28.06.2017
Diseases of the immune and endocrine system | Pancreas and adrenal glands
The first association with the word “adrenal glands” is adrenaline. This famous “macho man” in the world of hormones really comes from the adrenal glands. But he is not alone. In addition to it, small paired glands supply the body with a number of other, equally important secrets. To understand what the body loses if the adrenal glands stop coping with their task (and this is the so-called adrenal insufficiency or hypocortisolism), let’s take a closer look at the role of their hormones on the stage of the theater of life.
Most of the adrenal glands (about eighty to ninety percent) are in the so-called. the cortex is a “factory” for the production of corticosteroids. It is their deficiency that is to blame for the variety of clinical manifestations in people with adrenal insufficiency.
Glucocorticoids. The body cannot cope without them in a world full of surprises and dangers - stress, allergens, pathogens, obviously harmful or excessive physical and chemical factors, etc.
Mineralcorticoids. Without their participation, water-electrolyte metabolism and maintaining blood pressure within the physiological norm for a particular organism “here and now” is impossible.
Androgens. Significant participants in metabolic processes and regulators of sexual functions in the body of both men and women. However, the production of hormones of this class is not the sole responsibility of the adrenal glands. Therefore, they are not so significant from the perspective of our topic.
The medulla (the remaining ten to twenty percent) supplies the body with the number one stress hormone, adrenaline, and its brother, norepinephrine. The latter is primarily occupied with the honorable duty of mediating the transmission of nerve impulses. In addition, it is also involved in stress reactions, regulation of blood pressure and many other processes.
Primary adrenal insufficiency
Its manifestations are a consequence of the lack of intake into the body of the key participants in adaptation and metabolic processes discussed above - corticosteroids. And the reason for this is the destruction of the place of their formation - the bark.
This type of illness accounts for the lion's share of all cases of hypocortisolism (ninety-five percent). This disease is called Addison's disease, named after the English doctor Thomas Addison, who first described it in the mid-19th century.
The first symptoms most often appear in young and mature age (from twenty to fifty years).
What can destroy the adrenal cortex?
- The leading position is occupied by autoimmune processes (ninety-eight percent of cases).
- In second place is tuberculosis. Primary lesions in most cases are localized in the lungs, but with the bloodstream the pathogen reaches the adrenal glands and gradually destroys their tissue.
- In third place is the hereditary pathology of enzyme systems, manifested, among other things, by dystrophic processes in the glandular cortex - the so-called. adrenoleukodystrophy.
The destruction of organ tissue can also lead to:
- glandular infarction;
- metastases of neoplasms into glandular tissue;
- surgical interventions, etc.
Adrenal insufficiency (AI; adrenal insufficiency, hypocortisolism) is a clinical syndrome caused by insufficient secretion of hormones from the adrenal cortex as a result of dysfunction of one or more parts of the hypothalamic-pituitary-adrenal system (HPA).
Classification
According to the initial localization of the pathological process, NN is divided into primary (damage to the adrenal cortex itself; 1-NN) and central forms: secondary, resulting from a violation of the secretion of adrenocorticotropic hormone (ACTH), and tertiary, developing with a deficiency of corticotropin-releasing hormone (CRH). Secondary and tertiary NN are combined into central forms due to the complexity of their differential diagnosis in clinical practice. Often both are designated as secondary NN (2-NN).
I. Primary LV (1-LV)
1. Autoimmune destruction of the adrenal cortex:
1.1 Isolated 1-HH of autoimmune origin;
1.2 1-NN in the framework of autoimmune polyglandular syndromes (APS).
2. Tuberculosis of the adrenal glands.
3. Adrenoleukodystrophy.
4. Metastatic lesion of the adrenal cortex.
5. Damage to the adrenal glands due to disseminated fungal infections.
6. HIV-associated complex.
7. Iatrogenic 1-NN (bilateral adrenalectomy for Itsenko-Cushing’s disease, bilateral hemorrhage in the adrenal glands during anticoagulant therapy).
II. Central forms of NN (hypothalamic-pituitary diseases: panhypopituitarism, pituitary tumors, surgical interventions on the pituitary gland, etc.).
