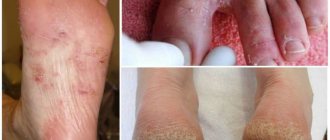
To treat fungal and bacterial skin diseases associated with inflammatory processes, various drugs are used, including Zinocap ointment. This is a non-hormonal drug for the treatment of dermatitis of various natures, eczema, psoriasis and other skin lesions. Used for several weeks until complete recovery.
Ointment "Zinocap": description, composition
The anti-inflammatory drug "Zinocap" is produced in 2 forms:
- aerosol (packaging – 58 g bottle);
- cream (packaging – tubes of 25 g or 50 g).
The active ingredient is zinc in the form of pyrithione. In both forms the mass fraction is 0.2%. Additional substances are:
- purified water;
- macrogol in the form of cetostearate;
- sodium dihydrogen phosphate in dihydrate form;
- liquid paraffin;
- silicon in the form of dioxide and others.
The product should be stored at room temperature up to 25 degrees Celsius or in the refrigerator. The place should be dark, without access to direct sunlight. Should be kept out of reach of children. The shelf life is 2 years - after this time the drug cannot be used.
Tsinocap
Zinocap® is 5 actions1 to defeat itching, flaking, irritation and dry skin. Without hormones and antibiotics. For adults and children from 1 year of age.
Zinocap® allows you to cope with various skin lesions caused by psoriasis, atopic or seborrheic dermatitis. Zinocap® is a broad-spectrum drug. Thanks to the active zinc contained in the composition (in the form of Zinc Pyrithione), Zinocap® has an antibacterial, antifungal, anti-inflammatory effect, which contributes to the rapid restoration of the skin and protection against infection. The drug also contains Dexpanthenol, which provides a regenerating effect, minimizes moisture loss by the skin, thereby reducing flaking and dryness, helping to restore a beautiful and healthy appearance.
Advantages of Zinocap®2
5 actions1 to defeat itching, peeling and irritation of the skin: antibacterial, antifungal, anti-inflammatory, protection against infection (when scratching), restoration and protection of the skin3.
- Without hormones and antibiotics. Zinocap® does not cause skin atrophy and “withdrawal dermatitis”4. It has a favorable safety profile5, and therefore can be used by both adults and children from 1 year of age.
- Possibility of long-term use . Unlike hormonal drugs, Zinocap®, if necessary, can be used for a long time without fear of local and systemic side effects.
- Wide range of applications . Zinocap® can be used for skin lesions even on large areas of the body, including particularly sensitive (face, neck) and hard-to-reach (folds and scalp);
- Ease of use. Zinocap® cream , with a lighter texture than regular ointments, is quickly absorbed6 and softens the skin, which is especially important for combating the manifestations of atopic dermatitis on the face, neck, and hands. Zinocap® aerosol is equipped with a special nozzle and is convenient for application to hard-to-reach areas of the skin (for example, on the scalp with seborrheic dermatitis).
Zinocap® - 5 actions1 for the treatment of skin dermatitis. Without hormones and antibiotics.
Indications for use
- atopic dermatitis (eczema, neurodermatitis)
- seborrheic dermatitis
- psoriasis
- dry skin
Release forms
- Aerosol 0.2% - 58 g
- cream 0.2% – 50 g
- cream 0.2% – 25 g
Question answer
At what age can you take Zinocap®?
Zinocap® is approved for use in children from 1 year of age and in adults.
Can Zinocap® be applied to the face?
Since Zinocap® is a non-hormonal drug, it can be applied to highly sensitive areas of the skin - face, neck area, folds, etc.
Zinocap® is available in 2 forms - aerosol and cream. How to use them for atopic dermatitis?
Both forms of the drug Zinocap® contain zinc pyrithione as the main active ingredient, which has anti-inflammatory, antibacterial, antifungal and antipruritic effects. Among the excipients, the aerosol includes ethyl alcohol, which has a drying effect during inflammation, and the cream contains dexpanthenol, which has an additional wound-healing effect. Therefore, the general rules are as follows: the use of an aerosol is indicated in the acute stage of inflammation in the presence of oozing phenomena, and the use of a cream in the subacute period after the acute inflammatory reaction has subsided or in case of moderately severe inflammation. To decide on the choice of the optimal form of the drug Zinocap®, we recommend consulting with a doctor who can assess the clinical symptoms, stage of the disease and prescribe adequate treatment.
