Home | About us | Delivery | Advertisers | Login | Registration
Delivery on Sundays and holidays does not work!
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2022 Pharmacy 84.
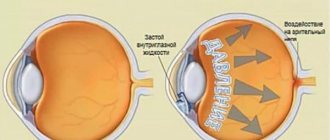
According to the European Glaucoma Society, glaucoma is a chronic progressive optical neuropathy, which combines a group of diseases with characteristic morphological changes in the head of the optic nerve (excavation) and the layer of retinal nerve fibers in the absence of other ophthalmopathology. Among the many factors involved in the development of the glaucomatous process, increased intraocular pressure (IOP) is given particular importance (for obvious reasons). Therefore, the strategic goal of drug treatment of glaucoma is to reduce IOP levels to safe values, which will ensure the preservation of visual functions and an acceptable quality of life.
There are many drugs used in the antihypertensive treatment of glaucoma. When making a choice in favor of a particular drug, it is necessary to take into account its effectiveness, safety, tolerability, impact on quality of life, assess possible adherence to treatment and the cost of the latter. The doctor takes into account the mechanism of action of the drug, possible adverse events, effects, takes into account concomitant pathology and associated contraindications to the use of each specific drug.
In recent years, trends in drug therapy are gradually changing due to the emergence of drugs from new pharmacological groups [1, 2]. One of the first registered first-line drugs were prostaglandin analogues due to their high efficiency and the absence of significant systemic and local adverse events.
Experimental and clinical studies have shown that the main mechanism of the hypotensive effect of prostaglandins is an increase in uveoscleral outflow, which can provide a decrease in IOP levels by 20-35% of initial values. After a single instillation, IOP begins to decrease after approximately 3-4 hours, achieving the greatest effect after 10-12 hours and maintaining it throughout the day. The maximum reduction in IOP levels occurs within 3-5 weeks after the start of therapy.
Latanoprost is most widely used in ophthalmological practice. This is an ester compound related to prostaglandin F2α analogues and is a prodrug. It is used in 0.005% concentration for instillation in the form of eye drops. The drug is registered by the European Agency for the Evaluation of Medicines .
EMA) and the Food and Drug Administration (
FDA) in the USA as a first-line drug for lowering IOP levels in patients with open-angle glaucoma or ocular hypertension [3] . For the first time, latanoprost 0.005% was registered under the brand name Xalatan (Pfizer) and was soon included in the WHO list of essential medicines.
Until recently, drug therapy for primary open-angle glaucoma (POAG) began with β-blockers (eg, timolol maleate). However, after the introduction into ophthalmological practice of prostaglandin analogues, such as latanoprost, travoprost, bimatoprost, tafluprost, the latter gradually began to take the place of β-blockers [4].
In its chemical structure, latanoprost is a complex molecular chain of isopropyl ether analogue of prostaglandin F2α (C26H40O5). Latanoprost is a colorless or yellowish oily liquid, highly soluble in alcohol and almost insoluble in water.
Pharmacodynamics of latanoprost
Of particular interest are the pharmacodynamic properties of latanoprost. It is known that the mechanism of the hypotensive effect of latanoprost, like all prostaglandins, is reduced to an increase in uveoscleral outflow [5]. Improving the outflow of chamber humor is an important element in the treatment of glaucoma, as it balances the ratio of production and outflow of intraocular fluid. The action of drugs of previous generations, for example cholinomimetics or partly α-2-sympathomimetics, was aimed mainly at improving the ease of outflow mainly along the trabecular pathway. Prostaglandins were the first drugs to activate outflow through the uveoscleral pathway. This mechanism of hypotensive action was confirmed in studies using radioisotopes in animals [6], as well as using fluorophotometry in humans [7].
The mechanism by which prostaglandins increase uveoscleral outflow is not fully understood. Perhaps they cause relaxation of the ciliary muscle and stimulate the formation of holes in the ciliary muscle bundles by collagenases and matrix metalloproteinases [8]. Possible mechanisms of action also include activation of the α1 and β2 integrin receptors of the ciliary muscle, which leads to contraction of the extracellular substance, or effects on the actin cytoskeleton and cell adhesion, which causes disruption of the extracellular matrix.