1-NN is a relatively rare disease, ranging from 40–60 to 100–110 new cases per 1 million adults per year. The true incidence of central forms of NN is unknown, but its most common cause is suppression of the HPA axis during chronic glucocorticoid therapy. Due to the fact that 1-NN is the most common type in clinical practice (more than 95%), we will focus on considering various aspects of this type of hypocortisolism. The clinical picture of the disease associated with the destruction of the adrenal glands by a pathological process was first and quite fully described in 1855 by the English physician Thomas Addison (1793–1860). Since then, 1-HH of tuberculous and autoimmune etiology has been designated as Addison's disease.
Etiology of primary hypocortisolism
Autoimmune destruction of the adrenal cortex is currently considered as the main cause of 1-HH. In the works of the early 1990s. It has been shown that specific immunological markers of autoimmune destruction of the adrenal cortex are antibodies to the enzymes of adrenal steroidogenesis: 21-hydroxylase (P450c21), 17a-hydroxylase (P450c17) and side chain cleavage enzyme (P450scc).
APS are considered an important aspect of the etiology of 1-NN. They mean primary damage by an autoimmune process to two or more peripheral endocrine glands, leading, as a rule, to their failure and often combined with various organ-specific non-endocrine diseases of autoimmune origin. Autoimmune polyglandular syndrome type 2 (APS-2) is the most common but less studied variant of APS. Its most common variant is Schmidt's syndrome, which is a combination of 1-HH and autoimmune thyropathies (autoimmune thyroiditis or Graves' disease). Less common is the combination of 1-NN with type 1 diabetes mellitus (Carpenter's syndrome).
Autoimmune polyglandular syndrome type 1 (APS-1; APECED-Autoimmune polyendocrinopathy-candidiasis-ectodermal-dystrophy, MEDAC-Multiple Endocrine Deficiency Autoimmune Candidiasis, candido-polyendocrine syndrome) is a rare disease with an autosomal recessive type of inheritance or even less common sporadically, for which is characterized by the classic triad described by Whiteker: mucocutaneous candidiasis, hypoparathyroidism, 1-NN.
Destruction of the adrenal cortex as a result of the tuberculosis process ranks second among the etiological factors of 1-NN. Adrenal tuberculosis develops due to hematogenous spread of mycobacteria. Typically, both the cortex and medulla are involved in the process.
Adrenoleukodystrophy (ALD, Siemerling-Creutzfeldt disease, melasma leukodystrophy) is the most common congenital peroxisomal disease with
An X-linked recessive type of inheritance, which is characterized by excessive accumulation of saturated long-chain fatty acids, mainly in myelin, and is manifested by predominant damage to the white matter of the central nervous system, adrenal cortex and testicles. There are several clinical phenotypes of ALD, from the severe childhood cerebral form to the asymptomatic course. In adrenomyeloneuropathy (35% of cases of ALD), which, as a rule, manifests itself in the 3rd–4th decade of life, NN against the background of progressive neurological symptoms (spastic paraparesis of the legs, impaired vibration sensitivity, impaired sphincter activity) develops in approximately 2/3 of patients. In 10–20% of cases of ALD, the only manifestation of the disease is NN without any signs of neurological dysfunction.
Pathogenesis and clinical manifestations of adrenal insufficiency
Primary LV
1-NN is based on an absolute deficiency of corticosteroids. Aldosterone deficiency leads to loss of sodium and water through the kidneys and gastrointestinal tract with the development of dehydration, hypovolemia, hypotension, and progressive hyperkalemia. Deficiency of cortisol, the main adaptogenic hormone of the human body, causes a decrease in resistance to various endo- and exogenous stressors, against the background of which (most often against the background of infections) decompensation of NN occurs.
The clinical picture of 1-NN was described quite fully by Thomas Addison. Over the past 150 years, only minor additions have been made to this description.
The disease usually manifests between the ages of 20 and 50 years. Hyperpigmentation of the skin and mucous membranes is the most well-known and typical symptom of Addison's disease. It is pathogenetically determined by the fact that with 1-NN, hypersecretion occurs not only of ACTH, but also of its precursor, propiomelanocortin (POMC), from which, in addition to ACTH, melanocyte-stimulating hormone is formed in excess.
