Pharmacodynamics and pharmacokinetics
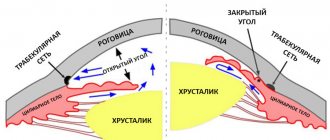
The active component of the drug is an analogue of prostaglandin F2a , its main selective antagonist , which has a high level of similarity to specific prostaglandin . The drug can reduce intraocular pressure and increase the outflow of fluid from the eye.
Approximately 120 minutes after using the drops, a decrease in intraocular pressure begins to occur, the maximum effect of the drug is observed after 12 hours. The effect of the medicine lasts about a day.
Clinical studies have shown that Travatan can be combined with other antiglaucoma drugs, such as Timolol and Brimonidine . In patients with open-angle glaucoma or high intraocular pressure, daily use reduced the pressure by approximately 30%.
The drug belongs to the group of essential prodrugs . After instillation, the active components are absorbed through the cornea of the eye, and the hydrolysis reaction of isopropyl ether with the formation of active free acid. Maximum concentrations of active metabolites are observed within 60-120 minutes after administration. The half-life is approximately 90 minutes.
The drug is excreted during metabolism through the kidneys. dosage adjustment is required for persons with renal or hepatic impairment .
Side effects
Clinical studies were conducted during which Travatan was used as monotherapy and in combination with other drugs. serious ophthalmic or systemic adverse reactions were identified.
The most common manifestations were:
- headache;
- hyperemia of the eye, conjunctiva or sclera ;
- photophobia , opalescence in the anterior chamber of the eye;
- eye irritation;
- decreased visual acuity and sensation of a foreign body in the eye;
- swelling, tearing;
- active eyelash growth or discoloration.
After some time, the hyperemia went away. In 80% of the patients studied, hyperemia was mild. Also, with prolonged use of the product, hyperpigmentation of the iris or skin around the eyes occurred.
When using the drug, the following were rarely observed:
- rapid heartbeat, bradycardia ;
- herpetic keratitis;
- change in blood pressure ;
- tinnitus, increased PSA ;
- allergic reactions;
- dysgeusia , dizziness ;
- dyspnea , asthma , cough, nasal congestion;
- corneal erosion , uveitis , keratitis , iridocyclitis ;
- dry eye syndrome , blepharitis , eye swelling, dilated pupils, cataracts , asthenopia ;
- erythema , hypertrichosis , allergic skin reactions, madarosis ;
- malaise, asthenia , muscle and bone pain.
Travatan 40mcg/ml eye drops 2.5ml No.1
A country
Belgium
Country of manufacture may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Active substance
Travoprost
Compound
macrogol glyceryl hydroxystearate - 2 mg, propylene glycol - 7.5 mg, boric acid - 3 mg, mannitol - 3 mg, sodium chloride - 3.5 mg, polydronium chloride - 0.01 mg, sodium hydroxide and/or hydrochloric acid - to adjust the pH, purified water - to 1 ml.
pharmachologic effect
Antiglaucoma drug. Synthetic analogue of prostaglandin F2?. It is a highly selective full agonist of prostaglandin FP receptors. Reduces intraocular pressure by increasing the uveoscleral outflow of aqueous humor through the trabecular meshwork and uveoscleral pathways. Intraocular pressure decreases approximately 2 hours after use of the drug, the maximum effect is achieved after 12 hours. A significant decrease in intraocular pressure can persist for 24 hours after a single use of the drug.
Indications for use
Reduction of increased intraocular pressure in: - open-angle glaucoma; - increased ophthalmotonus.
