pharmachologic effect
Pilocarpine is an antiglaucoma agent that stimulates m-cholinergic receptors. In this case, contraction of the ciliary muscle and the muscle constrictor pupil occurs. As a result of this, the iris moves away from the angle of the anterior chamber of the eye, and Schlemm's canal opens (with angle-closure glaucoma). In the case of open-angle glaucoma, Schlemm's canal and trabecular meshwork open due to a spasm of accommodation.
The hypotensive effect of Pilocarpine, which is due to the improvement of the outflow of aqueous humor, manifests itself after 10-30 minutes. The duration of the effect is about 4-6 hours.
What is presbyopia
Presbyopia (“senile vision”, age-related farsightedness, senile farsightedness) is a physiological insufficiency of accommodation associated with the aging of the eye, which leads to a gradual deterioration in the ability to clearly focus on close objects. Presbyopia, which affects people over 40 years of age, affects every fourth inhabitant of the Earth. The condition is similar to farsightedness (hyperopia), which begins in childhood and presents with symptoms of blurred vision at close distances.
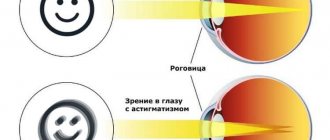
Presbyopia, a normal part of the aging process, occurs due to age-related changes in the lens (decreased elasticity and increased hardness) and the strength of the eye's ciliary muscle, causing the eye to focus light behind rather than on the retina when looking at close objects. Presbyopia is a type of refractive error along with nearsightedness, farsightedness and astigmatism.
Presbyopia can be corrected with glasses, contact lenses, multifocal intraocular lenses (artificial lenses), and refractive surgery that changes the curvature of the cornea to more accurately focus light on the retina: conductive keratoplasty, laser in situ keratomileusis (LASIK), laser subepithelial keratectomy ( LASEK), photorefractive keratectomy (PRK), insert lenses.
Mode of application
Pilocarpine is instilled into the conjunctival sac, 1-2 drops. The frequency of instillation varies depending on individual indicators.
In case of an acute attack of glaucoma, the drug is instilled 4 times during the first hour, then twice an hour for 2-3 hours. For the next 4-6 hours, Pilocarpine is instilled every hour, and then 3-6 times a day until the symptoms of the attack disappear.
For secondary types of glaucoma, Pilocarpine is prescribed 2-4 times a day. The same frequency of use is also used for open-angle glaucoma (used in combination treatment).
To narrow the pupil after using mydriatics, Pilocarpine is instilled once.
Main
Vuity (pilocarpine) is a new drug intended for the treatment of presbyopia in adults.
Viewity, developed by Allergan, an AbbVie company, is approved by the US Food and Drug Administration (FDA).
A 30-day course of Viewity eye drops will cost an American patient $86. The costs will have to be covered out of pocket as Viewity is unlikely to receive insurance coverage as it is not medically necessary due to the cheaper alternative of glasses.
“Viewity” will be in demand among people who are unhappy with the fact that they have to put on and take off reading glasses all day long if, for example, their work requires them to constantly switch between near and far vision.
"Vuity" copes with mild to moderate severity of presbyopia; the drug is less effective after the age of 65 years. Viewity should be used with caution when driving at night or performing hazardous work in poor lighting. There may also be some difficulty changing focus between near and far vision. Contact lenses should be removed before using Viuity, and then put back on 10 minutes after instillation of the drug.
Contraindications
The use of pilocarpine is contraindicated for:
- Irite;
- Iridocyclitis;
- Similar conditions where miosis can be dangerous;
- Hypersensitivity;
- Age less than 18 years;
- Conditions that predispose to retinal detachment;
- Retinal disinsertion.
Caution is required when prescribing Pilocarpine to young people with severe myopia. During pregnancy and while breastfeeding, Pilocarpine can be used only if there is a high expected benefit for the mother.
"Vuiti": effectiveness and safety of pilocarpine for presbyopia
Main results
The efficacy and safety of VUiti were tested in two phase III clinical trials, GEMINI 1 (NCT03804268) and GEMINI 2 (NCT03857542) (randomized, double-blind, placebo-controlled, multicenter), which enrolled adult (40–55 years) patients (n =750) with presbyopia.
Participants were given placebo or pilocarpine, one drop in each eye once a day for 30 days.
In both studies, the proportion of patients whose mesopic (dim-light) high-contrast binocular distance-corrected near visual acuity (DCNVA) improved by 3 or more lines (LogMAR) without losing more than 1 line (5 letters) of corrected near visual acuity distance visual acuity (CDVA) with the same refractive correction was statistically significantly greater in the pilocapine group compared to the placebo group: 31% and 26% versus 8% and 11% (p<0.01). The assessment was carried out on the 30th day of treatment, 3 hours after administration of the drug.
Functional near vision
Treatment with Viewity resulted in more patients achieving functional near vision status (DCNVA 20/40 or better) than those in the placebo group. At the same time, the peak effectiveness of treatment increased as therapy progressed, and tachyphylaxis (a rapid decrease in the therapeutic effect with repeated use of the drug, that is, the development of tolerance) was not observed.
Thus, on the 1st day of treatment in the Viuiti group, 73.9%, 82.4%, 87.5%, 77.7%, 71.0%, 72.1% and 66.5% of patients achieved this status - respectively, after 0.25, 0.5, 1, 3, 6, 8 and 10 hours after instillation of pilocarpine. In the placebo group, the proportions were as follows: 58.6% (p<0.0001), 63.8% (p<0.0001), 68.0% (p<0.0001), 64.6% (p=0 .0001), 63.3% (p=0.0264), 66.0% and 64.8%.