Hyperpigmentation is most noticeable on open parts of the body (face, arms, neck), places of friction (skin folds, places of contact with clothing) and natural accumulation of melanin, as well as on mucous membranes (oral cavity, gums, mucous membranes of the cheeks at the level of teeth, places of dental friction). prostheses).
Weight loss is another typical symptom of NN; a progressive increase in the patient's body weight practically excludes this diagnosis. Body weight loss is usually significant, reaching 5–20 kg. General and muscle weakness at the onset of the disease can be expressed moderately (decreased performance) and reaches a significant degree with decompensation of the disease (up to adynamia).
The cardinal symptom of 1-NN is arterial hypotension. Severe systolic and diastolic hypertension in most cases allows us to exclude the diagnosis. Dyspeptic disorders of varying severity with 1-NN are almost always observed. More often it is poor appetite and nausea; periodically there are diffuse abdominal pains; less often – vomiting, stool upset. A characteristic symptom of 1-NN, pathogenetically associated with severe sodium loss, is an addiction to salty foods.
None of the listed symptoms of 1-NN, taken separately, are specific for this disease - only their combination has diagnostic value.
Features of the clinical picture of secondary hypocortisolism (2-NN)
The most important pathogenetic difference between 2-NN is the absence of aldosterone deficiency. ACTH deficiency leads in this case to a deficiency of cortisol and androgens, but does not affect the production of aldosterone, which is practically independent of adenopituitary influences, the secretion of which is regulated by the renin-angiotensin-sodium-potassium system. In this regard, the symptoms of 2-NN are quite poor. Symptoms such as arterial hypotension, dyspeptic disorders, and addiction to salty foods are not expressed. An important difference between 2-NN and 1-NN is the absence of hyperpigmentation of the skin and mucous membranes. To the fore in the clinical picture
2-NN are general weakness, weight loss, and less commonly, hypoglycemic episodes. Diagnosis is facilitated by the presence of anamnestic or clinical data on pituitary pathology, operations on the pituitary gland, and long-term use of corticosteroids.
Acute hypocortisolism
The most common cause of acute hypocortisolism is decompensation or acute manifestation of chronic forms of NN, the etiology of which is discussed above. Thus, with acute hypocortisolism, severe symptoms characteristic of chronic NN almost always occur. Less commonly, we are talking about hemorrhagic adrenal infarction, the pathogenesis of which is based on DIC syndrome in septic conditions (Waterhouse-Friderichsen syndrome) and various coagulopathies. Circulatory failure and dehydration play a major role in the pathogenesis of acute hypocorticism. There are three main forms of acute NN.
- Cardiovascular. In this case, the phenomena of collapse and acute cardiovascular failure dominate.
- Gastrointestinal. Dyspeptic symptoms dominate: severe vomiting, diarrhea. This form must be differentiated from foodborne toxic infections.
- Cerebral (meningo-encephalic). Patients are prostrated, often in a delirious state; neurological symptoms are pronounced.
Diagnosis of NN
Studies such as determining the excretion of 17-hydroxycorticosteroids (17-OX), 11-hydroxycorticosteroids (11-OX) and 17-ketosteroids (17-KS) are considered uninformative and should not be used either for diagnosing NN or in clinical medicine in general. . Determining blood cortisol levels has limited diagnostic value, since in many patients with BN it is at the lower limit of normal. However, a pronounced decrease in this indicator is the level of cortisol
The “gold standard” in diagnosing 1-NN is a test with 1-24 ACTH.
Currently, there is only one 1-24 ACTH drug on the Russian pharmaceutical market - Synacthen depot (tetracosactide), intended for intramuscular administration and having a long-term effect, which makes it difficult to interpret the test results. At 8–9 a.m. on the 1st day, the patient’s blood cortisol level is determined (in principle, this study is not necessary, since it is not the relative increase that is important, but the peak level of cortisol); at 21–22 hours the patient is injected intramuscularly with Synacthen depot (250–1000 mcg of the drug). On the 2nd day, at 8–9 am, the plasma cortisol level is determined, and if it exceeds 500–550 nmol/l, chronic 1-NN can be excluded. This modification of the test should be considered only as a practical way out of the situation in which there is no 1-24 ACTH for intravenous administration, since the test protocol given is not generally accepted.