Mode of application
The drug is used topically. Prescribe 1 drop into the conjunctival sac of the eye (eye) 1 time/day, in the evening. To reduce the risk of developing systemic side effects, it is recommended to clamp the nasolacrimal duct after instillation of the drug by pressing in the area of its projection at the inner corner of the eye. If a dose of the drug is missed, treatment should be continued with the next dose. The daily dose of the drug should not exceed 1 drop into the conjunctival sac of the eye 1 time/day. Travatan® can be used in combination with other local ophthalmic drugs to reduce intraocular pressure. In this case, the interval between their use should be at least 5 minutes. If Travatan® is prescribed as a replacement for another ophthalmic drug for the treatment of glaucoma, the latter should be discontinued and the use of Travatan® should be started the next day. Dose adjustment is not required in patients with mild to severe hepatic impairment, as well as in patients with mild to severe renal impairment (with CC less than 14 ml/min). There are no or limited data on the use of Travatan® in pregnant women. Animal studies with travoprost have shown reproductive toxicity. There is no data on whether travoprost and/or metabolites are excreted in breast milk. Women during pregnancy, as well as women planning pregnancy, should avoid direct contact with substances containing prostaglandins. Prostaglandins and prostaglandin analogues are biologically active substances that can be absorbed through the skin. Women during pregnancy, as well as women planning pregnancy, should take appropriate precautions to prevent direct contact of the contents of the drug bottle with the skin. If a significant portion of the contents of the bottle does come into contact with the skin (which is unlikely), the area of skin on which the drug has come into contact should be immediately washed with water. No studies have been conducted to evaluate the effect of Travatan® on human fertility. Animal studies have shown that travoprost has no effect on fertility at doses greater than 250 times the maximum recommended human dose. The drug is contraindicated in children and adolescents under 18 years of age.
Interaction
No clinically significant drug interactions have been described.
Side effect
General profile of adverse reactions According to clinical studies, the most frequently occurring adverse events were conjunctival injection and iris hyperpigmentation, the incidence of which was 20% and 6%, respectively. The frequency of occurrence of adverse reactions is given in accordance with the following classification: very often (?1/10), often (from?1/100 to Infectious and parasitic diseases: rarely - herpetic keratitis, infection caused by Herpes simplex. On the part of the immune system: infrequently - hypersensitivity, seasonal allergies. Mental disorders: frequency unknown - depression, anxiety. From the nervous system: infrequently - headache, dizziness; rarely - dysgeusia. From the organ of vision: very often - conjunctival injection; often - hyperpigmentation of the iris, eye pain, eye discomfort, dry eye syndrome, eye irritation; uncommon - corneal erosion, uveitis, iritis, keratitis, punctate keratitis, photophobia, blepharitis, eye discharge, eyelid erythema, periorbital edema, eyelid itching, decreased visual acuity , blurred vision, lacrimation, conjunctivitis, ectropion, cataracts, crusts along the edges of the eyelids, increased eyelash growth, eyelash discoloration, asthenopia; rarely - photopsia, eczema of the eyelids, conjunctival edema, the appearance of rainbow circles around light sources, conjunctival folliculosis, eye hypoesthesia, meibomitis, pigment dispersion in the anterior chamber of the eye, mydriasis, thickening of eyelashes; frequency unknown - macular edema, retraction of the eyeballs. From the organ of hearing and labyrinthine disorders: frequency unknown - vertigo, tinnitus. From the cardiovascular system: infrequently - palpitations; rarely - irregular heartbeat, decreased heart rate; rarely - decreased diastolic blood pressure, increased systolic blood pressure, hypotension, hypertension; frequency unknown - chest pain, bradycardia, tachycardia. From the respiratory system: infrequently - dyspnea, asthma, nasal congestion, irritation in the throat; rarely - respiratory dysfunction, pain in the oropharynx, cough, dysphonia; frequency unknown - worsening of bronchial asthma. From the digestive system: rarely - constipation, dry mouth, exacerbation of stomach ulcers, disruption of the gastrointestinal tract; frequency unknown - diarrhea, abdominal pain, nausea. From the skin and subcutaneous tissues: uncommon - increased skin pigmentation in the periorbital area, skin discoloration, changes in the structure of vellus hair, hypertrichosis; rarely - allergic dermatitis, contact dermatitis, erythema, rash, discoloration of vellus hair, madarosis; frequency unknown - itching, abnormal growth of vellus hair. From the musculoskeletal system: rarely - musculoskeletal pain; frequency unknown - arthralgia. From the urinary system: frequency unknown - dysuria, urinary incontinence. Laboratory data: frequency unknown - increase in total PSA. Other: rarely - asthenia. Pediatric Adverse Event Profile In a 3-month phase III study and a 7-day pharmacokinetic study in 102 pediatric patients, the adverse event profile was consistent with that observed in adult patients. Short-term safety profiles were also similar in different subpopulations of the pediatric population. The most common adverse reactions in the pediatric population were conjunctival injection (16.9%) and increased eyelash growth (6.5%). In a similar 3-month study in adult patients, these adverse events occurred with a frequency of 11.4% and 0.0%, respectively. Additionally, in the pediatric population (n=77), a 3-month clinical study similar to that of adult patients (n=185) reported isolated cases of eyelid erythema, keratitis, lacrimation, and photophobia, for an overall adverse event rate of 1.3 % compared to 0.0% in the adult population.
