Zulbex®
Cytochrome P450 system
Rabeprazole sodium, like other PPIs, is metabolized by the cytochrome P450 (CYP450) system in the liver. In
vitro
studies on human liver microsomes have shown that rabeprazole sodium is metabolized by the isoenzymes CYP2C19 and CYP3A4.
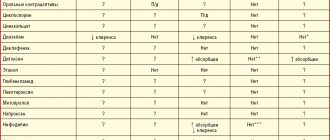
Studies in healthy volunteers have shown that rabeprazole sodium has no pharmacokinetic or clinically significant interactions with drugs that are metabolized by the cytochrome P450 system - warfarin, phenytoin, theophylline and diazepam (regardless of whether patients metabolize diazepam quickly or slowly).
A study of combination therapy with antibacterial drugs was conducted. This four-way crossover study involved 16 healthy volunteers who received rabeprazole 20 mg, amoxicillin 1000 mg, clarithromycin 500 mg, or a combination of these three drugs (RAC - rabeprazole, amoxicillin, clarithromycin). AUC and Cmax values for clarithromycin and amoxicillin were similar when combination therapy was compared with monotherapy. AUC and Cmax for rabeprazole increased by 11% and 34%, respectively, and for 14-hydroxy-clarithromycin (the active metabolite of clarithromycin), AUC and Cmax increased by 42% and 46%, respectively, for combination therapy compared with monotherapy. This increase in exposure rates for rabeprazole and clarithromycin was not considered clinically significant.
Interaction due to inhibition of gastric acid secretion
Rabeprazole sodium also causes long-term suppression of gastric juice secretion. Therefore, interactions may occur with substances for which absorption depends on the pH of gastric juice. When taken simultaneously with rabeprazole sodium, the absorption of ketoconazole is reduced by 30%, and the absorption of digoxin is increased by 22%. Therefore, when rabeprazole sodium is used concomitantly with ketoconazole, digoxin, or other drugs for which absorption is dependent on gastric pH, patients should be under medical supervision to determine whether dose adjustment is necessary.
Atazanavir
When atazanavir 300 mg/ritonavir 100 mg was coadministered with omeprazole (40 mg once daily) or atazanavir 400 mg with lansoprazole (60 mg once daily) in healthy volunteers, a significant reduction in atazanavir exposure was observed. Absorption of atazanavir depends on the pH of gastric juice. Although concomitant use with rabeprazole has not been studied, similar results are expected for other proton pump inhibitors. Therefore, concomitant use of atazanavir with PPIs, including rabeprazole, is not recommended.
Antacids
In clinical studies, antacid drugs were used concomitantly with rabeprazole sodium. No clinically significant interaction of rabeprazole sodium with aluminum hydroxide or magnesium hydroxide was observed.
Eating
In a clinical study, no clinically significant interactions were observed between rabeprazole sodium and a low-fat diet. Taking rabeprazole sodium simultaneously with a fat-enriched meal may slow down the absorption of rabeprazole by up to 4 hours or more, but Cmax and AUC do not change.
Cyclosporine
In
vitro studies using
human liver microsomes showed that rabeprazole inhibits the metabolism of cyclosporine with an IC50 of 62 μmol, i.e., at a concentration 50 times the Cmax for healthy volunteers after 20 days of administration of 20 mg rabeprazole. The degree of inhibition is similar to that of omeprazole at equivalent concentrations.
Methotrexate
Based on reports of adverse events, published pharmacokinetic studies and retrospective analysis, it can be assumed that the simultaneous use of PPIs and methotrexate (primarily in high doses) may lead to increased concentrations of methotrexate and/or its metabolite hydroxymethotrexate and increase T1/2. However, no specific drug interaction studies have been conducted between methotrexate and PPIs.
Impact on laboratory test results
PPI use leads to a decrease in gastric acidity, which may lead to an increase in serum chromogranin A (CgA) concentrations. Elevated CgA levels may lead to misinterpretation of laboratory results for the presence of a neuroendocrine tumor. To avoid this effect, use of Zulbex® should be temporarily discontinued at least 14 days before assessing serum CgA concentrations; repeating the test should be considered if the initial CgA level is high.
Zulbex, 14 pcs., 20 mg, enteric-coated tablets
Suction:
Zulbex® is a rabeprazole tablet coated with an enteric (stomach-resistant) coating. This form is due to the instability of rabeprazole in an acidic environment. Therefore, absorption of rabeprazole begins only after the tablet leaves the stomach. Absorption is fast; Cmax of rabeprazole in blood plasma is reached approximately 3.5 hours after oral administration of a dose of 20 mg. Cmax and AUC are linear in the dose range from 10 mg to 40 mg. The absolute bioavailability of an oral dose of 20 mg (compared to intravenous administration) is approximately 52%, largely due to first-pass metabolism. Bioavailability does not appear to increase with repeated use. In healthy people, T1/2 from blood plasma is approximately 1 hour (from 0.7 to 1.5 hours), and the total clearance is (283 ± 98) ml/min. There is no clinically significant interaction associated with food intake. Neither food nor the time of taking the drug affects the absorption of rabeprazole.