Is it possible to use Zinocap® for a long time - more than 2 weeks for psoriasis?
Since Zinocap® is a non-hormonal drug, it can be used for a long time without the risk of systemic and local side effects, for psoriasis for an average of 1-1.5 months.
What complications may occur during treatment with Zinocap®?
According to the instructions for medical use, the drug Zinocap® is well tolerated; local allergic reactions may occasionally occur. Since the drug is practically not absorbed and is found in the blood in trace amounts, Zinocap® does not cause systemic adverse reactions.
Pharmacodynamics
Cream and aerosol “Zinocap” has a complex effect:
- antibacterial;
- antifungal;
- anti-inflammatory;
- dermatoprojector (skin protection).
The active ingredient destroys gram-negative and gram-positive bacteria. The drug leads to disruption of cell wall potentials, which causes pathogenic microorganisms to die en masse. Thanks to this, the product eliminates flaking of the skin, including due to dandruff, and is used to treat seborrheic dermatitis and other diseases.
The absorption of the drug is small, it is found in the bloodstream only in small quantities. Therefore, the medicine can be used without harmful effects on health (subject to dosages).
Reviews
We invite you to read reviews from ordinary people about the use of Zinocap for acne and inflammation on the skin of the face, and if you still have any questions, you can always ask them to a cosmetologist or pharmacist on our forum.
The product has a light consistency that is easily and quickly absorbed, which is good for owners of both oily and combination skin. Zinocap is well distributed on the skin, which makes it very economical at such a relatively low price. I really liked it and will recommend it to my friends and acquaintances.
Olga, 25 years old, Moscow
I noticed the tsinocap and good reviews. To be honest, I never noticed the pronounced effects that they write about on various sites; I applied a thin layer to acne and noticed that they did not become smaller, and they did not go away any faster. However, what I definitely noticed was the excellent moisturizing effect and the effect of reducing redness around inflammation.
Angelika L. 27 years old, Kubinka
I have been using the cream for three months and am very pleased with the effect. It dries out perfectly and relieves redness; I bought it as a moisturizer while using Effezel gel, which just dries out the skin like crazy. And Tsinocap coped with his task with just five points. A huge plus is that it is non-comedogenic. During use, even after treatment with “heavy artillery,” I did not get a single black dot because of it. Except, of course, the standard ones, which appear in the same places.
Ksyusha, 23 years old, Shchelkovo
Indications for use
Cream and aerosol are used to treat various skin pathologies:
- neurodermatitis;
- dandruff;
- eczema;
- seborrhea;
- dermatitis of various nature;
- psoriasis;
- skin inflammation;
- peeling of the skin;
- pityriasis versicolor;
- Seborrheic dermatitis.
Contraindications and side effects
The product has virtually no contraindications - the gel and aerosol can be used by different patients, with the exception of children in the first year of life. Also, the drug cannot be used if there is an individual intolerance to the main or additional components.
In some cases, side effects are observed - itching, redness, burning and others. You should stop taking it - as a rule, the described symptoms go away on their own. In extreme cases, symptomatic treatment is indicated. To continue the course, you need to consult a doctor, as you may need to change the drug.
The effectiveness of Exomega cream for atopic dermatitis in children
N
The insufficient use of medicinal cosmetics for atopic dermatitis (AD) in children in our country is a consequence of stereotypes developed over decades not only among pediatricians, for whom caring for children’s skin has always remained outside the scope of their activities, but also among dermatologists. The use of external corticosteroids and medicinal cosmetics for hypertension are two positions that are most difficult for specialists to accept. The prescription of the most effective anti-inflammatory drugs - corticosteroids - is limited due to inadequate corticosteroid phobia, and medicinal cosmetics - due to an insufficiently serious attitude towards the need to constantly maintain the skin of patients with AD in a state of hydration and maximum elasticity. This situation exists despite the results of numerous controlled trials of modern corticosteroid drugs in children with AD and the vast experience of specialists around the world, demonstrating the need for the use of high-quality medicinal cosmetics that have not only a moisturizing, but also an anti-inflammatory effect during clinical remission and during moderate exacerbations. . However, anti-inflammatory therapy, such as topical corticosteroids and recently introduced topical immunosuppressants (tacrolimus, pimecrolimus, mycophenolate mofetil), are considered the mainstays in the treatment of AD. Their timely use in children with AD makes it possible to quickly achieve clinical remission and maintain it for a long time. Only careless or illiterate use of these drugs is associated with a potential risk of adverse reactions and complications [1].
