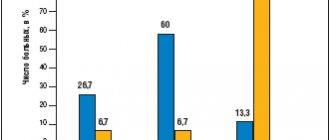
Effect of risperidone
The mechanism of action is caused by the effect on dopamine, serotonin, adrenergic and histamine receptors of nerve cells. Due to the fact that in many endogenous, organic mental diseases the functions of these receptor systems are changed, risperidone has a fairly broad therapeutic effect:
- For productive symptoms of psychosis and schizophrenia (delusions and hallucinations), for negative ones - apathy, isolation, lack of emotions, autism.
- Reduces pathologically elevated mood (mania).
- Calming, anti-anxiety, hypnotic effect.
- Reduces (or removes) aggressiveness, self-aggression.
- Anti-suicidal action.
- Reducing the pathological craving for drugs and alcohol in case of addiction.
- Removes obsessive thoughts, actions and sensations.
Release form
One of the advantages of this drug is the presence of all the dosage forms necessary for psychiatrists: tablets, drops, extended-release injections (depot).
Pills
2 or 4 milligrams per package of 10,20,30,50,60 or 100 pieces
Drops
. In bottles of 30 or 100 milliliters. A special graduated pipette is included, which is used to determine the amount of a single dose to take.
Depot
. In powder form for the preparation of a long-acting intramuscular suspension. Dosages: 25, 37.5 and 50 milligrams. It is used as an intramuscular injection, which will ensure slow release and entry of the drug into the blood over 2 - 3 weeks. Produced under the name RISPOLEPT CONST.
Rispolept Konsta por d/susp for IM injection prolong. 25mg fl incl. with r-lem
Registration Certificate Holder
JOHNSON & JOHNSON (Russia)
Dosage form
Medicine - Rispolept® Consta®
Description
Powder for the preparation of a suspension for intramuscular administration of prolonged action
white or almost white, free from visible foreign matter; the attached solvent is transparent, colorless, free from visible mechanical inclusions; the drug should easily form a suspension in the solvent; there should be no lumps or visible inclusions in the suspension; the suspension should pass through the needle smoothly, with little or no resistance.
1 g microgranules
1 fl.
risperidone (in the form of extended-release microgranules) 381 mg 25 mg
Excipients
: copolymer of lactic and glycolic acids - 619 mg (per 1 g of microgranules).
Solvent:
carmellose sodium 40 mPa.s - 22.5 mg, polysorbate 20 - 1 mg, sodium hydrogen phosphate dihydrate - 1.27 mg, anhydrous citric acid - 1 mg, sodium chloride - 6 mg, sodium hydroxide - 0.54 mg, water for injection - up to 1 ml .
Vials with pink stopper (1) complete with syringe filled with solvent (2 ml), 1 Alaris™ Smart Site® needle-free suspension device, Needle-Pro® safety needle (with safety device) for intramuscular injection ( 1 pc.) - contour cell packaging (1) - cardboard packs.
Indications
- treatment and prevention of exacerbations of schizophrenia and schizoaffective disorders.
Contraindications for use
- hypersensitivity to risperidone or any other ingredient of this drug;
- lactation period (breastfeeding);
- children and adolescents up to 18 years of age.
Carefully _
Use with caution in the following conditions:
- diseases of the cardiovascular system (chronic heart failure, previous myocardial infarction, cardiac muscle conduction disorders);
- dehydration and hypovolemia;
- cerebrovascular accidents;
- Parkinson's disease;
- seizures and epilepsy (including history);
- severe renal failure;
- liver failure;
- drug abuse or dependence;
- conditions predisposing to the development of tachycardia of the “pirouette” type (bradycardia, electrolyte imbalance, concomitant use of medications that prolong the QT interval);
- brain tumor, intestinal obstruction, cases of acute drug overdose, Reye's syndrome (the antiemetic effect of risperidone may mask the symptoms of these conditions);
- pregnancy.
pharmachologic effect
Risperidone is a selective monoaminergic antagonist. It has high affinity for serotonergic 5-HT2 receptors and dopaminergic D2 receptors. In addition, risperidone binds to alpha1-adrenergic receptors and, to a lesser extent, to H1-histamine and alpha2-adrenergic receptors. Risperidone does not bind to cholinergic receptors. Although risperidone is a potent D2 receptor antagonist, thereby improving the positive symptoms of schizophrenia, this drug, compared with typical antipsychotics, inhibits motor activity to a lesser extent and is less likely to cause catalepsy. Due to its balanced central antagonism of serotonin and dopamine receptors, risperidone is less likely to cause extrapyramidal side effects and has a therapeutic effect on the negative and affective symptoms of schizophrenia.
