Rabeloc, 14 pcs., 20 mg, enteric-coated tablets
Inside,
whole, without chewing or chopping. Time of day and food intake do not affect the activity of rabeprazole.
Tablets 10 mg
At a dose of 10 mg 1 time per day.
If there is no effect during the first 3 days of treatment, examination by a specialist is necessary.
The maximum course of treatment without consulting a doctor is 14 days.
Tablets 20 mg
Adults
For gastric ulcers in the acute stage and anastomotic ulcers
It is recommended to take 20 mg 1 time per day. Typically, the course of therapy is 6 weeks; in some cases, the duration of treatment can be increased by another 6 weeks.
With duodenal ulcer in the acute stage
It is recommended to take 20 mg 1 time per day. The duration of treatment is from 2 to 4 weeks. If necessary, the duration of treatment can be increased by another 4 weeks.
In the treatment of erosive GERD or reflux esophagitis
It is recommended to take 20 mg 1 time per day. The duration of treatment is from 4 to 8 weeks. If necessary, the duration of treatment can be increased by another 8 weeks.
During maintenance therapy for GERD
It is recommended to take 20 mg 1 time per day.
The duration of treatment depends on the patient's condition.
For non-erosive GERD
It is recommended to take 20 mg 1 time per day. If symptoms do not disappear after 4 weeks of treatment, the patient should be further examined. After relief of symptoms, the drug should be taken at a dose of 20 mg once a day as required.
For the treatment of Solinger-Ellison syndrome and other conditions characterized by pathological hypersecretion,
the dose is selected individually. The initial dose is 60 mg/day, then the dose is increased and the drug is prescribed at a dose of up to 100 mg/day with a single dose or 60 mg 2 times a day. Treatment should be carried out as clinically necessary. In some patients with Zollinger-Ellison syndrome, the duration of treatment with rabeprazole was up to one year.
For eradication of Helicobacter pylori
It is recommended to take 20 mg 2 times a day according to a specific regimen with the appropriate combination of antibiotics. The duration of treatment is 7 days.
Patients with renal failure and elderly patients
no dose adjustment is required. In patients with mild to moderate hepatic impairment, blood concentrations of rabeprazole are usually higher than in healthy patients. When prescribing the drug to patients with severe insufficiency, caution should be exercised.
Children.
The safety and effectiveness of rabeprazole in children aged 12 years and older has been established for short-term (up to 8 weeks) treatment of GERD. The recommended dose for children aged 12 years and older is 20 mg once daily for up to 8 weeks. The safety and effectiveness of rabeprazole for other indications has not been established in pediatric patients.
Rabeloc®
Cytochrome 450 system
Rabeprazole sodium, like other proton pump inhibitors, is metabolized via the cytochrome P450 (CYP450) system in the liver. In vitro studies on human liver microsomes have shown that rabeprazole sodium is metabolized by the isoenzymes CYP2C19 and CYP3A4.
Studies in healthy volunteers have shown that rabeprazole sodium has no pharmacokinetic or clinically significant interactions with drugs that are metabolized by the cytochrome P450 system - warfarin, phenytoin, theophylline and diazepam (regardless of whether patients metabolize diazepam extensively or poorly).
A study of combination therapy with antibacterial drugs was conducted. This four-way crossover study involved 16 healthy volunteers who received rabeprazole 20 mg, amoxicillin 1000 mg, clarithromycin 500 mg, or a combination of these three drugs (RAC - rabeprazole, amoxicillin, clarithromycin). AUC and Cmax values for clarithromycin and amoxicillin were similar when combination therapy was compared with monotherapy. AUC and Cmax for rabeprazole increased by 11% and 34%, respectively, and for 14-hydroxy-clarithromycin (the active metabolite of clarithromycin), AUC and Cmax increased by 42% and 46%, respectively, for combination therapy compared with monotherapy. This increase in exposure rates for rabeprazole and clarithromycin was not considered clinically significant.
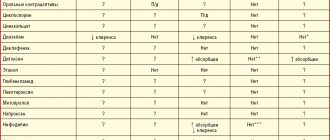
Interactions due to inhibition of gastric acid secretion
Rabeprazole sodium provides a stable and long-lasting suppression of gastric acid secretion. Thus, interactions may occur with substances for which absorption is pH dependent. When taken simultaneously with rabeprazole sodium, the absorption of ketoconazole is reduced by 30%, and the absorption of digoxin is increased by 22%. Therefore, some patients should be monitored to determine whether dose adjustments are necessary when taking rabeprazole sodium concomitantly with ketoconazole, digoxin, or other drugs for which absorption is pH dependent.
Atazanavir
When atazanavir 300 mg/ritonavir 100 mg was coadministered with omeprazole (40 mg once daily) or atazanavir 400 mg with lansoprazole (60 mg once daily) in healthy volunteers, a significant reduction in atazanavir exposure was observed. Absorption of atazanavir is pH dependent. Although concomitant use with rabeprazole has not been studied, similar results are expected for other proton pump inhibitors. Therefore, concomitant use of atazanavir with proton pump inhibitors, including rabeprazole, is not recommended.
