Glucovance tablets p/o 500mg/2.5mg No. 15x2
Name
Glucovance tablet p/pl.vol. 500mg/2.5mg per bl. in pack No. 15x2
Description
Main active ingredient
metformin glibenclamide
Release form
Pills
Dosage
500mg/2.5mg
special instructions
During treatment with Glucovance®, it is necessary to regularly monitor the level of glycemia on an empty stomach and after meals. Lactic acidosis Lactic acidosis is an extremely rare but serious (high mortality unless promptly treated) complication that can occur due to accumulation of metformin. Cases of lactic acidosis in patients receiving metformin occurred mainly in diabetic patients with severe renal failure. Other associated risk factors should be considered, such as poorly controlled diabetes, ketosis, prolonged fasting, excessive alcohol consumption, liver failure and any condition associated with severe hypoxia. The risk of developing lactic acidosis should be taken into account when nonspecific signs appear, such as muscle cramps accompanied by dyspeptic disorders, abdominal pain and severe malaise. In severe cases, acidotic dyspnea, hypoxia, hypothermia and coma may occur. Diagnostic laboratory parameters are: low blood pH, plasma lactate concentration above 5 mmol/L, increased anion gap and lactate/pyruvate ratio. Hypoglycemia Glucovance® contains glibenclamide, so taking the drug is accompanied by a risk of hypoglycemia in the patient. Gradual dose titration after initiation of treatment may prevent the occurrence of hypoglycemia. This treatment can only be prescribed to a patient who adheres to a regular diet (including breakfast). It is important that carbohydrate intake is regular, because... The risk of developing hypoglycemia increases with late meals, insufficient or unbalanced carbohydrate intake. The development of hypoglycemia is most likely during a hypocaloric diet, after intense or prolonged physical activity, when drinking alcohol, or when taking a combination of hypoglycemic agents. Due to compensatory reactions caused by hypoglycemia, sweating, fear, tachycardia, arterial hypertension, palpitations, angina pectoris and arrhythmia may occur. The latter symptoms may be absent if hypoglycemia develops slowly, in the case of autonomic neuropathy, or while taking beta-blockers, clonidine, reserpine, guanethidine or sympathomimetics. Other symptoms of hypoglycemia in patients with diabetes may include headache, hunger, nausea, vomiting, severe fatigue, sleep disturbances, restlessness, aggression, impaired concentration and psychomotor reactions, depression, confusion, speech impairment, visual impairment, trembling, paralysis. and paresthesia, dizziness, delirium, convulsions, somnolence, unconsciousness, shallow breathing and bradycardia. Careful prescribing, dose selection, and appropriate patient instructions are important to reduce the risk of hypoglycemia. If the patient has recurrent episodes of hypoglycemia that are either severe or due to unawareness of symptoms, treatment with other hypoglycemic agents should be considered. Factors contributing to the development of hypoglycemia: simultaneous consumption of alcohol, especially during fasting; refusal or (especially for older patients) inability of the patient to interact with the doctor and follow the recommendations set out in the instructions for use; poor nutrition, irregular eating, fasting, or changes in diet; imbalance between physical activity and carbohydrate intake; renal failure; severe liver failure; overdose of the drug Glucovance®; certain endocrine disorders: insufficiency of the thyroid gland, pituitary gland and adrenal glands; simultaneous use of individual medications. Renal and hepatic impairment Pharmacokinetics and/or pharmacodynamics may vary in patients with hepatic impairment or severe renal impairment. The hypoglycemia that occurs in such patients can be prolonged, in which case appropriate treatment should be started. Instability of blood glucose levels In the case of surgery or another cause of decompensation of diabetes mellitus, it is recommended to consider a temporary transition to insulin therapy. Symptoms of hyperglycemia include frequent urination, severe thirst, and dry skin. 48 hours before planned surgery or intravenous administration of an iodinated radiocontrast agent, Glucovance® should be discontinued. Treatment is recommended to be resumed after 48 hours and only after renal function has been assessed and found to be normal. Renal function Since metformin is excreted by the kidneys, before starting treatment and regularly thereafter it is necessary to determine CC and/or serum creatinine: at least once a year in patients with normal renal function, and 2-4 times a year in elderly patients, as well as in patients with CC on ULN. Particular caution is recommended in cases where renal function may be impaired, for example in elderly patients, or when initiating antihypertensive therapy, diuretics or NSAIDs. Other precautions The patient should inform the doctor about the occurrence of a bronchopulmonary infection or an infectious disease of the genitourinary organs. Impact on the ability to drive a car and operate machinery Patients should be informed about the risk of hypoglycemia and should take precautions when driving a car and operating machinery that requires increased concentration and speed of psychomotor reactions.
pharmachologic effect
Glucovance® is a fixed combination of two oral hypoglycemic agents of different pharmacological groups: metformin and glibenclamide. Metformin belongs to the group of biguanides and reduces the content of both basal and postprandial glucose in the blood plasma. Metformin does not stimulate insulin secretion and therefore does not cause hypoglycemia. Has 3 mechanisms of action: reduces liver glucose production by inhibiting gluconeogenesis and glycogenolysis; increases the sensitivity of peripheral receptors to insulin, the consumption and utilization of glucose by cells in the muscles; delays the absorption of glucose in the gastrointestinal tract. The drug also has a beneficial effect on blood lipid composition, reducing the level of total cholesterol, LDL and TG. Glibenclamide belongs to the group of sulfonylurea derivatives of the second generation. Glucose content when taking glibenclamide decreases as a result of stimulation of insulin secretion by pancreatic β-cells. Metformin and glibenclamide have different mechanisms of action, but complement each other's hypoglycemic activity. The combination of two hypoglycemic agents has a synergistic effect in reducing glucose levels.