The most significant test for diagnosing 2-NN is insulin hypoglycemia, in which short-acting insulin is administered intravenously at a dose of 0.1–0.15 U/kg. The test will have diagnostic value if the patient experiences a decrease in glycemic levels of less than 2.2 mmol/l and develops hypoglycemic symptoms. If, against this background, the blood cortisol level exceeds 20 mcg/dL (550 nmol/l), we can talk about the normal functioning of the HPA axis and the absence of both 2-NN and 1-NN. The test is contraindicated in patients with severe cardiac and other pathologies, arrhythmias, and epilepsy.
When laboratory confirmation of the presence of NN in a patient is carried out, the next step is to find out its causes. In clinical practice, having established a diagnosis of 1-NN, it is necessary to immediately exclude its tuberculous etiology. For these purposes, a chest x-ray and examination by a phthisiatrician are performed. In the absence of data indicating a tuberculosis process (and this is the most common situation), a diagnosis of idiopathic (autoimmune) Addison's disease is presumably made.
Instrumental methods that allow visualization of the adrenal glands (X-ray examination, computed tomography) traditionally play a small role in the etiological diagnosis of 1-NN. However, a number of authors report that in most cases of adrenal tuberculosis there is an enlargement of the adrenal glands according to CT and MRI, and in some, relatively rare cases, calcifications can be detected.
A laboratory marker of ALD is high levels of long-chain fatty acids. This study is especially indicated when combining
1-NN with various neurological symptoms, in particular peripheral neuropathy. To establish the diagnosis of ALD, electromyography, as well as CT and MRI of the brain, are of particular importance.
NN replacement therapy
Replacement therapy for acute hypocortisolism
- Without waiting for the results of laboratory tests (if possible, preliminary blood sampling to determine the level of cortisol, ACTH, potassium, general and biochemical blood tests), intravenous administration of 2-3 liters of saline should be started as early as possible, possibly in combination with 5-10% glucose solution. During the first day, at least 4 liters of liquid are administered. The administration of potassium-containing and hypotonic solutions, as well as diuretics, is contraindicated.
- Immediate administration of 100 mg hydrocortisone intravenously, then every 6 hours during the first 24 hours. As an alternative (during delivery to the clinic) - 4 mg of dexamethasone intravenously (or an equivalent dose of prednisolone - 40 mg), followed by transition to hydrocortisone therapy. In parallel, symptomatic therapy is most often antibiotic therapy for the infectious processes that caused decompensation of the disease.
- If the dynamics are positive, the dose of hydrocortisone is reduced to 150–200 mg/day on days 2–3 (with stable hemodynamics, the drug is administered intramuscularly). There is no need to prescribe mineralocorticoids (9alpha-fluorocortisol) until the daily dose of hydrocortisone is reduced to less than 100 mg/day.
With moderate severity of decompensation, as well as with newly diagnosed disease, therapy, as a rule, begins with intramuscular administration of 100–150 mg of hydrocortisone per day (for example, 75 mg in the morning, 50 mg at lunchtime, another 25 mg in the evening). After a few days, the dose of the drug is reduced and switched to maintenance replacement therapy with tableted corticosteroids. The pronounced positive effect of corticosteroid therapy has important diagnostic significance. In the presence of NN, patients already notice a noticeable improvement in their well-being within 1–3 days.
Replacement therapy for chronic hypocortisolism
1-NN replacement therapy involves the mandatory combined administration of gluco- and mineralocorticoids. With 2-NN, there is no mineralocorticoid deficiency and there is no need to prescribe minaralocorticoids (9alpha-fluorocortisol), unless the patient has severe arterial hypotension.