Contraindications
- pregnancy; - lactation period (breastfeeding); - children and adolescents up to 18 years of age; - hypersensitivity to the components of the drug. The drug should be prescribed with caution to patients with aphakia; pseudophakia due to rupture of the posterior capsule of the lens or anterior chamber intraocular lens; risk of developing cystoid macular edema; acute inflammatory phenomena of the organ of vision, as well as patients at risk of developing iritis, uveitis.
Overdose
Toxicity from overdose when applied topically is unlikely. Treatment: in case of accidental ingestion - symptomatic and supportive therapy. In case of overdose of the drug when applied topically, rinse the eyes with warm water.
special instructions
Changes in eye color Travatan® may cause a gradual change in eye color by increasing the number of melanosomes (pigment granules) in melanocytes. This effect is detected mainly in patients with mixed iris color, for example, blue-brown, gray-brown, green-brown or yellow-brown. This effect was also noted in patients with brown irises. Typically, brown pigmentation extends concentrically around the pupil to the periphery of the iris, and the entire iris or parts of it may become a more intense brown color. The long-term effect on melanocytes and the consequences of this influence are currently unknown. Changes in iris color occur slowly and may go unnoticed for months or years. Before starting treatment, patients should be informed about the possibility of permanent changes in eye color. If only one eye is treated, persistent heterochromia may develop. After completion of travoprost therapy, no further increase in brown pigmentation of the iris was observed. Changes in the skin of the periorbital area and eyelids In controlled clinical studies, darkening of the skin of the periorbital area and/or eyelids was observed with the use of Travatan® in 0.4% of patients. Travatan® may gradually change the eyelashes in the treated eye; These changes include increased length, thickness, increased pigmentation, and/or increased number of eyelashes. The mechanism of these changes, as well as their impact on the long-term safety of the drug, has not yet been established. Changes in the orbital region and eyelids, including deepening of the eyelid sulcus, have been noted with the use of prostaglandin analogues. Information about such changes in the periorbital region was obtained during studies in monkeys and was not noted during clinical studies in humans, which allows us to consider this effect to be species specific. There is no experience with the use of Travatan® in the treatment of inflammatory diseases of the organ of vision, neovascular glaucoma, closed-angle glaucoma, in patients with narrow-angle glaucoma or congenital glaucoma. Limited data are available on the use of the drug in the treatment of ocular manifestations of thyroid diseases, open-angle glaucoma with concomitant pseudophakia, pigmentary glaucoma, pseudoexfoliative glaucoma. Patients with aphakia During treatment with prostaglandin F2 analogues? macular edema was noted. Contact with skin Contact of the drug with skin should be avoided, because Transdermal absorption of travoprost was demonstrated in experiments on rabbits. Contact lenses Before using Travatan®, contact lenses should be removed and put back in no earlier than 15 minutes after using the drug. Excipients The drug contains propylene glycol, which may cause skin irritation. The drug contains macrogol glyceryl hydroxystearate, which may cause skin reactions. Use in pediatrics Information on the effectiveness and safety of the drug in the age group from 2 months to 3 years and older is limited. Information on the use of the drug in patients under 2 months of age is not available. In patients older than 3 years, who most often receive antihypertensive therapy for primary congenital glaucoma, surgical treatment (trabeculotomy/goniotomy) remains the first-line treatment. There is no information on the long-term safety of the drug in the pediatric population. Precautions Do not touch the tip of the dropper bottle to any surface to avoid contamination of the dropper bottle and its contents. The bottle must be closed after each use. Effect on the ability to drive vehicles and operate machinery Temporary blurred vision or other visual impairments after using the drug may affect the ability to drive a car or operate machinery. If blurred vision occurs after instillation of the drug, then the patient must wait until clear vision is restored before driving a vehicle or operating machinery.