Pharmacokinetics
Glibenclamide When taken orally, absorption from the gastrointestinal tract is more than 95%. Glibenclamide, which is part of the drug Glucovance®, is micronized. Cmax in plasma is achieved in approximately 4 hours. Vd is about 10 l. Communication with plasma proteins is 99%. Almost completely metabolized in the liver with the formation of two inactive metabolites, which are excreted by the kidneys (40%) and bile (60%). T1/2 - from 4 to 11 hours. Metformin After oral administration, it is absorbed from the gastrointestinal tract quite completely. Cmax in plasma is achieved within 2.5 hours. Absolute bioavailability ranges from 50 to 60%. Approximately 20-30% of metformin is excreted unchanged through the gastrointestinal tract. Metformin is quickly distributed in tissues and practically does not bind to plasma proteins. It is metabolized to a very weak extent and is excreted by the kidneys. T1/2 averages 6.5 hours. The combination of metformin and glibenclamide in one dosage form has the same bioavailability as when taking tablets containing metformin or glibenclamide alone. The bioavailability of metformin in combination with glibenclamide is not affected by food intake, nor is the bioavailability of glibenclamide. However, the rate of absorption of glibenclamide increases with food intake.
Indications for use
Type 2 diabetes mellitus in adults: with ineffective diet therapy, exercise and previous monotherapy with metformin or sulfonylurea derivatives; to replace previous therapy with two drugs (metformin and a sulfonylurea derivative) in patients with stable and well-controlled glycemic levels.
Directions for use and doses
The dose of the drug is determined by the doctor individually for each patient, depending on the level of glycemia. The initial dose is 1 tablet of Glucovance® 2.5 mg+500 mg or Glucovance® 5 mg+500 mg 1 time/day. To avoid hypoglycemia, the initial dose should not exceed the daily dose of glibenclamide (or an equivalent dose of another previously taken sulfonylurea) or metformin if used as first-line therapy. It is recommended to increase the dose by no more than 5 mg glibenclamide + 500 mg metformin per day every 2 weeks or more to achieve adequate blood glucose control. Replacement of previous combination therapy with metformin and glibenclamide: the initial dose should not exceed the daily dose of glibenclamide (or an equivalent dose of another sulfonylurea) and metformin previously taken. Every 2 or more weeks after the start of treatment, the dose of the drug is adjusted depending on the level of glycemia. The maximum daily dose is 4 tablets of Glucovance® 5 mg+500 mg or 6 tablets of Glucovance® 2.5 mg+500 mg. Dosage regimen The dosage regimen depends on the individual prescription: 1 time per day, in the morning during breakfast - when prescribing 1 tablet per day; 2 times a day, morning and evening - when prescribing 2 or 4 tablets per day. The tablets should be taken with meals. Each dose of the drug should be accompanied by a meal with a sufficiently high carbohydrate content to prevent the occurrence of hypoglycemia. Elderly patients The dose of the drug is selected based on the state of renal function. The initial dose should not exceed 1 tablet of Glucovance® 2.5 mg + 500 mg. Renal function should be assessed regularly. Children Glucovance® is not recommended for use in children.
Use during pregnancy and lactation
The use of the drug is contraindicated during pregnancy. The patient should be warned that during treatment with Glucovance® it is necessary to inform the doctor about the planned pregnancy and the occurrence of pregnancy. When planning pregnancy, as well as in the event of pregnancy while taking Glucovance®, the drug should be discontinued and treatment with insulin should be prescribed. Glucovance® is contraindicated during breastfeeding, since there is no data on its ability to pass into breast milk.