Mineralocorticoid replacement therapy. The adrenal glands have two vital functions - maintaining water-salt balance and adapting the body to environmental stressors. If the first of them, which is provided by mineralocorticoids, is violated, the body dies as a result of dehydration (loss of sodium and water) and increasing hyperkalemia. At the same time, glucocorticoid deficiency without stress influences may, in principle, have no effect. However, a typical mistake when carrying out replacement therapy for chronic 1-NN is the prescription of glucorticoid monotherapy, at best cortisone, but most often prednisolone. In this situation, despite the increasing dose of the drug, the patients' condition does not normalize, and the dose of the drug continues to increase, which in some cases leads to the development of exogenous Cushing's syndrome.
Modern 1-HH mineralocorticoid replacement therapy involves the use of only one drug - 9-alpha-fluorocortisol (fludrocortisone, Cortineff). Cortineff is prescribed once a day at a dose of 0.05–0.1 mg (usually daily in the morning). In clinical practice, it is recommended to use the following criteria for the adequacy of 9alpha-fluorocortisol therapy:
- normal plasma potassium and sodium levels;
- normal or moderately increased level of plasma renin activity;
- normal (comfortable) blood pressure;
- absence of swelling, fluid retention (signs of drug overdose).
The main advantages of Cortineff compared to other drugs with mineralocorticoid effects are their effectiveness when administered orally, as well as a powerful salt-retaining effect comparable to that of aldosterone. The half-life of Cortineff is 18–36 hours, which allows it to simulate virtually non-circadian aldosterone secretion with a single daily dose of 0.05–0.2 mg of the drug. The glucocorticoid effects of Cortineff can be neglected in practice, since they become noticeable if the dose of the drug exceeds 0.5 mg/day.
After starting Cortineff therapy, some patients develop mild, transient swelling and a tendency to retain fluid. These phenomena should not be taken as grounds for discontinuing the drug. After a few days (maximum a week), they usually resolve on their own. The dose of Cortineff sometimes has to be increased in the summer (especially in hot climates) due to increased losses of sodium and water through sweat.
Some features of Cortineff replacement therapy during pregnancy should be borne in mind, during which there is a gradual and significant increase in the level of progesterone, which, like spironolactone, is a mineralocorticoid antagonist. Therefore, during pregnancy, the dose of Cortineff may increase significantly. Dose selection should be based on plasma potassium levels and blood pressure. Cases have been described when, in accordance with the listed criteria, the dose of Cortineff had to be increased to 0.3 and even 0.6 mg per day.
Along with NN Cortineff, due to its powerful mineralocorticoid and salt-retaining properties, it is used in the treatment of idiopathic hypotension and orthostatic arterial hypotension.
Glucocorticoid replacement therapy. The doctor has a large number of glucocorticoid drugs in his arsenal that can be used for replacement therapy for NN. The latter can be carried out using the following schemes:
- using short-acting drugs (hydrocortisone - 10-20 mg in the morning and 5-10 mg in the afternoon);
- using drugs of medium duration of action (prednisolone - 5 mg in the morning and 2.5 mg in the afternoon).
All over the world, tableted hydrocortisone is most often used for replacement therapy of NN. A negative property of synthetic drugs is their relatively narrow therapeutic range. Hydrocortisone is considered the drug of choice in children and adolescents, since growth retardation in children with NN was noted in a number of studies during therapy with synthetic drugs. In this regard, when using synthetic glucocorticoids, a higher incidence of osteopenia syndrome can be expected than when using hydrocortisone therapy.
On the other hand, when treating with hydrocortisone and cortisone acetate, certain difficulties are created by the relatively short period of their action. With the classic two-time use of these drugs, patient complaints of weakness in the evening and early morning before taking them are quite typical. The following criteria for the adequacy of the glucocorticoid component of NN replacement therapy can be distinguished:
- minimally expressed complaints of weakness and low performance;
- absence of pronounced hyperpigmentation of the skin and its gradual regression;
- maintaining normal body weight, no complaints of constant hunger and no signs of overdose (obesity, Cushingoidization, osteopenia, osteoporosis).
Reliable objective (laboratory) criteria for the adequacy of NN replacement therapy with glucocorticodia are currently absent, and the selection of therapy is based almost exclusively on clinical picture data and the doctor’s experience.