Pharmacokinetics of latanoprost
Data on the pharmacokinetics of latanoprost when administered topically and intravenously were obtained using samples of the drug labeled with radioactive tritium. Radioactivity was determined by the liquid scintillation method and high-performance liquid chromatography in real time [9]. The systemic pharmacokinetic profile of latanoprost was studied [9] using tritium-labeled eye drops (50 μg/ml) instilled into healthy male volunteers; each of them received latanoprost at a dose of 1.5 mcg. After local administration of a radiolabeled drug, its half-life (t½) was 17 minutes, the area under the curve (AUC) was 34 pg h/ml, and the volume of distribution was 0.36 l/kg. The maximum plasma concentration (Cmax) was 53 pg/ml after 5 minutes (Tmax). The systemic bioavailability of latanoprost after topical administration was approximately 45%, with approximately 88% of the radiolabeled drug found in urine.
Latanoprost, as an esterified prodrug, is more lipophilic than the parent drug, and therefore has better ability to penetrate the cornea. Instillation of latanoprost into the eyes of primates revealed that the drug actively penetrates into the corneal tissue, with a maximum radioactivity of 0.6 μg per 1 mg of tissue. After this peak, radioactive isotopes move from the cornea to the anterior chamber and other structures of the eye. The maximum concentration of radioactivity in the iris, in the anterior chamber humor and ciliary body was 0.6 μg/mg 1 hour after instillation of the drug, and the half-life from the eye tissue was 3-4 hours [10].
Metabolism
After entering the corneal tissue, latanoprost undergoes 100% hydrolysis under the action of esterases, mainly in the corneal epithelium [10]. Latanoprost does not undergo further metabolism in ocular tissues and, unlike other phenyl analogues, is not a substrate for 15-hydroxyprostaglandin dehydrogenase.
Following intravenous administration of 210 μg of [3H]-latanoprost to 4 healthy volunteers, the initial (t½α) and terminal (t½β) half-lives of total radioactivity were 6.6 and 69 min, respectively.
Radioactivity in plasma samples was not determined 9 hours after intravenous and 6 hours after topical administration of the drug (3 μg). In primates, the main systemic route of metabolism was oxidation of the drug in the liver. In humans, the drug was excreted primarily by the kidneys (88 and 97% of the released radioactivity after topical application and intravenous administration, respectively), and some of it was excreted in the feces (15 and 16%, respectively) [10].
Clinical effectiveness
The critical importance of latanoprost for the treatment of glaucoma is confirmed by the fact that over 10 years, more than 1,400 original articles and about 150 reviews on the clinical effectiveness of the drug have been published. The most effective regimen for using latanoprost is instillation of one drop of the drug per day at a concentration of 0.005%.
The maximum reduction in IOP levels under the influence of latanoprost occurs 8-12 hours after instillation of the drug [11]. Thus, in patients using the drug in the evening, when measuring IOP in the morning, the maximum effect of latanoprost is observed.
A systematic meta-analysis assessed the hypotensive effect and tolerability of latanoprost, bimatoprost and travoprost [12]. This meta-analysis used data from eight prospective studies involving a total of 1610 patients: one study used all three prostaglandins [13], four studies used latanoprost and bimatoprost [14, 15], two used travoprost and bimatoprost [16 , 17] and in one - latanoprost and travoprost [18].
The reduction in IOP levels from baseline was significantly greater with latanoprost than with bimatoprost at all IOP control time points. Data obtained for latanoprost and travoprost indicate that these two drugs have a similar degree of reduction in IOP levels during all hours of IOP control; however, there were no statistically significant differences between them.
In their meta-analysis, W. Stewart et al. [19] summarized data from 11 studies with 24-hour IOP monitoring in 386 patients with POAG, exfoliative glaucoma, or ocular hypertension. Prostaglandins were most effective in reducing IOP levels compared to baseline values. The effectiveness of latanoprost during the day was higher than during the night.
Van der Valk et al. [20] in a meta-analysis of 28 studies (7000 patients) showed the dynamics of IOP levels when using the most commonly prescribed drugs for the treatment of glaucoma (latanoprost, travoprost, bimatoprost, betaxolol, timolol, dorzolamide, brinzolamide and brimonidine) and placebo. When evaluating drugs for the maximum reduction in IOP levels, latanoprost took second place (31%). Compared with 0.5% timolol maleate administered twice daily, latanoprost was significantly more effective. This is the result of three out of four large ( n
=163—267) double-blind randomized studies conducted in Scandinavia, the USA and Japan. According to a study conducted in the UK, latanoprost and timolol were equally effective. Continuation of these studies for 1 and 2 years [21, 22] showed that the hypotensive effect of latanoprost persists for a long time.