Interaction with other drugs
Contraindicated combinations Associated with the use of glibenclamide Miconazole can provoke the development of hypoglycemia (up to the development of coma). Metformin-related Iodinated contrast media: Depending on renal function, the drug should be discontinued 48 hours before or after IV administration of iodinated contrast media. Combinations not recommended Related to the use of sulfonylurea derivatives Alcohol. Disulfiram-like reactions (alcohol intolerance) are very rarely observed when taking alcohol and glibenclamide simultaneously. Alcohol intake may increase the hypoglycemic effect (by inhibiting compensatory reactions or delaying its metabolic inactivation), which may contribute to the development of hypoglycemic coma. During treatment with Glucovance®, you should avoid drinking alcohol and medications containing ethanol. Phenylbutazone increases the hypoglycemic effect of sulfonylurea derivatives (by replacing sulfonylurea derivatives at protein binding sites and/or reducing their elimination). It is preferable to use other anti-inflammatory drugs that exhibit less interaction, or to warn the patient about the need to self-monitor glycemic levels. If necessary, the dose should be adjusted when using an anti-inflammatory drug together and after its discontinuation. Associated with the use of glibenclamide Bosentan in combination with glibenclamide increases the risk of hepatotoxicity. It is recommended to avoid taking these drugs together. The hypoglycemic effect of glibenclamide may also be reduced. Associated with the use of metformin Alcohol. The risk of developing lactic acidosis increases with acute alcohol intoxication, especially in the case of fasting, or poor nutrition, or liver failure. During treatment with Glucovance®, you should avoid drinking alcohol and medications containing ethanol. Combinations requiring caution Associated with the use of all hypoglycemic agents Chlorpromazine in high doses (100 mg/day) causes an increase in glycemic levels (reducing insulin release). Precautions: the patient should be warned about the need to independently monitor blood glucose levels; If necessary, the dose of the hypoglycemic drug should be adjusted during simultaneous use of the antipsychotic and after discontinuation of its use. GCS and tetracosactide: an increase in blood glucose levels, sometimes accompanied by ketosis (GCS cause a decrease in glucose tolerance). Precautions: the patient should be warned about the need to independently monitor blood glucose levels; if necessary, the dose of the hypoglycemic agent should be adjusted during the simultaneous use of GCS and after discontinuation of their use. Danazol has a hyperglycemic effect. If treatment with danazol is necessary and when taking the latter, a dose adjustment of the drug Glucovance is required under the control of glycemic levels. Beta2-adrenergic agonists, by stimulating beta2-adrenergic receptors, increase the concentration of glucose in the blood. Precautions: it is necessary to warn the patient and establish control of blood glucose levels; transfer to insulin therapy is possible. Diuretics: increase blood glucose levels. Precautions: the patient should be warned about the need to independently monitor blood glucose levels; It may be necessary to adjust the dose of the hypoglycemic agent during simultaneous use with diuretics and after discontinuation of their use. ACE inhibitors: the use of ACE inhibitors (captopril, enalapril) helps reduce blood glucose levels. If necessary, the dose of Glucovance should be adjusted during simultaneous use with ACE inhibitors and after discontinuation of their use. Associated with the use of metformin Diuretics: lactic acidosis that occurs when taking metformin against the background of functional renal failure caused by taking diuretics, especially loop diuretics. Associated with the use of glibenclamide, beta-blockers, clonidine, reserpine, guanethidine and sympathomimetics mask some symptoms of hypoglycemia: palpitations and tachycardia; Most non-selective beta-blockers increase the incidence and severity of hypoglycemia. The patient should be warned about the need to independently monitor blood glucose levels, especially at the beginning of treatment. Fluconazole: an increase in T1/2 of glibenclamide with the possible occurrence of manifestations of hypoglycemia. The patient should be warned about the need to self-monitor blood glucose levels; It may be necessary to adjust the dose of hypoglycemic drugs during concomitant treatment with fluconazole and after discontinuation of its use. Bile acid sequestrants: simultaneous use with Glucovance® reduces the concentration of glibenclamide in the blood plasma, which can lead to a decrease in the hypoglycemic effect. Glucovance should be taken at least 4 hours before taking a bile acid sequestrant. Combinations to consider Glibenclamide-related Desmopressin: Glucovance® may reduce the antidiuretic effect of desmopressin. Antibacterial drugs (drugs) from the sulfonamide group, fluoroquinolones, anticoagulants (coumarin derivatives), MAO inhibitors, chloramphenicol, pentoxifylline, lipid-lowering drugs from the group of fibrates, disopyramide - the risk of developing hypoglycemia during the use of glibenclamide.
Contraindications
hypersensitivity to metformin, glibenclamide or other sulfonylurea derivatives, as well as to excipients; type 1 diabetes mellitus; diabetic ketoacidosis; diabetic precoma, diabetic coma; renal failure or impaired renal function (KR
Compound
glibenclamide 2.5 mg metformin hydrochloride 500 mg Excipients: croscarmellose sodium - 14 mg, povidone K30 - 20 mg, microcrystalline cellulose - 56.5 mg, magnesium stearate - 7 mg. Shell composition: opadry OY-L-24808 pink - 12 mg (lactose monohydrate - 36%, hypromellose 15cP - 28%, titanium dioxide - 24.39%, macrogol - 10%, iron oxide yellow - 1.3%, iron oxide red - 0.3% , iron oxide black - 0.01%), purified water - qs
Overdose
In case of overdose, hypoglycemia may develop due to the presence of a sulfonylurea derivative in the drug. Mild to moderate symptoms of hypoglycemia without loss of consciousness or neurological manifestations can be corrected by immediate consumption of sugar. It is necessary to adjust the dose and/or change the diet. The occurrence of severe hypoglycemic reactions in patients with diabetes mellitus, accompanied by coma, paroxysm or other neurological disorders, requires emergency medical care. It is necessary to administer intravenous dextrose solution immediately after diagnosis or suspicion of hypoglycemia, before hospitalization of the patient. After regaining consciousness, it is necessary to give the patient food rich in easily digestible carbohydrates (to avoid re-development of hypoglycemia). Lactic acidosis is a medical emergency; Lactic acidosis should be treated in a clinic. The most effective treatment method for removing lactate and metformin is hemodialysis. Plasma clearance of glibenclamide may be increased in patients with liver disease. Since glibenclamide actively binds to blood proteins, the drug is not eliminated during dialysis. Long-term overdose or the presence of associated risk factors can provoke the development of lactic acidosis, because The drug contains metformin.