Antacids
In clinical studies, antacid substances were used in conjunction with rabeprazole sodium. Clinically significant interactions of rabeprazole sodium with aluminum hydroxide gel or magnesium hydroxide were not observed.
Eating
In a clinical study, no clinically significant interactions were observed when rabeprazole sodium was taken with a low-fat meal. Taking rabeprazole sodium simultaneously with a fat-enriched meal may slow down the absorption of rabeprazole by up to 4 hours or more, but Cmax and AUC do not change.
Cyclosporine
In vitro experiments using human liver microsomes showed that rabeprazole inhibits the metabolism of cyclosporine with an IC50 of 62 μmol, i.e., at a concentration 50 times the Cmax for healthy volunteers after 14 days of administration of 20 mg rabeprazole. The degree of inhibition is similar to that of omeprazole for equivalent concentrations.
Methotrexate
Adverse event reports, published pharmacokinetic studies, and retrospective analyzes suggest that concomitant use of PPIs and methotrexate (primarily at high doses) may result in increased concentrations of methotrexate and/or its metabolite hydroxymethotrexate and prolong the elimination half-life. However, no specific drug interaction studies have been conducted between methotrexate and PPIs.
Impact on laboratory results
The use of PPIs leads to a decrease in gastric acidity, which can lead to an increase in serum chromogranin A (CgA). Elevated CgA levels may lead to misinterpretation of laboratory results for the presence of a neuroendocrine tumor. To avoid this effect, use of Rabeloc® should be temporarily discontinued at least 14 days before assessing CgA levels; repeating the test should be considered if the initial CgA level is high.
Rabeloc Lyophilisate, bottle, 1 piece, 20 mg, for intravenous administration, for adults
Interaction with other drugs
Drugs metabolized via cytochrome CYP450 isoenzymes
Rabeprazole sodium, like other proton pump inhibitors (PPIs), is metabolized via the cytochrome P450 (CYP450) system in the liver. In vitro studies on human liver microsomes have shown that rabeprazole sodium is metabolized by isoenzymes CYP2C19 and CYP3A4.
Studies in healthy volunteers have shown that rabeprazole sodium has no pharmacokinetic or clinically significant interactions with drugs metabolized by the cytochrome P450 system - warfarin phenytoin theophylline and diazepam (regardless of whether patients metabolize diazepam extensively or poorly).
Warfarin
There are reports of prolongation of the international normalized ratio (INR) and prothrombin time (PT) in patients receiving concomitant proton pump inhibitors including rabeprazole and warfarin. Prolongation of INR and PT may lead to an increased risk of bleeding.
Cyclosporine
In in vitro studies with human liver microsomes, rabeprazole was shown to inhibit the metabolism of cyclosporine with an inhibitory concentration of 50-62 μM, which is more than 50 times higher than the maximum concentration in healthy volunteers after administration of 20 mg of rabeprazole. This degree of inhibition was similar to that of omeprazole at equivalent concentrations.
Drugs whose absorption depends on gastric pH
Rabeprazole sodium provides a stable and long-lasting suppression of gastric acid secretion. Thus, interaction with substances for which absorption depends on pH can occur. When taken simultaneously with rabeprazole sodium, the absorption of ketoconazole is reduced by 30% and the absorption of digoxin is increased by 22%. Therefore, some patients should be monitored to determine whether dose adjustment is necessary when taking rabeprazole sodium concomitantly with ketoconazole, digoxin, or other drugs for which absorption is pH dependent.
The simultaneous use of rabeprazole and antacids does not lead to clinically significant changes in the plasma concentration of rabeprazole.
With simultaneous use of atazanavir with proton pump inhibitors, a significant decrease in the concentration of atazanavir is expected, which leads to a decrease in its therapeutic effect; therefore, simultaneous use of atazanavir with proton pump inhibitors (including rabeprazole) is not recommended.
With simultaneous use of rabeprazole amoxicillin and clarithromycin, the AUC and Cmax values for clarithromycin and amoxicillin were similar when comparing combination therapy with monotherapy. The AUC and Cmax of rabeprazole increased by 11% and 34%, respectively, and the AUC and Cmax of 14-hydroxyclarithromycin (the active metabolite of clarithromycin) increased by 42% and 46%, respectively. This increase in indicators was not considered clinically significant.
Methotrexate
Based on reports of adverse events, published pharmacokinetic studies and retrospective analysis, it can be assumed that the simultaneous use of proton pump inhibitors and methotrexate (primarily in high doses) may lead to increased concentrations of methotrexate and/or its metabolite hydroxymethotrexate and increase the half-life. However, no specific drug interaction studies have been conducted between methotrexate and proton pump inhibitors.
Medicines metabolized by the CYP2C19 isoenzyme
In a clinical study in Japan evaluating rabeprazole in patients according to CYP2C19 genotype (n=6 in each genotype category), acid suppression was greater in poor metabolizers compared to rapid metabolizers, which may be due to higher rabeprazole concentrations in poor metabolizers. Differences between slow and rapid metabolizers when interacting with other drugs metabolized by CYP2C19 have not been studied.