Latanoprost also caused a greater reduction in IOP than brimonidine 0.2% given twice daily. This was confirmed in two large, unmasked, 6-month comparison studies [23] and one small, 6-week, double-blind study [24]. Latanoprost had a stable effect throughout the day (for example, the proportion of patients with a mean decrease in IOP <18 mm Hg at 10 and 17 hours was 43% with latanoprost in both cases and 28 and 19%, respectively, with brimonidine) [23 ].
Local adverse events
Conjunctival hyperemia is a common side effect with topical use of prostaglandins. It usually appears within the first two days after starting latanoprost therapy and its severity decreases over time (after 2-4 weeks). Most patients experience only mild hyperemia. Presumably, it occurs more frequently with bimatoprost than with latanoprost [25–27]. The reason for this is probably related to the peculiarity of the latanoprost molecule and its pharmacological profile of interaction with receptors [28].
Conjunctival hyperemia is a problem because it can affect the patient's decision to use the drug as directed by the physician, reducing patient compliance.
E. Arcieri et al found a significant increase in the degree of hyperemia in the latanoprost, bimatoprost and travoprost groups 1 week after the initial examination [29]. The degree of hyperemia reached its maximum value 15 days after the start of treatment and decreased approximately 1 month after that. Thus, it is important to note that in clinical practice, conjunctival hyperemia may decrease with daily use of prostaglandin analogues.
Increased iris pigmentation has also been reported in 5% to 25% of patients with glaucoma who received latanoprost. This effect was usually observed in patients with mixed eye colors and rarely in those with blue, green, gray or brown eyes. These changes are observed within 3 months after the start of therapy. Iris spots and nevi remain unchanged [25]. The pathogenesis of darkening of the iris has not been fully studied, but it is assumed that this phenomenon is related to an increase in melanin synthesis under the influence of prostaglandins.
Studies investigating the increase in iris pigmentation induced by latanoprost are of great importance. Concerns have been raised about the possibility of malignancy of melanocytes, as well as increased IOP levels as a result of pigmentation of the trabecular meshwork, which may reduce the outflow of aqueous humor. And although such complications have not yet been reported [30-33], E. Arranz-Marquez and co-authors, based on the results of histological examination, indicated that more significant changes occur in the iris than just an increase in the melanin content in melanocytes [34] . In this regard, patients with an increase in iris pigmentation that occurs during treatment with latanoprost should be closely monitored.
A rare phenomenon, the direct connection of which with prostaglandin treatment has not been established, is the formation of iris cysts. A connection with the use of prostaglandins was suggested by Krohn and Hove in a case report, after which a number of further reports were received indicating latanoprost as a possible cause of the formation of pigment epithelial cysts [35]. In all cases, after stopping the use of latanoprost, a decrease in the size of the cysts was observed, and after a few months they completely disappeared [35, 36]. There were no subsequent relapses.
Although the significance of this small number of reported cases must be interpreted with caution, the relationship between the occurrence of iris cysts and prostaglandin treatment is of interest. Krohn and Hove suggested that this may be due to hydrodynamic pressure resulting from increased outflow along the uveoscleral pathway. Inflammation and cell proliferation have also been reported as mechanisms of iris cyst formation [37], but it is unlikely that these mechanisms explain the possible occurrence of iris cysts associated with prostaglandin use since no signs of iritis were reported in these cases [36]. In addition, the relatively rapid reverse development after discontinuation of drops in two described cases [35], as well as the absence of relapses [35-37], indicate an unlikely connection between the formation of cysts and proliferation phenomena. The assumption of a uveoscleral mechanism for the formation of iris cysts when using prostaglandins was also met with skepticism, based on anatomical and functional premises [38]. If this effect is indeed due to latanoprost, it is unlikely that it is due to exposure to this prostaglandin alone, although no reports of iris cysts have been found with other prostaglandins.
Hypertrichosis in the form of excessive eyelash growth is considered a frequently occurring side effect with topical use of prostaglandin analogues in ophthalmology. It is assumed that hypertrichosis is associated with the induction of one of the growth phases in eyelash follicles, accelerating the resting phase [39]. Prostaglandin analogues may also increase the duration of the so-called anagen phase of eyelashes, resulting in increased eyelash length [40].