Side effect
During treatment with Glucovance®, the following side effects may occur. The frequency of side effects of the drug is assessed as follows: very common: (≥1/10) common: (≥1/100,
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 30 °C.
Buy Glucovance tablet p/pl.ob. 500mg/2.5mg per bl. in pack No. 15x2 in the pharmacy
Price for Glucovance tablet p/pl.ob. 500mg/2.5mg per bl. in pack No. 15x2
Instructions for use for Glucovance tablet p/pl.ob. 500mg/2.5mg per bl. in pack No. 15x2
Glucovance, 5 mg+500 mg, film-coated tablets, 60 pcs.
During treatment with Glucovance®, it is necessary to regularly monitor the level of glycemia on an empty stomach and after meals.
Lactic acidosis
Lactic acidosis is an extremely rare but serious (high mortality rate unless promptly treated) complication that can occur due to accumulation of metformin. Cases of lactic acidosis in patients receiving metformin occurred mainly in diabetic patients with severe renal failure.
Other associated risk factors should be considered, such as poorly controlled diabetes, ketosis, prolonged fasting, excessive alcohol consumption, liver failure and any condition associated with severe hypoxia.
The risk of developing lactic acidosis should be taken into account when nonspecific signs appear, such as muscle cramps accompanied by dyspeptic disorders, abdominal pain and severe malaise. In severe cases, acidotic dyspnea, hypoxia, hypothermia and coma may occur.
Diagnostic laboratory parameters are: low blood pH, plasma lactate concentration above 5 mmol/L, increased anion gap and lactate/pyruvate ratio.
Hypoglycemia
Since Glucovance® contains glibenclamide, taking the drug is accompanied by a risk of hypoglycemia in the patient. Gradual dose titration after initiation of treatment may prevent the occurrence of hypoglycemia. This treatment can only be prescribed to a patient who adheres to a regular diet (including breakfast). It is important that carbohydrate intake is regular, as the risk of hypoglycemia increases with late meals, insufficient or unbalanced carbohydrate intake. The development of hypoglycemia is most likely during a hypocaloric diet, after intense or prolonged physical activity, when drinking alcohol or taking a combination of hypoglycemic agents.
Due to compensatory reactions caused by hypoglycemia, sweating, fear, tachycardia, hypertension, palpitations, angina pectoris and arrhythmia may occur. The latter symptoms may be absent if hypoglycemia develops slowly, in the case of autonomic neuropathy, or while taking beta-blockers, clonidine, reserpine, guanethidine or sympathomimetics.
Other symptoms of hypoglycemia in patients with diabetes may include headache, hunger, nausea, vomiting, severe fatigue, sleep disturbance, restlessness, aggression, impaired concentration and psychomotor reactions, depression, confusion, speech impairment, visual impairment, trembling, paralysis. and paresthesia, dizziness, delirium, convulsions, somnolence, unconsciousness, shallow breathing and bradycardia.
Careful prescribing, dose selection, and appropriate patient instructions are important to reduce the risk of hypoglycemia. If the patient has recurrent episodes of hypoglycemia that are either severe or due to unawareness of symptoms, treatment with other hypoglycemic agents should be considered.
Factors contributing to the development of hypoglycemia:
- simultaneous consumption of alcohol, especially during fasting;
- refusal or (especially for older patients) inability of the patient to interact with the doctor and follow the recommendations set out in the instructions for use;
- poor nutrition, irregular eating, fasting or changes in diet;
- imbalance between physical activity and carbohydrate intake;
- renal failure;
- severe liver failure;
— overdose of the drug Glucovance®;
- certain endocrine disorders: insufficiency of the thyroid gland, pituitary gland and adrenal glands;
- simultaneous use of individual medications.
Kidney and liver failure
Pharmacokinetics and/or pharmacodynamics may vary in patients with hepatic impairment or severe renal impairment. The hypoglycemia that occurs in such patients can be prolonged, in which case appropriate treatment should be started.
Instability of blood glucose levels
In the case of surgery or other cause of decompensation of diabetes, it is recommended to consider a temporary transition to insulin therapy. Symptoms of hyperglycemia include frequent urination, severe thirst, and dry skin.
48 hours before planned surgery or intravenous administration of an iodinated radiocontrast agent, Glucovance® should be discontinued. Treatment is recommended to be resumed after 48 hours and only after renal function has been assessed and found to be normal.