Distribution:
In humans, rabeprazole is approximately 97% bound to plasma proteins.
Metabolism and excretion:
rabeprazole, like other members of the class of proton pump inhibitors, is metabolized in the liver with the participation of cytochrome P450 (CYP450).
In vitro
studies with human liver microsomes have shown that rabeprazole is metabolized by CYP450 isoenzymes (CYP2C19 and CYP3A4). In these studies, rabeprazole at expected plasma concentrations in humans did not suppress or stimulate CYP3A4. These results indicate that no interaction is expected between rabeprazole and cyclosporine. In humans, the main metabolites found in plasma are the thioester (M1) and carboxylic acid (M6), with the sulfonic metabolite (M2), desmethylthioester (M4) and mercapturic acid conjugate (M5) detected in smaller quantities. Only the desmethyl metabolite (M3) has little antisecretory activity, but it is not detected in plasma.
After a single oral dose of 20 mg of 14C-labeled rabeprazole, unchanged rabeprazole is not excreted by the kidneys. Approximately 90% of the dose taken is excreted by the kidneys in the form of two metabolites: a conjugate with mercapturic acid (M5) and carboxylic acid (M6), as well as in the form of two unknown metabolites. The rest of the administered drug is found in the intestinal contents.
Floor:
Taking into account adjustments for height and body weight, no sex differences were identified in the pharmacokinetic parameters of rabeprazole at a dose of 20 mg.
Renal dysfunction:
in patients with renal failure requiring hemodialysis (Cl creatinine less than 5 ml/min/1.73 m2), the distribution of rabeprazole was similar to its distribution in healthy volunteers. AUC and Cmax in such patients were approximately 35% lower than the corresponding values in healthy volunteers. The average T1/2 of rabeprazole was 0.82 hours in healthy volunteers, 0.95 hours in patients undergoing hemodialysis and 3.6 hours after hemodialysis. Rabeprazole creatinine Cl in patients with impaired renal function requiring maintenance hemodialysis was approximately 2 times higher than in healthy volunteers.
Liver dysfunction:
after a single dose of rabeprazole 20 mg in patients with mild or moderate liver disease, the AUC increased by 2 times, and the T1/2 of rabeprazole increased by 2-3 times compared to healthy volunteers. However, after daily oral administration of a dose of 20 mg for 7 days, AUC increased only 1.5 times, and Cmax increased only 1.2 times. T1/2 of rabeprazole in patients with impaired liver function was 12.3 hours compared to 2.1 hours in healthy volunteers. The pharmacodynamic response (control of gastric pH) in the two groups was clinically comparable.
Elderly patients:
in elderly patients, the excretion of rabeprazole is slightly reduced. After using rabeprazole for 7 days at a daily dose of 20 mg, AUC increased approximately 2 times, Cmax increased by 60%, T1/2 was increased by 30% compared to healthy young volunteers. There were no signs of accumulation of rabeprazole.
CYP2C19 polymorphism:
after oral administration of rabeprazole at a dose of 20 mg in people with slow CYP2C19 metabolism, AUC and T1/2 were approximately 1.9 and 1.6 times higher than the corresponding parameters in people with active metabolism, while Cmax increased only by 40% .
Zulbex enteric tablets 20 mg 14 pcs.