Drug interactions
The interactions of Rispolept Consta® with other drugs have not been systematically assessed. The interaction data presented in this section is based on studies of the oral formulation of Rispolept®.
Interactions related to the pharmacodynamics of the drug
Centrally acting drugs and alcohol
Rispolept Konsta®
increases the severity of the inhibitory effect on the central nervous system of opioid analgesics, hypnotics, anxiolytics, tricyclic antidepressants, general anesthesia, and alcohol.
Levodopa and dopamine receptor agonists
Rispolept Konsta® may weaken the effect of levodopa and other dopamine receptor agonists. If concurrent use is necessary, especially in patients with end-stage Parkinson's disease, the minimum effective dose of each drug should be prescribed.
Antihypertensive drugs
Clinically significant arterial hypotension is observed when risperidone is used concomitantly with antihypertensive agents.
Drugs that prolong the QT interval
Caution should be exercised when using Rispolept Konsta together.®
with drugs that increase the QT interval, such as antiarrhythmics (quinidine, disopyramide, procainamide, propafenone, amiodarone, sotalol), tricyclic antidepressants (amitriptyline), tetracyclic antidepressants (maprotiline), some antihistamines, other antipsychotics, some antimalarials ( quinine, mefloquine), drugs that cause electrolyte imbalance (hypokalemia, hypomagnesemia), bradycardia and drugs that inhibit the metabolism of risperidone in the liver.
Paliperidone
Because paliperidone is an active metabolite of risperidone, caution should be exercised when Xeplion is coadministered with risperidone or oral paliperidone for an extended period of time. Data on the safety of Xeplion and other antipsychotics are limited.
Interactions related to drug pharmacokinetics
Risperidone is primarily metabolized by CYP2D6 and to a lesser extent by CYP3A4. Both risperidone and its active metabolite 9-hydroxyrisperidone are substrates of P-glycoprotein (P-GP). Substances that alter the activity of CYP2D6, or substances that potently inhibit or induce the activity of CYP3A4 and/or P-GP, may affect the pharmacokinetics of risperidone and the active antipsychotic fraction.
Strong CYP2D6 inhibitors
Concomitant use of Rispolept Consta® with a strong CYP2D6 inhibitor may increase the plasma concentration of risperidone, but to a lesser extent the active antipsychotic fraction. Higher doses of a strong CYP2D6 inhibitor may increase concentrations of risperidone and the active antipsychotic fraction (eg, paroxetine, see below). It is expected that other CYP2D6 inhibitors, such as quinidine, may affect risperidone plasma concentrations in a similar manner. When using paroxetine, quinidine or another strong CYP2D6 inhibitor, especially at higher doses, the dose of Rispolept Consta® should be adjusted.
CYP3A4 and/or P-GP inhibitors
Co-administration of Rispolept Consta with a strong CYP3A4 and/or P-GP inhibitor may significantly increase plasma concentrations of risperidone and the active antipsychotic fraction. When co-administering itraconazole or another strong inhibitor of CYP3A4 and/or P-GP, the dose of Rispolept Consta® should be adjusted.
Inducers of CYP3A4 and/or P-GP
Concomitant use of Rispolept Consta® with a strong inducer of CYP3A4 and/or P-GP may reduce plasma concentrations of risperidone and the active antipsychotic fraction. When using carbamazepine or another strong inducer of CYP3A4 and/or P-GP simultaneously, the dose of Rispolept Consta® should be adjusted. The degree of induction may vary over time, with the maximum effect achieved up to 2 weeks after administration and a decrease in induction up to 2 weeks after discontinuation of the drug.
Drugs that bind tightly to plasma proteins
When used together with drugs that are highly bound to plasma proteins, no clinically significant displacement of the drug from plasma proteins is observed.
If used concomitantly, please refer to the instructions for use of the relevant drug and, if necessary, adjust the dosage of the drugs taken.
Antibacterial drugs
Erythromycin, a moderate CYP3A4 inhibitor and P-GP inhibitor, does not alter the pharmacokinetics of risperidone and the active antipsychotic fraction.
Rifampin, a strong inducer of CYP3A4 and P-GP, reduces plasma concentrations of the active antipsychotic fraction.
Anticholinesterase drugs
Donepezil and galantamine, substrates of CYP2D6 and CYP3A4, do not have a clinically significant effect on the pharmacokinetics of risperidone and the active antipsychotic fraction.