Kidney function
Since metformin is excreted by the kidneys, before starting treatment and regularly thereafter, it is necessary to determine creatinine Cl and/or serum creatinine levels: at least once a year in patients with normal renal function, and 2-4 times a year in elderly patients, and also in patients with creatinine Cl at ULN.
Particular caution is recommended in cases where renal function may be impaired, such as in elderly patients or when starting antihypertensive therapy, diuretics or NSAIDs.
Other Precautions
The patient should inform the doctor about the occurrence of a bronchopulmonary infection or infectious disease of the genitourinary organs.
Impact on the ability to drive a car and operate machinery.
Patients should be informed about the risk of hypoglycemia and take precautions when driving a car and operating machinery that requires increased concentration and speed of psychomotor reactions.
Glucovance®
Contraindicated combinations
Associated with the use of glibenclamide
Miconazole
increases the hypoglycemic effect, can provoke the development of hypoglycemia up to the development of coma.
Bosentan
in combination with glibenclamide increases the risk of hepatotoxicity and reduces the hypoglycemic effect of glibenclamide. Concomitant use of bosentan and glibenclamide is not recommended.
Associated with metformin use
Iodinated contrast
media
: The drug should be discontinued 48 hours before intravenous administration of iodinated contrast media and can be resumed no earlier than 48 hours after administration if there is no deterioration in renal function upon re-evaluation.
Combinations not recommended
Related to the use of sulfonylurea derivatives
Alcohol
Disulfiram-like reactions (alcohol intolerance) are very rarely observed when taking alcohol and glibenclamide simultaneously.
Alcohol intake may increase the hypoglycemic effect (by inhibiting compensatory reactions or delaying its metabolic inactivation), which may contribute to the development of hypoglycemic coma. During the period of use of the combination of glibenclamide and metformin, alcohol and medications containing ethanol should be avoided.
Phenylbutazone
(with systemic administration) increases the hypoglycemic effect of sulfonylurea derivatives (by replacing sulfonylurea derivatives at protein binding sites and/or reducing their elimination). It is preferable to use other anti-inflammatory drugs that exhibit fewer interactions, or to warn the patient about the need to self-monitor glycemic levels; If necessary, the dose should be adjusted when using an anti-inflammatory drug together and after its discontinuation.
Associated with metformin use
Alcohol
: The risk of developing lactic acidosis increases with acute alcohol intoxication, especially in the case of fasting, or poor nutrition, or liver failure. During the period of use of the combination of glibenclamide and metformin, alcohol and medications containing ethanol should be avoided.
Combinations requiring precautions during use
Associated with metformin use
Danazol
: simultaneous use of danazol is not recommended to avoid the hyperglycemic effect of the latter. If treatment with danazol is necessary and after stopping the latter, a dose adjustment of the drug Glucovance is required under the control of blood glucose concentrations.
Chlorpromazine
: when taken in large doses (100 mg per day), it increases the concentration of glucose in the blood, reducing the release of insulin. When treated with antipsychotics and after stopping the latter, dose adjustment of the drug is required under the control of blood glucose concentrations.
Glucocorticosteroids (GCS)
Systemic and local action reduces glucose tolerance, increases blood glucose concentrations, sometimes causing ketosis. During GCS therapy and after its cessation, a dose adjustment of Glucovance® is required under the control of blood glucose concentrations.
Diuretics
Some drugs may have a negative effect on kidney function, which may increase the risk of lactic acidosis, such as non-steroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase (COX) II inhibitors, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists and diuretics, especially "loop" diuretics. Close monitoring of renal function is necessary when initiating or using these drugs in combination with metformin.
Beta2-agonists prescribed by injection:
increase the concentration of glucose in the blood due to stimulation of beta2-adrenergic receptors. In this case, monitoring of blood glucose concentration is necessary. If necessary, insulin administration is recommended. When using the above drugs simultaneously, more frequent monitoring of blood glucose concentrations may be required, especially at the beginning of treatment. If necessary, the dose of metformin can be adjusted during treatment and after its cessation.
Antihypertensive drugs, with the exception of
ACE inhibitors
, can reduce blood glucose concentrations. If necessary, the dose of metformin should be adjusted.
When using the drug Glucovance® simultaneously with sulfonylurea derivatives, insulin, acarbose, salicylates
hypoglycemia may develop.
Nifedipine
increases absorption and Cmax of metformin.
Cationic drugs
(amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim and vancomycin), secreted in the renal tubules, compete with metformin for tubular transport systems and can lead to an increase in its Cmax.
Organic cation transporters (OCTs)
Metformin is a substrate of both OCT1 and OCT2 transporters.
Concomitant use of metformin with:
- OCT1 inhibitors (verapamil) may reduce the effectiveness of metformin;
- OCT1 inducers (rifampicin) can increase the absorption of metformin in the gastrointestinal tract and its effectiveness;
- OCT2 inhibitors (cimetidine, dolutegravir, ranolazine, trimethoprim, crizotinib, olaparib, daclatasvir, vandetanib) may reduce the renal elimination of metformin and thus lead to an increase in plasma concentrations of metformin.
