrated analysis of studies HZC102970 and HZC102871 at week 52, fluticasone furoate/vilanterol 92/22 mcg showed an improvement in adjusted mean trough FEV1 compared with vilanterol 22 mcg (42 mL 95% CI:
- 19, 64 ml, p<0.001). The 24-hour bronchodilator effect of the fluticasone furoate/vilanterol combination was maintained from the first dose through the 1-year treatment period. There were no signs of loss of effectiveness (tachyphylaxis was absent).
Taken together, in the two studies, a history of cardiovascular disease or risk factors for cardiovascular disease was noted in 2009 (62%) patients during the selection process. The incidence of cardiovascular disease/risk factors was similar in all treatment groups, with the most commonly reported conditions being hypertension (46%), hypercholesterolemia (29%), and diabetes mellitus (12%). Similar reductions in moderate and severe exacerbations were observed in this subgroup compared with the overall population. In patients with a history of cardiovascular disease and risk factors, the combination of fluticasone furoate/vilanterol 92/22 mcg resulted in a significant reduction in the annual incidence of moderate/severe exacerbations of COPD compared with vilanterol (adjusted mean annual incidence rates of 0.83 and 1.18, respectively , 30% reduction (95% CI 16, 42%, p<0.001)). At week 52, fluticasone furoate/vilanterol 92/22 mcg also demonstrated an improvement in adjusted mean trough FEV1 compared with vilanterol 22 mcg (44 mL 95% CI:
- 15, 73 ml, (p=0.003)).
Comparative studies with salmeterol/fluticasone propionate combination
In a 12-week study (HZC113107) in patients with COPD, improvements in pulmonary function compared to baseline were observed with both the combination of fluticasone furoate/vilanterol 92/22 mcg once daily in the morning and with the combination of salmeterol/FP 50/ 500 mcg 2 times/day. Adjusted mean increases in weighted mean FEV1 over the period 0-24 hours of 130 ml during therapy compared to baseline (fluticasone furoate/vilanterol combination) and 108 ml (salmeterol/FP combination) indicated an overall improvement in pulmonary function over the course of treatment. 24 hours when using both types of therapy. Adjusted mean difference by treatment between groups 22 ml (95% CI:
- -18, 63 ml) was not statistically significant (p=0.282). The adjusted mean change from baseline in trough FEV1 at Day 85 was 111 mL in the fluticasone furoate/vilanterol group and 88 mL in the salmeterol/FP group;
- the difference of 23 ml (95% CI: -20, 66) between treatment groups was not clinically or statistically significant (p=0.294).
Comparative studies with salmeterol/FP combinations or other conventional bronchodilators have not been conducted to adequately compare the effect on exacerbations of COPD.
Children
The European Medicines Agency has waived the mandatory reporting of results from studies of Relvar™ Ellipta™ in all subsets of the pediatric COPD population (see Dosage Regimen for information on use in pediatric patients).
The European Medicines Agency has deferred the mandatory reporting of results from studies of Relvar™ Ellipta™ in all subsets of the pediatric population in relation to asthma (for information on use in pediatric patients, see Dosage Regimen).
How to use the drug
It's actually very easy to use. Once a day in the morning or evening, open the lid, exhale to the side and inhale the dose through the mouthpiece and hold your breath for 10-15 seconds. After this, be sure to gargle and gargle your mouth, otherwise it will hurt!
Ready to use Relvar Ellipta
Guide on how to use Relvar Ellipta
How much does Relvar Ellipta cost?
Prices vary in different pharmacies. Prices vary from 1650 to 2000 rubles for a small dosage, and from 2000 to 2500 rubles for a large dosage. I usually use the services of apteka.ru. Usually it’s cheaper there, and you can sometimes order it at a discount at the nearest pharmacy. By the way, I have an article on how to buy medicines cheaper , you can read it by following the link.
Price of the drug Relvar Ellipta on apteka.ru