The use of medicinal cosmetics for hypertension in children is based on several serious points arising from a whole group of scientifically proven facts. AD is a multifactorial, polygenic skin disease that affects up to 20% of the child population in Western countries.
who have not reached puberty [1]. Despite the fact that AD is considered an immunological disease, factors that are not immunological in the strict sense of the term take part in its occurrence, development and chronicity [2,3].
A large number of studies indicate that the barrier function of the skin is impaired in AD. This occurs due to constant scratching of the affected itchy areas of the skin, which maintains the activation of inflammation. There is also evidence that patients with AD have reduced levels of ceramides in the skin, which may lead to a reduced ability to bind water, high transepidermal water loss and, as a consequence, reduced water content in the skin [4]. These changes increase the likelihood of antigens being absorbed into the skin, thereby creating a vicious cycle leading to further activation of the immune system and the maintenance of chronic inflammation. A number of changes occurring in the skin of children suffering from AD lead to the appearance of one of the most persistent, chronic, and difficult to overcome symptoms of the disease - dry skin.
. Dryness is associated both with the presence of inflammation and with insufficient activity of the enzyme one of the most important stages of the metabolism of polyunsaturated fatty acids in skin cells - delta-6 desaturase. However, dryness, in turn, plays a significant role in the pathogenesis of AD. Disruption of the structure of the stratum corneum of the skin, clinically manifested by its dryness, leads to a further increase in the evaporation of water from the skin surface and a decrease in barrier function. As is known, the three key components of skin lipids include stratum corneum ceramides, cholesterol and free fatty acids. To perform the normal barrier function of the skin, it is necessary to have a sufficient amount of extracellular lipids, which form a lamellar bilayer system, while in AD, a correlation has been identified between the severity of barrier function impairment and a global decrease in the ceramide fraction of lipids in the stratum corneum [5,6]. Recent studies have revealed the ability of externally applied mixtures - analogues of three key lipid components of the skin (ceramides, cholesterol and free fatty acids) with their molar ratio in the optimal proportion (3:1:1) to enhance the restoration of the barrier function of the skin after various types of external influences [7, 8].
Moisturizers and emollients of inappropriate (wrong) composition can increase impairment of the skin barrier function, maintain the course of an existing disease and cause exacerbations of initially inactive dermatoses. [7,9]. For example, unfortunately, some moisturizers widely used by our dermatologists and pediatricians, prepared according to the “water in oil” principle and considered as the main ones in the symptomatic treatment of AD, do not correct the underlying lipid abnormality of the stratum corneum [10,11]. Moreover, such moisturizers increase the impairment of skin barrier function due to their suppression of the formation of essential unsaturated fatty acids in the skin.
The use of medicinal cosmetics does not require those controlled trials that are necessary for approval for the use of pharmaceuticals. However, before recommending this or that cosmetic product in pediatric practice, and especially one that has a declared therapeutic effect, leading experts are obliged to refer not only and not so much to their experience, but to those more or less controlled studies that can be carried out in a pediatric clinic, consultative or inpatient.
The above fully applies to both foreign and domestic medicinal cosmetics, and the first of them have long proven themselves in the world and are subject to the strictest inspection in the manufacturing countries.
The authors of the article have been using products from the French company Pierre-Fabre Dermocosmetic for more than 4 years. In 2001, the company's representative office in Russia offered Exomega
, which was positioned as having anti-inflammatory, moisturizing and antipruritic effects.
In Russia, Exomega cream is approved for use in children from birth and is a registered drug that has passed the necessary certification. Exomega is produced in France by Pierre Fabre Dermocosmetic, Ducret laboratory. Our experience has shown fairly high effectiveness and excellent tolerability of Exomega cream
. To maximize the objectification of these data, we decided to conduct an open, non-comparative clinical study possible in a pediatric clinic to determine the clinical effectiveness and tolerability of Exomega cream for external use in children with AD.