The mechanism by which latanoprost causes eyelash hypertrichosis, increased eyelash pigmentation, and changes in the skin around the eyes is not well understood. Prostaglandins are strong stimulators of melanogenesis, and FP receptors, to which latanoprost binds, are detected in all tissues of the eye, as well as in hair follicles [28]. Melanin in the skin, which is produced by its melanocytes, is transferred to neighboring keratinocytes in the basal layer of the epidermis. As keratinocytes move toward the outer surface, the melanin partially disintegrates and then disappears as the horny epithelium is renewed. This mechanism may explain why latanoprost-induced changes in the skin around the eyes are reversible. Similarly, pigment-containing melanosomes of the hair follicle bulb can migrate into hair keratinocytes. The effects of prostaglandin on the skin and eyelashes can be minimized by avoiding contact of eye drops with the skin [41].
The most discussed problems associated with the use of prostaglandins are cystoid macular edema [42] and anterior uveitis.
It is known that prostaglandins in high concentrations are mediators of inflammation, induce disruption of the barrier between blood and aqueous humor and cause an increase in IOP levels. The development of prostaglandin analogues as antihypertensive drugs for the treatment of glaucoma involved not only the use of low concentrations, but also modification of the chemical compounds to achieve the safest profile while maintaining effective hypotensive effect. In relation to latanoprost, this is a phenyl substitution of prostaglandin F2α-isopropyl ether, resulting in the formation of PhXA34, the precursor of latanoprost (PhXA41). In clinical studies using PhXA34 and latanoprost, no signs of blood-ocular barrier disruption were observed in volunteers [28]. However, latanoprost has been found to cause disruption of the blood-ocular barrier after cataract surgery [29]. Several cases of anterior uveitis associated with latanoprost treatment have been described. In eyes with pseudophakia and aphakia, persistent disruption of the blood-ophthalmic barrier is possible as a result of disruption or destruction of tight junctions of the ciliary epithelium or due to disruption of the blood-ophthalmic barrier of the retinal pigment epithelium, which leads to an increase in the flow of inflammatory cells and cytokines. It is also possible that some patients may have increased sensitivity of receptors to prostaglandin analogues, accompanied by an increase in the release of arachidonic acid and increased formation of pro-inflammatory eicosanoids. Apart from identifying risk factors from the patient's medical history, there are no other ways to identify them, so the doctor must be aware of the possibility of this rare adverse event and be attentive to it [43].
To gain a better understanding of the relationship between keratitis and topical treatment with prostaglandin analogues, claims received from almost 10,000 patients with glaucoma over a 6-year period (from 1996 to 2002) were reviewed, among which 411 cases were identified with the phenomenon of “viral ocular damage” herpes simplex" [44]. This prevalence (0.004%) is similar to the incidence in the general population. In addition, there was no correlation with prostaglandin use.
In conclusion, it should be noted that the risk of reactivation of herpes simplex virus (HSV) infection after initiation of treatment with prostaglandin analogues is quite low. However, based on rare case reports and laboratory studies, it is prudent to evaluate patients with glaucoma for the presence of HSV keratitis before prescribing a new antihypertensive drug, and if there is a history of it, consider treatment options other than prostaglandin analogues.
The use of prostaglandin F2α analogues and prostamids over time can lead to a deepening of the fold above the upper eyelid. This change disappears after discontinuation of these drugs [45–50]. J. Park et al. confirmed that in some patients treated with latanoprost, deepening of the fold over the upper eyelid is explained by atrophy of the orbital fat [51]. The authors studied the mean adipocyte density in patients treated with bimatoprost, latanoprost, or travoprost. They found that in these patients this indicator was significantly higher than in eyes without treatment. The mean adipocyte density was highest in the bimatoprost group, followed in descending order by the travoprost and latanoprost groups.
Restoration of the upper eyelid fold after discontinuation of prostaglandin analogues did not occur in all patients; it is possible that other, irreversible factors are associated with this undesirable effect.
Four cases of dendritic epitheliopathy-type corneal lesions have been described after treatment with latanoprost in patients aged 63 years or older with glaucoma, two of whom had diabetes mellitus. Clinical symptoms disappeared after discontinuation of the drug.
Cases (2 patients aged 79 and 84 years) of herpetic dermatitis of the skin around the eyes associated with treatment with latanoprost have also been described [52].