Secondary adrenal insufficiency
Its manifestations (which we will discuss in more detail below) are partially similar to the primary form of the disease. But the reason is different, and it is rooted “at the top” - in the hypothalamic-pituitary system. More specifically, in the insufficient production of the “stimulating factor” by the adenohypophysis for the endocrine activity of the adrenal cortex. It's called adrenocorticotropic hormone.
What causes this condition?
- Benign (craniopharyngiomas) or malignant processes in the pituitary gland and/or hypothalamus.
- Injuries and surgical interventions in the hypothalamic-pituitary region.
- Necrotic lesion of the pituitary gland after childbirth (so-called Sheehan syndrome).
Causes of adrenal insufficiency
Adrenal insufficiency can be acute or chronic. Chronic adrenal insufficiency can be primary or secondary. Primary adrenal insufficiency (Addison's disease) occurs as a result of destruction of the tissue of the adrenal gland itself. Primary adrenal insufficiency develops when less than 10-15% of the adrenal tissue is preserved and functioning. Predisposing factors for primary adrenal insufficiency include: infectious diseases (syphilis, tuberculosis, fungal diseases of the adrenal glands); adrenal amyloidosis; HIV infection; idiopathic atrophy of the adrenal cortex (an autoimmune process, in which the body’s immune control system is disrupted for an unknown reason and autoantibodies are formed that destroy the cells of its own adrenal glands). Secondary insufficiency of the adrenal cortex occurs in diseases of the brain with damage to the pituitary gland or hypothalamus (brain tumors, traumatic brain injuries, after brain surgery, after radiation therapy, during various intoxications), which normally control the functioning of the adrenal glands. Acute adrenal insufficiency, or Addisonian crisis, is an acute coma. Acute adrenal insufficiency can develop:
- against the background of an existing chronic deficiency of adrenal hormones;
- with surgical removal of the adrenal glands due to Itsenko-Cushing's disease (Nelson syndrome);
- with abrupt withdrawal of glucocorticoids prescribed for treatment.
The cause of acute adrenal insufficiency can be autoimmune thyroiditis (Schmidt's syndrome). Acute adrenal insufficiency can occur in a newborn due to hemorrhage in the adrenal glands during difficult and protracted labor, due to birth trauma or exposure to various infections. This condition is called Waterhouse-Friderichsen syndrome. In adults and elderly people, hemorrhage into the adrenal glands can occur due to injuries to the abdomen and chest, with an overdose of anticoagulants, during surgical interventions, sepsis, peritonitis, and burns. With insufficiency of the adrenal cortex, the content of their hormones in the blood - glucocorticoids and mineralocorticoids - drops sharply. In this case, the body loses the ability to adapt to a stressful situation.
Symptoms of adrenal insufficiency
Clinical manifestations are caused by an acute shortage of hormone regulators in the body. Their severity is determined by the degree of hormonal deficiency and the preservation of compensatory mechanisms.
The primary form of the disease is characterized by:
- Loss of body weight (moderate to severe).
- Asthenic syndrome - weakness, fatigue. Muscle weakness is especially noticeable.
- Lethargy, depression, irritability.
- Tendency to depression.
- Decreased sexual desire.
- Reducing blood pressure (in hypertensive patients to normal, in people with previously normal blood pressure to hypotension levels).
- Fainting caused by stressful situations.
- Disorders in the gastrointestinal tract: pain, nausea, loss of appetite, upset stool, etc.
- Passion for salty things.
- Large amount of urine discharge.
- Severe pigmentation of the mucous membranes and skin (starting from open areas with a gradual expansion of the affected areas).
- Reducing the amount of hair in the armpits and pubic areas.
Secondary adrenal insufficiency has a milder course. The reason is that in this case the production of only glucocorticoids is disrupted, while mineralcorticoids are formed in sufficient quantities.
It is characterized by:
- asthenia;
- hypoglycemia, expressed in a transient malaise that occurs several hours after eating.
Chronic adrenal insufficiency
Both types of disturbances in the production of hormones by the adrenal cortex discussed above are of the chronic type.