Dispensing conditions in pharmacies
On prescription
Instructions for use of Travatan (Method and dosage)
The drug is intended for ophthalmic use.
Eye drops are used once a day. The medicine is instilled into the conjunctival sac , best before bedtime.
Before the first use, you need to break the tip of the dropper bottle.
After instillation, it is recommended to close the eye or perform nasolacrimal occlusion to reduce the likelihood of developing systemic adverse reactions.
The course of treatment is determined by the attending physician.
If you additionally use another medicine in the form of drops, you can instill it after 5 minutes.
After completing a course of treatment with another ophthalmic drug, you need to take a break for a day, then you can use Travatan drops.
Avoid contact of the dropper with the mucous membrane of the eye or eyelid.
If there are problems with the liver or kidneys, no adjustment of the daily dosage is required.
TRAVATAN (drops)
I.
Every year, vision became worse and worse, if anyone knows that with open-angle glaucoma, vision decreases and cannot be restored, this is why it is dangerous. In short, after fifteen years of treatment, my father was treated to such a state that one eye stopped seeing altogether, and the other saw only three percent. I had to turn to a practicing ophthalmologist I know (a good friend of my brother), he suggested doing surgery on the eye in which I can still see, since he was afraid that situations could be different, and suggested replacing the lens. Of course, we agreed; there is no way out of this situation anymore. There is a little more than a month left before the operation, the operation should be in early September. In the meantime, before the operation, I prescribed two types of drops, and I’ll tell you about some of them now. I will add that there has always been a problem with high intraocular pressure, and if it was possible to reduce it, it was for a while and with great difficulty.
Alcon "Travatan" eye drops (photo): Alcon "Travatan" eye drops are in a very ordinary cardboard box.
On the edge of the package there is information about the composition of the medicinal product (photo):
The active substance is travoprost, which is probably where the name of the eye drops comes from.
Below is information about the manufacturer; you can understand that the product was made in Belgium.
The following edge shows the storage conditions for eye drops (photo):
There is an important note: eye drops must be used within four weeks or, more simply, within one month after opening the bottle.
On the lid of the package there is information about the date of manufacture of the medicine and the expiration date of the eye drops (photo):
Let's see what a bottle of Travatan eye drops looks like (photo):
The bottle is made of durable soft plastic and closes with a regular screw cap. The bottle is small because it contains only 2.5 mg of the drug.
View of the medicine bottle from the back (photo): There is information, but it is very small and does not repeat the information that is on the package.
The bottle of medicine is equipped with a special dropper for convenient use (photo):
Eye drops must be accompanied by instructions for using the drug (photo): I will not give the instructions in full, but I will try to highlight the main important points (photo):
Indications for the use of eye drops. Contraindications.
Dosage regimen (strictly individual) (photo):
There is a small warning: after using eye drops, vision decreases, so driving is contraindicated (photo):
Expiration date of the product; storage conditions; conditions of dispensing in pharmacies; manufacturer (photo):
Now about the drug itself, eye drops “Travatan”. My father has been using eye drops for exactly two months now. By the way, the bottle, although very small, is enough for exactly one month of use. Now I will indicate the dosage that was prescribed to him: once a day, one drop in both eyes at eight o’clock in the evening every day.
The results of the application became noticeable after a month of daily instillation of eye drops, the pressure decreased significantly, and then almost returned to normal. The usual standard medications, which had been prescribed for years, did not provide such a therapeutic effect and led to loss of vision. I would like to note that my father uses not only these drops, but also others called “Azopt” from the same manufacturer.
Trovatan eye drops are expensive, one package of eye drops costs from 530 rubles to 700 rubles depending on the pharmacy chain, they are worth it if vision is at stake.
It’s difficult for me to give any recommendations in this case; everyone decides for themselves what is more important and necessary for them.
special instructions
Eye drops may cause changes in the color of the iris. This occurs due to an increase in the number of melanosomes . Therefore, before starting treatment, the patient must be notified of the possibility of such irreversible changes. Eye color changes can occur over several years. As a rule, blue-gray, blue-brown, yellow-brown or green-brown eyes become dark brown. The process stops after stopping use of the drug.
Also, the medicine, with long-term and systematic use, can change the structure of eyelashes, increase their length, thickness, number and change color.