In this regard, caution is recommended, especially in patients with renal failure, when these drugs are taken concomitantly with metformin, as the plasma concentration of metformin may increase. If necessary, a dose adjustment of metformin may be considered, as OCT inhibitors/inducers may alter the effectiveness of metformin.
Some drugs can have a hyperglycemic effect and lead to worsening glycemic control. Such drugs include phenothiazides, glucagon, estrogens, oral contraceptives, phenytoin, sympathomimetics, nicotinic acid, isoniazid, slow calcium channel blockers, thyroid hormones. With the simultaneous use of the above drugs in patients receiving metformin, the hypoglycemic effect may be reduced.
Associated with the use of glibenclamide
β-blockers
mask some symptoms of hypoglycemia: palpitations and tachycardia; Most non-selective beta-blockers increase the incidence and severity of hypoglycemia.
The patient should be warned about the need to independently monitor blood glucose concentrations, especially at the beginning of treatment.
Clonidine, reserpine, guanethidine
and sympathomimetics mask the warning signs of hypoglycemia. The patient should be warned about the need to independently monitor blood glucose concentrations, especially at the beginning of treatment.
Fluconazole
: increase in the half-life of glibenclamide with the possible occurrence of manifestations of hypoglycemia. The patient should be warned about the need to independently monitor blood glucose concentrations; It may be necessary to adjust the dose of hypoglycemic drugs during concomitant treatment with fluconazole and after discontinuation of its use.
Desmopressin
: When used together, the combination of glibenclamide + metformin reduces the antidiuretic effect of desmopressin.
Kolesevelam
: simultaneous use with the drug Glucovance® reduces the concentration of glibenclamide in the blood plasma, which can lead to a decrease in the hypoglycemic effect. This effect was not observed if glibenclamide was taken separately before taking colesevelam. Glucovance should be taken at least 4 hours before colesevelam.
ACE inhibitors (captopril, enalapril):
the use of ACE inhibitors helps reduce blood glucose concentrations. If necessary, the dose of Glucovance should be adjusted during simultaneous use with ACE inhibitors and after discontinuation of their use.
Place of the combined drug Glucovance in the treatment of type 2 diabetes mellitus
Type 2 diabetes mellitus is a chronic progressive disease based on peripheral insulin resistance and impaired insulin secretion. Modern approaches to the treatment of diabetes mellitus involve early diagnosis of the disease (at the stage of impaired glucose tolerance), aggressive treatment tactics aimed at early achievement of target glycemic values, the predominant use of combination therapy, and active insulin therapy to achieve compensation for carbohydrate metabolism.
Figure 1. Relationship between decreased effectiveness of monotherapy and disease duration
Figure 2. Structure of Glucovance
Figure 3. Change in plasma glibenclamide concentration during the day when taking Glucovance and a combination of metformin and glibenclamide
Figure 4. Canadian Diabetes Association 1998 Guidelines for Achieving Glycemic Control (Oral Antidiabetic Drugs). New EASD recommendations have been in effect since 2005
In 2005, the International Diabetes Federation proposed the following target values for achieving diabetes compensation: fasting blood glucose below 6.0 mmol/l 2 hours after meals below 8 mmol/l, glycated hemoglobin (HbA1c) below 6.5%, normolipidemia, blood pressure below 140/90 mm. Hg Art., body mass index below 25 kg/m2. The results of the DCCT study showed that achieving such indicators will minimize the risk of developing diabetes complications (Global Guadeline for type 2 Diabetes. IDF. Brussels, 2005).
Oral antidiabetic monotherapy directly affects only one of the links in the pathogenesis of type 2 diabetes. In many patients, this treatment does not provide sufficient long-term control of blood glucose levels and a need for combination therapy arises. According to the UKPDS results (Turner RC et al. 1999), monotherapy with oral hypoglycemic drugs was effective in only 50% of patients after 3 years from the start of treatment, and in only 25% after 9 years (Figure 1). This has led to growing interest in various combination therapy regimens.
Combination therapy (a combination of drugs from different groups) is carried out in case of ineffectiveness of monotherapy with the first glucose-lowering drug prescribed at the maximum dose. It is advisable to use a combination of drugs that affect both insulin secretion and the sensitivity of peripheral tissues to the action of insulin.
Already at the onset of type 2 diabetes, there is a significant decrease in both tissue sensitivity to insulin (by 70%) and insulin secretion (by 50%) (Haffner SM Diabbetes Care, 1999). In this regard, the use of a combination of drugs that affect both links of pathogenesis already in the first years of the disease is effective and appropriate. A similar combination is a combination of biguanides and sulfonylurea derivatives. Sulfonylureas have played an important role in the treatment of type 2 diabetes for more than 30 years. The action of drugs in this group is associated with increased insulin secretion and an increase in circulating insulin levels, but over time they lose the ability to maintain glycemic control and b-cell function (Rachman J., Payne MJ et al., 1998). Biguanides improve tissue sensitivity to the action of insulin. To date, the only representative of this class of drugs is metformin. The main mechanism of action of metformin is aimed at eliminating insulin resistance of the liver tissue and reducing excess glucose production by the liver (blocks gluconeogenesis enzymes). In the presence of insulin, metformin increases peripheral glucose utilization by muscles by activating insulin receptor tyrosine kinase and the translocation of GLUT4 and GLUT1 in muscle cells. By enhancing anaerobic glycolysis, metformin increases the utilization of glucose by the intestines, which is manifested in a decrease in the level of glucose in the blood flowing from the intestines. Long-term use of metformin leads to a decrease in the level of cholesterol and triglycerides in the blood, and has a positive effect on the fibrinolytic properties of the blood.