Acute adrenal insufficiency
An acute situation most often occurs due to a failure of the mechanisms that compensate for the lack of hormones in Addison's disease. In this case they talk about the so-called. Addison's crisis. In other cases, acute failure may result from:
- acute injury to both glands;
- removal of the adrenal gland affected by the tumor;
- hemorrhage into the tissue of both glands (during childbirth, with fulminant sepsis, heparin overdose).
Manifestations:
- From the cardiovascular system (essentially a state of shock up to the development of collapse): hypotension; cardiopalmus; pallor of the face, cyanosis of the nasolabial triangle; cold extremities; lack of urination; fainting.
- From the digestive system (reminiscent of acute food poisoning): spasms; sharp pain; nausea; bloating; diarrhea; severe vomiting.
- From the nervous system: headache; convulsions; delirium; lethargy to the point of stupor.
Adrenal insufficiency in children
Children are diagnosed with both primary and secondary forms of adrenal insufficiency. Both of them are congenital and acquired.
Childhood illness has its own characteristics. Due to the anatomical and physiological underdevelopment of the glands in children (up to the third year of life), the risk of an acute situation is increased. This can be caused by:
- birth injury;
- acute infection;
- acute psycho-emotional stress;
- hemorrhagic infarction of the glands due to trauma, surgery, infection;
- overdose of drugs (for example, heparin);
- abrupt withdrawal of hormonal therapy.
Otherwise, the causes and clinical manifestations of childhood illness are similar to those in adult patients.
Diagnosis and treatment of adrenal diseases at GUTA CLINIC
GUTA CLINIC uses the most modern methods of diagnosis and treatment of adrenal diseases
. We specialize not only in the treatment of adrenal diseases, but also in the diagnosis and treatment of other endocrine pathologies - thyroid diseases, metabolic disorders, obesity, menopausal syndrome, diabetes.
Laboratory diagnostics are carried out
hormone levels in the blood and urine, special tests and instrumental studies (ultrasound, computed tomography, etc.)
Because adrenal disease
are mainly associated with decreased or, conversely, increased levels of adrenal hormones, the main method of treating adrenal disease is hormone-corrective therapy with dynamic monitoring of the patient’s general health.
Hormonal therapy is prescribed strictly according to indications after a thorough diagnosis. Gender, age, character, severity, course and form of the disease, as well as other factors are taken into account.
In some cases, adrenalectomy (removal of the adrenal glands) is prescribed according to indications. Surgical treatment of diseases is carried out at the level of world standards, allowing the patient to maintain a high quality of life.
About adrenal function
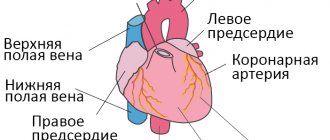
The adrenal glands are two endocrine glands located on top of the kidneys. Although the size of these glands is very small and their total weight is up to 12 grams, they perform very important functions in the body. The functioning of the entire body depends on the proper functioning of the adrenal glands.
What are the adrenal glands for?
The adrenal glands are made up of an outer layer (cortex) and an inner layer (medulla). The cortex occupies about 90% of the volume of the entire gland, and only 10% is the medulla. The hormones produced by the cortex and medulla are completely different.
The cortex synthesizes:
- hormones responsible for water-salt metabolism
- hormones responsible for carbohydrate metabolism
- sex hormones.
The medulla produces:
- adrenalin
- norepinephrine
- peptides.
Each of the functions of the adrenal glands is critically important for the human body, and a failure in the synthesis of any hormone can lead to death or disability.
Causes of adrenal disease
Pathology of the cortical layer can be caused by dysfunction of the brain, which affects the activity of hormone production in the adrenal gland itself. As a result of a violation of this mechanism, either increased secretion of hormones occurs (their excess occurs in the body), or suppressed (deficiency).
The presence of a tumor in the adrenal gland may be the cause of constant hyperproduction of the hormone in the area of production of which the tumor arose.
Congenital pathology, inflammatory diseases, circulatory disorders in the adrenal gland become the trigger point in the development of such a life-threatening condition as adrenal insufficiency.