Rabeprazole belongs to the class of antisecretory drugs, substituted benzimidazoles, which do not have anticholinergic or antihistamine (H2) properties, but suppress gastric secretion by inhibiting the enzyme H+/K+-ATPase (proton pump). The effect of the drug depends on the dose and leads to the suppression of basal and stimulated secretion of hydrochloric acid in the stomach, regardless of stimulating factors. Animal studies have shown that rabeprazole rapidly disappears from the plasma and gastric mucosa. Being a weak base, rabeprazole is rapidly absorbed in all dosages and accumulates in the acidic environment of the parietal cells of the stomach. Rabeprazole is converted to the sulfenamide form by protonation and then interacts with available cysteine molecules in the proton pump. Antisecretory effect: after oral administration of 20 mg of rabeprazole, the antisecretory effect begins to develop within 1 hour, reaching a maximum after 2-4 hours. Suppression of basal and food-stimulated secretion of hydrochloric acid in the stomach 23 hours after taking the first dose of rabeprazole is 69% and 82% , respectively, and lasts up to 48 hours. The inhibitory effect of rabeprazole on the secretion of hydrochloric acid when taking repeated doses increases slightly, reaching an equilibrium state after 3 days. After discontinuation of the drug, the secretory activity of the stomach is restored after 2-3 days. In vitro, rabeprazole was found to have a bactericidal effect against Helicobacter pylori. Eradication of Helicobacter pylori with rabeprazole and antimicrobial drugs leads to a high degree of healing of mucosal lesions. According to the results of clinical studies, it was found that taking 20 mg of rabeprazole 2 times a day in combination with two antibiotics, for example clarithromycin and amoxicillin or clarithromycin and metronidazole for 1 week, can achieve a Helicobacter pylori eradication rate of more than 80% in patients with gastroduodenal ulcers. When choosing an appropriate combination for Helicobacter pylori eradication, one should be guided by approved treatment standards. In patients with persistent infection (in the presence of initially sensitive strains of microorganisms), it is necessary to take into account the possibility of developing secondary resistance to antibacterial drugs when choosing a dosage regimen. Effect on serum gastrin: In clinical studies, patients received rabeprazole 10 or 20 mg once daily for up to 43 months. Serum gastrin concentrations increased during the first 2 to 8 weeks of dosing, reflecting a suppressive effect on hydrochloric acid secretion, and then remained stable with continued therapy. Gastrin concentrations returned to baseline values usually within 1-2 weeks after discontinuation of therapy. Biopsy specimens from the antrum and fundus of the stomach obtained from more than 500 patients receiving rabeprazole or comparator treatment for up to 8 weeks showed no changes in ECL cellularity, histology, grade of gastritis, incidence of atrophic gastritis, intestinal metaplasia or extent infection with H. pylori in more than 250 patients observed over 36 months of therapy, no significant changes in the initially existing conditions were detected. Other effects: the systemic effect of rabeprazole on the central nervous system, cardiovascular and respiratory systems has not been identified to date. Rabeprazole, administered orally at a dose of 20 mg for 2 weeks, had no effect on thyroid function, carbohydrate metabolism or circulating concentrations of parathyroid hormone, cortisol, estrogens, testosterone, prolactin, cholecystokinin, secretin, glucagon, follicle-stimulating hormone (FSH), luteinizing hormone (LH), renin, aldosterone or growth hormone. Clinical studies have shown that rabeprazole does not interact clinically with amoxicillin and does not have a negative effect on the plasma concentrations of amoxicillin or clarithromycin when these drugs are used simultaneously to eradicate H. pylori from the upper gastrointestinal tract.
Zulbex
World Health Organization (WHO) side effect frequency classification:
very often >1/10
often > 1/100 to
uncommon > 1/1000 to
rarely from >1/10000 to
very rarely from
In each group, adverse events while taking the drug are listed in order of decreasing severity.
From the hematopoietic system: rarely: neutropenia, leukopenia, thrombocytopenia, leukocytosis.
From the immune system: rarely: hypersensitivity reactions.
Metabolic and nutritional disorders: rarely: anorexia, weight gain; very rarely: hyponatremia.
From the nervous system: often: headache, dizziness, insomnia; uncommon: drowsiness, nervousness; rarely: depression; very rarely: confusion.
From the senses: rarely: visual disturbances.
From the cardiovascular system: very rarely: peripheral edema.
From the respiratory system: often: cough, pharyngitis, rhinitis; uncommon: bronchitis, sinusitis.
From the digestive system: often: diarrhea, vomiting, nausea, abdominal pain, constipation, flatulence; uncommon: dyspepsia, dry oral mucosa, belching; rarely: gastritis, stomatitis, taste changes, hepatitis, jaundice, hepatic encephalopathy.
From the skin: uncommon: rash, erythema; rarely: itching, sweating, bullous rash; very rare: erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome.
From the musculoskeletal system: often: nonspecific pain, back pain; uncommon: myalgia, calf muscle cramps, arthralgia.
From the urinary system: uncommon: urinary tract infections; rarely: interstitial nephritis.
From the reproductive system: very rarely: gynecomastia.
Laboratory indicators: uncommon: increased activity of liver enzymes.
Other: often: asthenia, influenza-like illness.
Current experience with intentional or accidental overdose with rabeprazole is limited. The maximum established amount of the drug taken did not exceed 60 mg twice a day or 160 mg once a day. The effects were insignificant and corresponded to the known range of adverse reactions that resolved spontaneously without any additional medical intervention. A specific antidote is unknown. Dialysis is ineffective. Treatment is symptomatic.