Metformin is a drug that significantly reduces the overall incidence of macro- and microvascular diabetic complications and affects the life expectancy of patients with type 2 diabetes. The results of a prospective study conducted in the United Kingdom (UKPDS) showed that the use of metformin (the original metformin Glucophage was used in the study) from the time of diagnosis reduced mortality from diabetes-related causes by 42%, all-cause mortality by 36%, and the incidence of diabetic complications is 32% (Stratton IM, Adler AL et al., 2000).
Recently, the tablet drug Glucovance, well known in Europe and many countries around the world, entered the Russian pharmaceutical market (manufactured by Merck SANTE, France, represented in Russia by Nycomed Austria GmbH), the effectiveness and safety of which have been well studied in extensive, well-planned clinical studies.
Glucovance is a combined tablet preparation that contains metformin and glibenclamide in micronized form. Currently, two dosage forms of the drug are available in Russia, containing in one tablet: metformin 500 mg and glibenclamide 5 mg and metformin 500 mg and glibenclamide 2.5 mg.
The production technology of Glucovance is unique (Donahue SR; Turner KC; Patel S., 2002): glibenclamide in the form of particles of a strictly defined size is evenly distributed in the matrix of soluble metformin (Figure 2). This structure determines the rate at which glibenclamide enters the bloodstream. When taking Glucovance, glibenclamide appears in the blood faster than when taking glibenclamide as a separate tablet. The earlier onset of peak plasma glibenclamide concentrations when taking Glucovance allows for more effective control of glucose levels after meals compared to monotherapy with its components (Donahue SR, et al.2002). Glucovance significantly increases insulin secretion 2 hours after a meal, without affecting fasting insulin levels. Thus, increased insulin secretion occurs at the optimal time to reduce the increase in glucose concentration after a meal (Figure 3).
A study of the effectiveness of Glucovance was conducted in groups of patients who did not achieve adequate glycemic control during monotherapy with glibenclamide and metformin (Marre M; Howlett H; Lehert P; Allavoine T, 2002). Thus, in a double-blind multicenter study, 411 patients who had decompensated carbohydrate metabolism while taking metformin were randomized and received therapy for 16 weeks with metformin, glibenclamide (5 mg tablets) and Glucovance in the form: metformin 500 mg/glibenclamide 2.5 mg and metformin 500 mg/glibenclamide 5 mg. To achieve target glycemic values (fasting plasma glucose ≤7.0 mmol/l), drug doses were gradually increased. The results of the study showed that the best results were achieved in the groups of patients taking Glucovance. After 16 weeks of treatment, the level of HbA1c and fasting plasma glucose in the group of patients taking Glucovance with a metformin-glibenclamide ratio of 500 mg/2.5 mg decreased by 1.2% and 2.62 mmol/l, with a metformin-glibenclamide ratio of 500 mg/5 mg by 0.91% and 2.43 mmol/l, while in the group of patients taking metformin, these indicators decreased only by 0.19% and 0.57 mmol/l, and in the group of patients taking glibenclamide by 0.33% and 0.73 mmol/l, respectively. At the same time, a higher effect of the combined drug was achieved with lower final doses of metformin and glibenclamide compared to the doses used in monotherapy. Thus, for the combination drug, the maximum doses of metformin and glibenclamide were 1225 mg/6.1 mg and 1170 mg/11.7 mg (depending on the dosage form of the drug), while with monotherapy the maximum doses of metformin and glibenclamide were 1660 mg and 13.4 mg. Thus, despite the lower dose of antidiabetic drugs, the synergistic interaction of metformin and glibenclamide, used as a combination tablet, provides a greater reduction in blood glucose levels than monotherapy. The results of numerous studies have also shown that a greater effect of the combination drug was achieved with lower final doses of metformin and glibenclamide compared with doses used in monotherapy.
A retrospective analysis also showed that Glucovance was more effective in reducing HbAlc levels than the combined use of glucophage and glibenclamide. The results of the study showed that when patients were transferred from the combined use of glucophage and glibenclamide to taking Glucovance, a significant decrease in HbAlc levels was observed (on average - 0.6%), and the effect was most pronounced in patients who had an initial HbAlc level >8%. It was also shown that Glucovance controlled postprandial glycemia more effectively than co-administration of glibenclamide and metformin (Donahue SR, et al., 2003). A stronger decrease in glucose and HbA1c levels when using Glucovance may be due to better patient compliance with the prescribed treatment (better compliance). In addition, the unique structure of Glucovance may be of some importance, namely, that when taking Glucovance, glibenclamide is absorbed faster than after taking a glibenclamide tablet, although the systemic concentration of glibenclamide with these two methods of administration is almost the same. Since Glucovance is taken with food, this helps to achieve optimal effectiveness of the drug against both meal-associated hyperglycemia and fasting hyperglycemia.