On the 30th day of treatment in the Viewity group, 80.9%, 86.6%, 92.7%, 87.6%, 79.6%, 78.2% and 74.9% of patients certified functional vision status near - respectively, after 0.25, 0.5, 1, 3, 6, 8 and 10 hours after instillation of pilocarpine. In the placebo group, the proportions were as follows: 74.5% (p=0.0415), 77.1% (p=0.0011), 78.9% (p<0.0001), 75.2% (p<0 .0001), 76.3%, 74.0% and 73.8%.
Severity of presbyopia
The therapeutic efficacy of Viewity correlated with the initial severity of presbyopia. Thus, when treated with VIUITY, patients with more severe presbyopia were more likely to see more lines, while patients with less severe presbyopia were more likely to achieve functional near vision (DCNVA 20/40 or better).
For example, after 1 hour of pilocarpine instillation, when therapeutic efficacy had reached its peak, the proportion of subjects in the subgroup with an initial presbyopia severity of 20/40 or better who showed an improvement of 1 or more lines in DCNVA was 75.0%, whereas Initial severity 20/80 or worse, this proportion was 93.2%. An improvement in DCNVA of 3 or more lines was true for 24.7% and 59.6% of patients in the 20/50 and 20/100 subgroups, respectively.
After 1 hour after instillation of pilocarpine, 85.4%, 69.5% and 46.6% of people in the subgroups 20/50, 20/63 and 20/80 of the initial severity of presbyopia achieved functional near vision status. After 3 hours - 75.3%, 46.3% and 35.2%, after 6 hours - 62.9%, 37.8% and 20.5%.
Mid-distance vision
Viewity provided improved vision at an average distance, based on a distance of 66 cm - the distance at which computer work is usually carried out.
Thus, according to distance-corrected visual acuity at average distance (DCIVA), which was assessed on the 30th day of treatment, the appointment of “Vuiti” led to an improvement in vision by 7.7, 6.5, 4.8, 4. 3 and 4.0 letters (5 letters - 1 line) - respectively, after 1, 3, 6, 8 and 10 hours after instillation of pilocarpine. In the placebo group, the results are as follows: 3.3 (p<0.0001), 3.0 (p<0.0001), 2.9 (p<0.0001), 2.8 (p=0.0001) and 2.4 letters (p<0.0001).
LASIK
Viewity was able to improve near visual acuity in presbyopic patients who had previously undergone the laser refractive surgery procedure LASIK.
Thus, on the 30th day of treatment, the DCNVA indicator improved by 3 or more lines in 16.7%, 38.9%, 41.7%, 37.8%, 16.2%, 13.9% and 8.3 % of patients in the Viewity group - respectively, after 0.25, 0.5, 1, 3, 6, 8 and 10 hours after instillation of pilocarpine. In the placebo group, the proportions of participants were as follows: 0.0% (p=0.0087), 2.6% (p=0.0001), 10.5% (p=0.0022), 5.1% (p =0.0005), 7.7%, 2.6% and 0.0%.
This improvement in near visual acuity was not statistically significantly different between the subgroups of patients who had previously undergone LASIK and those who had not undergone this procedure: 16.8%, 32.7%, 39.0%, 28.0%, 17.4 %, 12.6% and 10.5%.
Patient Experience
In a survey of participants (n=125) in the GEMINI clinical trial, 76% of those treated with Viewity stopped using reading glasses within the 10-hour treatment period; 84% expressed confidence that they would use Viewity when it went on sale; 83% reported that they intended to instill pilocarpine at least 5 days a week.
Interactions
Pilocarpine, when combined with beta-blockers, sympathomimetics and carbonic anhydrase inhibitors, enhances their hypotensive effect. When co-administered with adrenergic agonists, an antagonistic effect on pupil size may be observed.
The effect is inhibited by the simultaneous administration of tricyclic antidepressants, chlorprothixene, phenothiazine, clozapine. Efficiency increases when combined with cholinesterase inhibitors.
During anesthesia with halothane, bradycardia and hypotension may occur.
"Vuity": the mechanism of action of pilocarpine in presbyopia
Pilocarpine (AGN-190584), implemented in the eye drop formulation, is a cholinergic muscarinic agonist that activates muscarinic receptors located on smooth muscles such as the orbicularis iris muscle (pupillary sphincter) and ciliary muscle. Contraction of the first leads to a constriction of the pupil, which improves visual acuity at near and intermediate distances while maintaining some pupillary response to light. Contraction of the second moves the eye into a more myopic state.
Viewity's know-how is represented by an optimized formulation of pilocarpine hydrochloride (1.25%), delivered using pHast technology, which allows the ophthalmic solution to quickly adapt to the physiological pH of the tear film in order to improve patient comfort, enhance drug tolerance, and minimize blurred vision.
The effect of "Vuity", which begins to act within 15 minutes after instillation into the eyes, lasts up to 6 hours, improving near and intermediate vision without affecting distance vision.
Pilocarpine overdose, symptoms and treatment
Pilocarpine in the form of eye drops is safe due to its low systemic bioavailability and rapid elimination from the bloodstream. The administration of 20 drops of 1% solution for 1 hour did not cause the development of pronounced systemic side effects. Nausea and bradycardia are most likely. Treatment is symptomatic. Atropine can be used as a specific antidote. In case of severe bradycardia or when asystole occurs, 0.5–2 mg of atropine is administered parenterally.
List of pharmacies where you can buy Pilocarpine:
- Moscow
- Saint Petersburg