Antiepileptic drugs
Carbamazepine, a strong inducer of CYP3A4 and P-GP, reduces the plasma concentration of the active antipsychotic fraction of risperidone. A similar effect is observed with the use of phenytoin and phenobarbital, which are also inducers of CYP3A4 and P-GP.
Topiramate moderately reduces the bioavailability of risperidone, but not the active antipsychotic fraction. This interaction is not considered clinically significant.
Risperidone does not have a clinically significant effect on the pharmacokinetics of valproic acid or topiramate.
Antifungal drugs
Itraconazole, a strong inhibitor of CYP3A4 and P-GP, at a dose of 200 mg/day increases the plasma concentration of the active antipsychotic fraction by approximately 70% when using risperidone at a dose of 2 to 8 mg/day.
Ketoconazole, a strong inhibitor of CYP3A4 and P-GP, at a dose of 200 mg/day increases plasma concentrations of risperidone and reduces plasma concentrations of 9-hydroxyrisperidone.
Antipsychotic drugs
Phenothiazines may increase plasma concentrations of risperidone, but to a lesser extent the concentration of the active antipsychotic fraction.
Aripiprazole, a CYP2D6 and CYP3A4 substrate: Risperidone has no effect on the pharmacokinetics of aripiprazole and its active metabolite, dihydroaripiprazole.
Antiviral drugs
Protease inhibitors: No formal study data available. Because ritonavir is a strong inhibitor of CYP3A4 and a weak inhibitor of CYP2D6, ritonavir and ritonavir-boosted protease inhibitors may result in increased concentrations of risperidone and the active antipsychotic fraction.
Beta blockers
Some beta blockers may increase plasma concentrations of risperidone, but not the active antipsychotic fraction.
Blockers of "slow" calcium channels
Verapamil, a moderate inhibitor of CYP3A4 and P-GP, increases plasma concentrations of risperidone and the active antipsychotic fraction.
Cardiac glycosides
Risperidone does not have a clinically significant effect on the pharmacokinetics of digoxin.
Diuretics
See Cautions section for increased mortality in elderly patients with dementia when furosemide is coadministered with oral risperidone.
Gastrointestinal drugs
H2 receptor antagonists: cimetidine and ranitidine, which are weak inhibitors of CYP2D6 and CYP3A4, increase the bioavailability of risperidone, but have a minimal effect on the concentration of the active antipsychotic fraction.
Lithium preparations
Risperidone does not have a clinically significant effect on the pharmacokinetics of lithium preparations.
Serotonin reuptake inhibitors and tricyclic antidepressants
Fluoxetine, a strong CYP2D6 inhibitor, increases plasma concentrations of risperidone but has less effect on the concentration of the active antipsychotic fraction.
Paroxetine, a strong CYP2D6 inhibitor, increases plasma concentrations of risperidone, but at doses up to 20 mg/day has a lesser effect on the concentration of the active antipsychotic fraction. However, higher doses of paroxetine may increase the concentration of the active antipsychotic fraction of risperidone.
Tricyclic antidepressants may increase plasma concentrations of risperidone but do not affect the concentration of the active antipsychotic fraction. Amitriptyline does not affect the pharmacokinetics of risperidone or the active antipsychotic fraction.
Sertraline is a weak inhibitor of CYP2D6, and fluvoxamine is a weak inhibitor of CYP3A4. At doses up to 100 mg/day, sertraline and fluvoxamine do not have a clinically significant effect on the concentration of the active antipsychotic fraction of risperidone. However, the use of doses above 100 mg/day may lead to increased concentrations of risperidone and the active antipsychotic fraction.
Dosage regimen
In patients who have not previously received risperidone, it is recommended to determine the tolerability of oral dosage forms of risperidone before starting treatment with Rispolept Consta®.
Rispolept Konsta® is administered once every 2 weeks by deep injection into the gluteal or deltoid muscle, using the sterile needle supplied with the syringe. For injection into the deltoid muscle, a 25 mm long needle is used, and for injection into the gluteal muscle, a 51 mm long needle is used. Injections should be made alternately into the right and left gluteal or deltoid muscles. The drug cannot be administered intravenously.
Instructions for use are given below in the “Instructions for Use” subsection.
Adults (over 18 years old)
The recommended dose is 25-50 mg intramuscularly once every 2 weeks.
It is recommended to transfer patients taking risperidone orally at a fixed dose for two weeks or more to Rispolept Consta® according to the following scheme. Patients taking oral risperidone at a dose of 4 mg or less should be switched to Rispolept Consta® at a dose of 25 mg. Patients taking oral risperidone at a dose of more than 4 mg should be switched to Rispolept Consta® at a dose of 37.5 mg.