Exomega emollient cream, intended for the care of the skin of patients with AD, contains a complex of Realba oat extract and Omega-6 fatty acids. The description of Exomega cream states that the synergistic effect of Realba complex oat extract and Omega-6 fatty acids helps restore the skin barrier, reduces skin dryness and has an anti-inflammatory effect. The anti-inflammatory effect of Exomega cream is due to the incorporation of essential fatty acids into the layer of membrane phospholipids and intercellular cement of the stratum corneum of the skin, which suppresses the synthesis of prostaglandins and leukotrienes B4. The consequence of this is a decrease in the severity of the inflammatory response. In this regard, we expected that applying Exomega cream to the skin would not only help reduce the severity of dry skin, but also have an anti-inflammatory effect, accelerating the onset of remission of the disease and prolonging it.
Exomega cream contains the following components:
complex of Realba oat extract and Omega fatty acids – 63.6%, glycerin – 5%, moisturizing and protective base in a long-lasting aqueous phase, vitamin E – 0.5% (all types of its effects, including antioxidant, are widely known).
During the study, we determined the dynamics of the main clinical symptoms of blood pressure in children during treatment with Exomega cream, the frequency and nature of side effects of the cream, and assessed the acceptability (convenience) of its use. The study was conducted in 20 children aged 0 to 15 years who suffered from AD in the stage of incomplete clinical remission. Patients were included in the study if they had a substantiated diagnosis of AD in accordance with the criteria of JM Hanifin and G. Rajka [12]. All children were discontinued from antihistamines and corticosteroids for both systemic and external use at least 5 days before the start of the study. Egzomega cream was used only in those patients whose dermatitis was in a chronic or subacute phase; patients with acute dermatitis were not included in the study. All children were hospitalized in the department of allergology and dermatology of the Moscow Research Institute of Pediatrics and Pediatric Surgery of the Ministry of Health of the Russian Federation. The atopic genesis of dermatitis was confirmed in all children by the presence of close relatives (maternal or paternal) who suffered from one or another atopic disease, moderately or significantly increased levels of total serum immunoglobulin E (more than the average age value plus 1 to 2 or more standard deviations) , positive skin prick tests with household and/or food allergens. At the same time, in all children, according to the anamnesis, a clear connection was found between the exacerbation of the disease and exposure to a causally significant allergen.
The initial condition of the patients was assessed on the 1st day of the study before the administration of Exomega cream. The attending physician recorded the following symptoms: erythema, swelling/papules, weeping/crusting, excoriation, lichenification, dryness. The SCORAD scale was used to assess the effectiveness of Exomega cream. The SCORAD index, which is a digital expression of the score for the severity of skin lesions, was calculated using the formula: A/5+7B/2+C, where A is the prevalence of skin lesions as a percentage; B – intensity of signs of damage in points from 0 to 3 (erythema, swelling/formation of papules, weeping/crusts, excoriation, lichenification, dryness of unaffected areas of the skin); C – subjective symptoms: itching + loss of sleep (the severity of each of the subjective symptoms is from 0 to 10). This assessment was carried out once every 5 days throughout treatment with Exomega cream. The day of completion of treatment was determined by the onset of remission of the disease, but in all cases did not exceed 45 days.
In addition to the doctor’s assessment of the patient’s condition, parents and older patients made their own assessment of the acceptability of use, based on their subjective feelings from the use (yes, no, acceptable), taking into account the child’s negative reaction to the use of the drug. The cosmetic properties of Exomega cream were assessed at the end of the trial according to several criteria. These are ease of application (good, poor), ease of use (convenient, satisfactory, unsatisfactory), smell (pleasant, unpleasant, neutral, absent). Patients independently or with the help of their parents applied Exomega cream to all affected areas of the skin 2 times a day - morning and evening.
The study was interrupted in only one 3-year-old child (a girl) due to the occurrence of a local reaction in the form of erythema, itching at the sites where Exomega cream was applied, which occurred 5 minutes after application of the cream and persisted for up to 1.5 hours. No special measures were required to eliminate this reaction. Since at the end of the test the skin redness and itching disappeared completely, the connection with the use of Exomega cream was assessed as significant. In the remaining patients in the study group, no adverse events were identified during the study. Thus, the study was successfully completed in 19 children.
The dynamics of the SCORAD index during treatment with Exomega cream are presented in Table 1. From the table it follows that already on the 5th day of treatment, the number of children with moderate and high SCORAD index decreased from 15 to 3. In one child, the increase in the index value on the 10th day was due to errors in the diet. On days 10–15, most children showed a significant decrease in the SCORAD index, and only 2 children with initially severe skin lesions had a SCORAD index of more than 40 on the 15th day of treatment.