During therapy with Glucovance, a significant and prolonged decrease in HbA1c levels was observed. The HbA1c level achieved during Glucovance therapy – 7% – remained virtually unchanged over the next 52 weeks of observation (Garber AJ, Bruce S, Fiedorek FT. Clin Ther, 2002).
The indication for prescribing Glucovance is type 2 diabetes mellitus in adults - in case of ineffectiveness of previous monotherapy with metformin or glibenclamide, as well as for replacing previous therapy with two drugs - metformin and glibenclamide. Contraindications to the use of metformin and glibenclamide are also contraindications to the use of Glucovance.
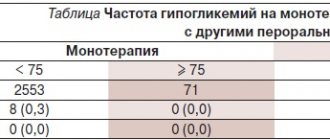
The main problems with tolerability of Glucovance as a combination drug containing glibenclamide and metformin are symptoms of hypoglycemia and side effects from the gastrointestinal tract. Reducing the dose of antidiabetic drugs, as well as the presence of a micronized form of glibenclamide, helps reduce the incidence of side effects. The incidence of hypoglycemia and dyspeptic disorders in patients who had not previously received tableted hypoglycemic drugs when taking Glucovance was significantly lower than with monotherapy with glibenclamide and metformin. In patients who had previously received metformin or sulfonylureas, the frequency of these side effects when taking Glucovance was generally the same as when taking monotherapy with its individual components. More often, symptoms of hypoglycemia during therapy with glibenclamide (both as a monotherapy and in a combination form) were observed in patients with an initial HbA1c level below 8 mmol/l. It was also shown that in elderly people there was no increase in the frequency of hypoglycemia when treated with Glucovance.
Poor compliance with doctor's recommendations is one of the main obstacles to the successful treatment of patients with various pathologies, including type 2 diabetes. The results of numerous studies show that only a third of patients with type 2 diabetes sufficiently comply with recommended therapy. The need to take several medications at the same time significantly impairs patients’ compliance with all doctor’s recommendations and affects the quality of treatment. A retrospective analysis was conducted of 1920 patients switched from oral metformin or glibenclamide monotherapy to concomitant use of these drugs and to the metformin/glibenclamide combination drug. The study results showed that among patients switched to the combination drug, the rate of treatment compliance was significantly higher than among patients switched to concomitant use of metformin and glibenclamide (77% and 54%, respectively). When transferring patients from monotherapy directly to a combination drug, there was an improvement in the degree of compliance with treatment (from 71% to 87%) (H. Howlett, F. Porte et al. Current Med. Res. And Opinions, 2003).
Glucovance is taken with meals. The dose of the drug is determined by the doctor individually for each patient, depending on the level of glycemia. Usually the initial dose is 1 tablet. Glucovance 500/2.5 mg per day. When replacing previous combination therapy with metformin and glibenclamide, the initial dose is 1-2 tablets. 500/2.5 mg depending on previous doses of single drugs. The dose of the drug is adjusted every 1-2 weeks after the start of treatment, depending on the glucose level. The maximum daily dose is 4 tablets. Glucovance 500/2.5 mg or 2 tablets. Glucovance 500/5 mg.
The following algorithm for transferring to Glucovance therapy has been proposed:
- if metformin monotherapy is ineffective, you must first gradually increase the dose of metformin to the optimal dose (for most, usually 2000 mg/day);
- in cases where monotherapy is ineffective, transfer to Glucovance at a dose of 1 tablet/day. (500/2.5), and then the dose of the drug is increased;
- in the case when transferring from the combined use of metformin and glibenclamide to Glucovance, start with a dose of 1-2 tablets/day. (500/2.5).
* When switching from Glucovance 500/2.5 mg to Glucovance 500/5 mg, the latter is started at a dose not exceeding the previously used dose.
Thus, the goal of treatment of type 2 diabetes is to achieve and maintain effective control of blood glucose levels, since the risk of development and progression of complications of type 2 diabetes and the prognosis of the disease are directly dependent on the quality of glycemic control and HbA1c levels. To achieve compensation for carbohydrate metabolism, the following treatment algorithm for patients with type 2 diabetes can be proposed, depending on the level of glycated hemoglobin (Figure 4). Combination therapy is one of the main stages in the treatment of patients with type 2 diabetes and should be used at earlier stages than is usually prescribed, since this allows for maximum effectiveness in glycemic control, as well as an effective effect on metabolic syndrome.
Thus, Glucovance is:
- The only combination drug containing a micronized form of glibenclamide, which reduces the risk of hypoglycemia.
- It has a balanced combination of metformin 500 mg and glibenclamide 2.5 and 5 mg in one tablet.
- Provides a choice of dosages - the ability to titrate doses.
- It is highly effective in small doses, which ensures better tolerability of this combination drug compared to monotherapy of each component.
- Convenient administration of the drug (good compliance).