In clinical studies, no increase in efficacy was observed with 75 mg. The maximum dose should not exceed 50 mg once every 2 weeks.
During the 3-week period after the first administration of Rispolept Consta® and during exacerbation of schizophrenia, the patient should take an effective antipsychotic drug. (oral risperidone or previously used antipsychotic).
The dose of the drug can be increased no more often than once every 4 weeks. The effect of such an increase in dose should be expected no earlier than 3 weeks after the first injection of the increased dose.
Children (18 years and under)
Rispolept Konsta® has not been studied in children under 18 years of age.
Elderly patients (65 years and older)
The recommended dose is 25 mg intramuscularly once every 2 weeks. During the 3-week period after the first injection of Rispolept Consta®, the patient must take an effective antipsychotic drug. Clinical data on the use of Rispolept Consta® in elderly patients are limited, so the drug should be used with caution in this category of patients.
Patients with impaired liver or kidney function
Currently, there is no data on the use of Rispolept Consta® in patients with impaired liver or kidney function.
If it is necessary to treat a patient with impaired liver or kidney function with Rispolept Consta®, it is recommended to take 0.5 mg of the oral dosage form of risperidone twice a day in the first week. During the second week, the patient can take 1 mg of risperidone twice daily or 2 mg of risperidone once daily. If the patient tolerates an oral dose of at least 2 mg well, then 25 mg of Rispolept Consta® can be administered intramuscularly once every 2 weeks.
Directions for use
Important information
The use of the drug Rispolept Konsta® requires strict adherence to the instructions for preparing the suspension to ensure accurate administration of the drug and avoid possible errors.
You should remove the package of Rispolept Consta® from the refrigerator and allow it to warm to room temperature for at least 30 minutes before preparing the suspension.
Do not heat in any other way.
The components of this kit are specially designed for the use of the drug Rispolept Konsta®. To prepare a suspension from the Rispolept Konsta® extended-release microgranules contained in the bottle, you can only use the solvent included in the kit.
The components contained in the package must not be replaced with any other products.
Do not store the suspension after preparation.
The drug should be administered immediately after preparing the suspension.
To ensure the full dose of risperidone is administered, the entire contents of the vial must be administered. The introduction of part of the contents of the bottle cannot ensure that the patient receives the required dose of the drug.
Do not reuse: This device is intended for one-time use only. Any attempt at subsequent reuse may adversely affect the integrity of the device itself or result in deterioration in its performance.
1. Assemble the device components
Connect the needleless device to the bottle
Remove the cap from the bottle: - remove the colored plastic cap from the bottle. - Wipe the unopened bottle with an alcohol wipe and let it dry. — do not remove the gray rubber plug.
Prepare the needleless device - Holding the sterile blister with one hand, pull back with the other and remove the paper backing. — Do not remove the needleless device from the blister. — To avoid contamination, do not touch the sharp tip of the device.
Connect the needle-free device to the bottle - Place the bottle on a hard surface and hold the base of the bottle. Place the needleless device vertically on the bottle so that the sharp tip is located in the center of the rubber stopper. Using downward pressure, push the sharp tip of the needleless device through the center of the rubber stopper of the bottle until the device is securely attached to the top of the bottle. — Do not connect the needleless device at an angle, as the solvent may leak when poured into the bottle.
Connect the pre-filled syringe to the needleless device
Remove the sterile blister. Important!
The sterile blister of the needle-free device should be removed only when you are ready to remove the white cap from the syringe. — The bottle should be held vertically to avoid leakage. Hold the base of the bottle and pull on the blister to remove it. - Do not shake. — To prevent contamination, do not touch the luer lock. — Hold the syringe by the white collar. — Do not hold the syringe by the glass base.
Remove cap
- Hold the syringe by the white collar and break off the white cap. — Do not unscrew or cut off the white cap. — To prevent contamination, do not touch the tip of the syringe. — The broken cap can be thrown away.
Connect the syringe and needleless device
- To prevent rotation during connection, the “skirt” of the needleless device must be held firmly. — Holding the syringe by the white collar, insert the tip of the syringe into the luer lock of the needleless device. — Do not hold the syringe by the glass base. This can lead to a white collar disconnect. — Screw the syringe firmly onto the needleless device in a clockwise direction. - Avoid twisting. This may cause the syringe to fail.