The dynamics of the reduction in the area of skin lesions during treatment with Exomega cream are presented in Table 2. A reduction in the area of lesions of less than 10% of the skin surface was observed in the majority of children (13 patients) by the 10th day of treatment. Moreover, on the 15th day of treatment, the lesion area of more than 10% remained in only 4 children. The results of assessing the ease of use (cosmetic properties) of Exomega cream are shown in Table 3. Thus, all patients assessed the texture of the cream and the ease of its application positively. 4 patients rated the ease of use as satisfactory, 15 as significant. The majority (15 patients) rated the odor of the cream as pleasant or neutral, and only 4 as unpleasant. There were no cases of refusal to use Exomega cream due to its organoleptic properties.
As a result of the study, the following conclusions could be drawn :
1. A decrease in the severity of blood pressure symptoms with the use of Exomega cream was observed already by the 5th day of therapy.
2. To achieve the maximum therapeutic and cosmetic effects of Exomega cream, it is advisable to use it for 2–4 weeks.
3. The greatest effectiveness of the drug was observed in patients with moderate to mild AD, accompanied by significant dry skin, outside the period of exacerbation.
4. Exomega cream is well tolerated by children suffering from atopic dermatitis and has good cosmetic properties.
Such an open, non-comparative study of one of the most effective and well-proven medicinal cosmetics in pediatric clinics allowed us not only to once again verify in our own practice the feasibility and justification of its use for blood pressure, but also to recommend it to our colleagues - pediatricians and dermatologists.
References:
1. Laughter D., Istvan J., Tofte S., Hanifin J. The prevalence of atopic dermatitis in Oregon schoolchildren. J. Am. Acad. Dermatol., 2000;43:649–655
2. Leung DY Atopic dermatitis: new insights and opportunities for therapeutic intervention. J. Allergy Clin. Immunol. 2000;105:860–876
3. Cooper KD Atopic dermatitis: recent trends in pathogenesis and therapy. J. Invest. Dermatol., 1994;102:128–137
4. Imokawa G., Abe A., Jin K., Higaki Y., Kawashima M., Hidano A. Decreased level of ceramides in the stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J. Invest. Dermatol., 1991;96:523–526
5. Matsumoto Y., Hamashima H., Masuda K., Shiojima K., Sasatsu M., Arai T. The antibacterial activity of plaunotol against Staphylococcus aureus isolated from the skin of patients with atopic dermatitis. Microbios 1998;96:149–155
6. Di Nardo A., Wertz P., Giannetti A., Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm. Venereol., 1998;78:27–30
7. Mao–Quiang M., Feingold KR, Elias PM Exogenous lipids influence permeability barrier recovery in acetone treated murine skin. Arch. Dermatol., 1993;129:728–738
8. Ghadially R., Brown BE, Hanley K., Reed JT, Feingold KR, Elias PM Decreased epidermal lipid synthesis accounts for altered barrier function in aged mice. J. Invest. Dermatol., 1996;106:1064–1069
9. Mao-Qiang M., Brown BE, Wu-Pong S., Feingold KR, Elias PM Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch. Dermatol. 1995;131:809–816
10. Cooper KD Atopic dermatitis: recent trends in pathogenesis and therapy. J. Invest. Dermatol., 1994;102:128–137
11. Denda M., Sato J., Tsuchiya T., Elias PM, Feingold KR Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J. Invest. Dermatol., 1998;111:873–878
12. Hanifin JM, Rajka G.: Diagnostic features of atopic dermatitis. Acta Dermatol 1980;92:44–47
Cream "Zinocap": instructions for use
The product can be used by adult patients, including pregnant women, as well as lactating women. The drug can also be prescribed to children over 1 year of age.
The aerosol is shaken before use, then sprayed onto the affected area (it is first washed and wiped dry). The procedure is repeated 2 or 3 times a day, the total duration depends on how quickly the therapeutic effect is noticeable. In this case, after the symptoms disappear, treatment should be continued for at least 7 days. To treat the scalp, use a special attachment, which is included in the kit.
The cream is applied to the affected area in a small layer and rubbed in with gentle movements. The procedure must also be repeated 2 or 3 times a day until complete recovery. After this, the course of treatment is continued for 7 days.