If drops get on the skin, wash them off with water to avoid systemic absorption of the drug.
Before using the product, gently remove contact lenses and put them on 15 minutes after the procedure.
If the patient experiences blurred vision after using the medicine, then you should not drive in this condition.
TRAVATAN eye drops 0.04 mg/ml vial-cap. 2.5 ml
special instructions
TRAVATAN may cause a gradual change in eye color by increasing the amount of brown pigment in the iris.
This effect is detected predominantly in patients with mixed iris color, for example, blue-brown, gray-brown, green-brown or yellow-brown, which is explained by an increase in the melanin content in the stromal melanocytes of the iris. This effect was also noted in patients with brown eyes. Typically, brown pigmentation extends concentrically around the pupil to the periphery of the iris, and the entire iris or parts of it may become a more intense brown color. The long-term effect on melanocytes and the consequences of this influence are currently unknown. Changes in iris color occur slowly and may go unnoticed for months or years. After discontinuation of the drug, no further increase in the amount of brown pigment was observed, however, the color change that has already developed may be irreversible.
In the presence of nevi or lentigo on the iris, no changes were noted under the influence of therapy
drug TRAVATAN.
The drug may cause darkening, thickening and lengthening of eyelashes/or an increase in their number, rarely darkening of the skin of the eyelids. The mechanism of these changes is currently unknown.
Before starting treatment, patients should be informed about the possibility of changes in eye color. Treatment of only one eye can lead to permanent heterochromia.
It is necessary to avoid contact of the drug with the skin, since transdermal absorption of travoprost was demonstrated in experiments on rabbits.
Prostaglandins and prostaglandin analogs are biologically active substances that can be absorbed through the skin. Women during pregnancy, as well as women planning pregnancy, should refrain from direct contact with substances containing
prostaglandins. If a large amount of the drug comes into contact with the skin, immediately rinse the area with water.
Before using the drug, contact lenses should be removed and put back no earlier than 15 minutes after using the drug. The product contains propylene glycol, which may cause skin irritation!
The drug contains macrogol glyceryl hydroxystearate, which can cause skin reactions!
Do not touch the tip of the dropper bottle to any surface to avoid contamination of the dropper bottle and its contents. The bottle must be closed after each use.
Reviews about Travatan
They respond well to the drug. The medicine quickly and permanently reduces intraocular pressure . Some people use it for several years. The most common adverse reactions are eye discomfort and irritation.
Some women use Travatan for eyelash growth; they do not drop the product directly into the eyes, but apply a thin layer to the growth line, which helps many.
Reviews of Travatan eye drops
- “... I’ve been using these drops for a couple of years now. I am very pleased, they are not addictive, intraocular pressure is reduced and kept normal. From using this drug, the eyelashes became thicker, denser and blacker, but the eyes did not change color”;
- “... I used the drops for 2 months, they relieve intraocular pressure, but relief does not come immediately, you need to wait for some time. I stopped using the drops because my eyes remained red and irritated throughout the day”;
- “... I regularly use Travatan to reduce intraocular pressure, it helps a lot. A funny effect: after using these drops, my eye color changed to brown.”
Travatana price, where to buy
The price of Travatan eye drops is about 600 rubles per bottle.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Travatan eye drops 40 µg/ml vial-cap.
2.5 ml 3 pcs.a. Alkon-Couvreur n.v. 1660 rub. order
Pharmacy Dialogue
- Travatan (eye drops 40 µg/ml 2.5 ml No. 1) S.A. Alkon-Kuvrer n.v.
RUR 715 order
- Travatan (eye drops 40 µg/ml 2.5 ml No. 1) S.A. Alkon-Kuvrer n.v.
RUR 773 order
- Travatan 40 µg/ml eye drops 2.5 ml No. 1Alcon
RUR 726 order
- Travatan eye drops 40 µg/ml 2.5 ml No. 3Alcon
RUB 1,582 order
show more
Pharmacy24
- Travatan 40 mcg/ml 2.5 ml eye drops Alkon-Cuvrior, Belgium
281 UAH.order
PaniPharmacy
- Travatan liquid Travatan h/c 40 µg 2.5 ml Belgium, Alcon-Couvreur
285 UAH. order
show more