Within 5 minutes after instillation of latanoprost, a transient decrease in the sensitivity of the central parts of the cornea to mechanical influences is possible [53]. Decreased corneal sensitivity has been reported with the use of prostaglandin synthesis inhibitors. Therefore, the decrease in sensitivity may not be a direct effect of prostaglandin analogues, but may be related to other components of the study drugs, such as benzalkonium chloride, which is known to be toxic to the cornea and impair the stability of the precorneal tear film. The correlation between the decrease in sensitivity and the results of the Schirmer and Norn tests is consistent with the described effect of the drug on the development of dry eye syndrome.
Systemic adverse effects
Approximately 80% of topical drops enter the nasolacrimal duct immediately after instillation, from where the drug can enter the systemic circulation. In adults, no systemic effects have been observed following topical latanoprost administration, so data on more detailed systemic adverse events are limited [54]. However, two phase I studies and a pharmacokinetic study conducted during drug development provided a possible therapeutic range for the occurrence of systemic adverse effects in adults. Therefore, latanoprost plasma concentration, the primary outcome measure of the study, was used as an endpoint to assess systemic safety. When extrapolating data obtained in adults, it was assumed that the therapeutic range was wider in children aged 3 years, in whom the observed systemic concentration of the drug was 5-7 times higher. The wide therapeutic range indicates a low likelihood of adverse systemic events at plasma concentrations observed in neonates, children and adults. Latanoprost has been described to have rapid plasma clearance, predominantly through hepatic metabolism, and a small volume of distribution [54]. For drugs with high hepatic metabolism, such as latanoprost, clearance is determined primarily by hepatic blood flow [55, 56].
In elderly people, glaucoma and obstructive diseases of the upper respiratory tract are more common than in young people, and the use of β-blockers for glaucoma may lead to a worsening of obstructive diseases of the upper respiratory tract. Prostaglandins D2 and F2α can induce thromboxane-mediated bronchoconstrictive effects in the lungs. However, it is unlikely that latanoprost has an effect on pulmonary function since it is a relatively selective prostaglandin F2α receptor agonist and has little or no interaction with thromboxane receptors.
No significant effects were found when peak expiratory flow (PEF) determined by peak flowmetry, asthma symptoms and the need for anti-asthmatic drugs in patients with asthma (not receiving corticosteroids) were treated with latanoprost in a double-blind, randomized, placebo-controlled crossover study. There were also no changes in spirometry (PEF, forced expiratory volume in 1 s (FEV1) and FEV1/forced ventilatory capacity in 33 patients with newly diagnosed glaucoma who received latanoprost for 3 months in a prospective comparative study (patient age was not reported) [57].
The most common (4%) adverse events observed during clinical trials were upper respiratory tract infections (ARVI, influenza). Other systemic events, the incidence of which was 1-2%, included angina-type chest pain, muscle pain, joint pain, back pain, and rash (allergic in nature?) on the skin.
Isolated cases of angina [58], arterial hypertension [54] and tachycardia have been reported after initiation of latanoprost in patients with glaucoma. In addition, three patients (aged ≥54 years) with open-angle glaucoma who had no previous history of migraine and/or headache experienced the onset of migraine after latanoprost use [59]. A randomized trial showed that headache was more common in patients receiving latanoprost compared with those in the bimatoprost group (4.4 vs. 0%), but these differences were not statistically significant [14].
Conclusion
The appearance of latanoprost in clinical practice allowed for a completely new approach to the treatment of glaucoma, since the mechanism of action of this highly effective molecule affected the previously unknown uveoscleral outflow tract, which makes it possible to significantly reduce the level of IOP. In contrast to previously available drugs, the use of latanoprost led to a persistent decrease in intraocular pressure fluctuations throughout the day, while a longer-lasting decrease in IOP levels occurred compared to previously used drugs [60]. In addition, latanoprost has the significant advantage of being administered once daily, which allows ophthalmologists to alleviate the problem of improving compliance. This, in turn, will help reduce the risk of glaucoma progression and, consequently, preserve visual functions [61].
As more and more phase III studies were completed, the efficacy and safety of latanoprost were confirmed, and it became the first-line treatment for glaucoma. Based on the results of a number of analyses, it can be concluded that latanoprost has a very high systemic tolerability; Local adverse events are often minor. The pro-inflammatory mechanism of action of latanoprost should be remembered and, therefore, used with caution in some patients (for example, in those with uveitis and cystoid macular edema).
The authors declare no conflict of interest.
Information about authors
Erichev Valery Petrovich
- Dr. med. Sci., Professor, Head of the Department of Glaucoma