The total duration depends on the disease:
- psoriasis – 4-6 weeks, during an exacerbation the course is repeated;
- atopic dermatitis – 3-4 weeks;
- seborrheic dermatitis – 2 weeks.
No cases of overdose have been reported. When used in the indicated quantities, the drug is safe for health. There is also no information about the interaction of Zinocan with other drugs. Therefore, it can be taken simultaneously with different drugs.
Zinocap cream with D-panthenol for dermatitis, psoriasis 0.2% 25g
Active substance:
100 g of cream contains: zinc pyrithione (in terms of 100% substance) – 0.2 g (0.2%)
Excipients:
Macrogol cetostearate, cetostearyl alcohol, liquid paraffin (vaseline oil), propylene glycol, dexpanthenol (D-Panthenol) , sodium dihydrogen phosphate dihydrate, sodium hydrogen phosphate dodecahydrate, purified water
Description:
For the skin of mothers, children and fathers! Zinocap® is a line of non-hormonal, broad-spectrum agents for the treatment of skin dermatitis in adults and children from 1 year of age.
Zinocap® - treatment of dermatitis without hormones and antibiotics; Zinocap® - against itching, irritation, dryness and flaking*.
The use of Zinocap® in the treatment of patients with seborrheic dermatitis*: - reduces the intensity of itching by up to 2 times already in the first week of therapy; - reduces indicators reflecting the severity of seborrheic dermatitis: dermatological index of the DCSS symptom scale and dermatological quality of life index DLQI; - helps to prolong remission; — has a favorable safety profile*.
The use of Zinocap in the treatment of patients with psoriasis*: - causes a decrease in the psoriasis coverage and severity index PASI, skin lesion area index BSA, dermatological quality of life index DLQI; — significantly improves the quality of life of patients with psoriasis; - helps to prolong remission; — has a favorable safety profile*.
Pyrithione in the treatment of patients with atopic dermatitis*: - reduces the intensity of skin itching; - reduces the need for topical steroids; - reduces the need to take antihistamines.
Pyrithione in the treatment of children with atopic dermatitis*: - promotes the onset of clinical remission in children with mild atopic dermatitis; — in case of moderate severity: 58% of children achieved clinical remission; 42% had a significant improvement in the course of the disease; — in children with a severe, persistent course, improvement was recorded; — significantly reduces skin colonization with S. aureus and Trichophyton, which support allergic inflammation in atopic dermatitis.
Zinocap® is a dermatoprotector with an improved composition: it contains dexpanthenol*, which has an additional healing effect*; Does not contain hormones and antibiotics - does not provoke skin atrophy and “withdrawal dermatitis” *; Suitable for both adults and children from 1 year*; Can be used on large affected areas, sensitive areas of the skin (face, neck, folds), as well as weeping and infected areas*.
Two forms of release provide convenience of therapy: - CREAM for dermatitis with dry skin: 25 g - starter pack, for lesions of small areas of the skin; 50 g - course packaging; — AEROSOL: suitable for treating the scalp with the drug. The package includes a convenient removable nozzle.
*Open randomized comparative study of the therapeutic efficacy and safety of Zinocap and Skin-cap. TsNIKVI 2008; An open randomized clinical study of the therapeutic equivalence of Zinocap cream and Skin-cap cream in patients with psoriasis. Federal State Institution State Scientific Center for Dermatology of the Federal Agency for High-Tech Medical Care, 2008, Doctor of Medical Sciences. Professor A.A. Kubanova et al; Pyrithione zinc in the treatment of atopic dermatitis in children (according to the results of the Russian multi-center study "CADET"). Russian allergological journal, No. 2, 2007; Modern external therapy of chronic inflammatory skin diseases in children (Experience of clinical use of the drug Skin-cap). Moscow / Pediatrics, 2008. No. 4.S. 92 -97; UTI drug Zinocap (cream, aerosol); Kungurov N.V., Kokhan M.M., Keniksfest Yu.V. Med. Council 2013, Pediatrics, p. 20-22 for atopic dermatitis; Clinical effectiveness and safety of external therapy for atopic dermatitis. N.V. Kungurov, M.M. Kokhan, Yu.V. Kenixfest. Medical advice. Pediatrics. 2013. pp. 18-22.
Release form:
The cream is white or white with a yellowish tint, odorless or with a weak specific odor. Cream for external use 0.2%. 25 g in aluminum tubes. The tube, along with instructions for use, is placed in a cardboard pack.