2. Dissolve the microbeads
Introduce solvent
- Inject the entire contents of the syringe with solvent into the bottle. Important!
The contents of the bottle will now be under pressure. You need to hold the syringe plunger with your thumb.
Suspend microbeads in solvent
- Holding the syringe plunger with your thumb, shake the contents of the bottle vigorously for at least 10 seconds until a homogeneous suspension is formed. After proper mixing, the suspension becomes homogeneous, thick, and milky in color. Microgranules may be visible in the liquid, but no dry microgranules should remain unwetted with solvent. - Proceed to the next step immediately as the suspension may separate.
Transfer the suspension into a syringe
- Turn the bottle upside down and SLOWLY draw the entire contents of the bottle into the syringe.
Remove needleless device
- Holding the syringe by the white collar, unscrew the syringe from the needleless device. — Separate part of the label from the bottle along the perforation line and glue it to the syringe (for identification). — Dispose of the bottle and needle-free device in accordance with local waste disposal regulations.
3. Attach the needle
Choose the right needle
— Select a needle depending on the injection site (gluteal or deltoid).
Attach the needle
- Open the blister pack and grasp the base of the needle. — While still holding the syringe by the white collar, secure the syringe tightly into the luer lock cannula of the needle guard by pressing and turning clockwise. — To prevent contamination, do not touch the luer lock of the safety device.
Resuspend microbeads
- Remove the blister completely. — Immediately before administering the drug, it is necessary to resuspend the microgranules, since after preparing the suspension in the vial, some of the microgranules may settle. You need to shake the syringe vigorously.
4.Administer the drug
Remove the transparent case from the needle
- Pull the needle guard in the opposite direction from the syringe. Holding the syringe by the white collar, remove the transparent case from the needle. DO NOT bend the case, because the luer lock connection may be damaged.
Remove air bubbles
- Lightly tap the syringe with your finger so that the air bubbles in it rise up. Slightly moving the piston up, remove air bubbles from the syringe and needle, holding the syringe so that the needle is pointing vertically upward.
Inject the drug
- Immediately inject the entire contents of the syringe intramuscularly into the patient's gluteal or deltoid muscle. - The gluteal injection should be given in the upper outer quadrant of the gluteal region. — The suspension cannot be administered intravenously.
Place the needle in the safety device
- With one hand, position the guard on a flat surface at a 45-degree angle, and quickly push downward until the needle enters the guard.
Warning:
- Do not use both hands. — Do not disassemble the needle guard. — Do not attempt to straighten the needle or touch the needle guard if the needle is bent or damaged.
Dispose of the needle properly
- Before discarding the needle, make sure that the needle is firmly secured in the needle guard. — Dispose of in accordance with local regulations for the disposal of such waste. — You should also discard the unused needle provided in the kit.
Overdose
Overdose is less likely to occur with parenteral risperidone formulations than with oral formulations (film-coated tablets and oral solution) and therefore information is provided here for oral formulations.
Symptoms:
symptoms observed in overdose are exaggerated by known pharmacological effects.
They include sedation, drowsiness, tachycardia, low blood pressure and extrapyramidal disorders. QT interval prolongation and seizures have been observed. Bidirectional ventricular tachycardia has been observed during concomitant use of increased doses of oral risperidone and paroxetine. In case of overdose, the possibility of multiple drug involvement should be considered.
Treatment:
provide and maintain airway patency, adequate oxygenation and ventilation.
Monitoring of cardiovascular function is necessary, which should include continuous ECG monitoring to identify possible arrhythmias. Rispolept ®
does not have a specific antidote, and therefore treatment should be aimed at maintaining the function of the central nervous system and cardiovascular system, and detoxification therapy should also be carried out. For severe extrapyramidal symptoms, anticholinergic drugs should be administered. Medical supervision and monitoring must be continued until signs of overdose disappear.
Side effect
The most common side effects (≥1/10) are: insomnia, anxiety, headache, upper respiratory tract infections, parkinsonism, depression and akathisia.
During post-marketing surveillance, serious injection site reactions were observed, including necrosis, abscess, subcutaneous fat inflammation, ulceration, hematoma, cyst and nodular thickenings. The incidence of these reactions is unknown (the frequency cannot be estimated from the available data). In some cases, surgery was required.
Below are the side effects of the drug Rispolept Consta®, which were observed during clinical trials and during the post-marketing observation period. The frequency of side effects was classified as follows: very common (≥1/10), common (≥1/100 and <1/10), uncommon (≥1/1000 and <1/100), rare (≥1/10000 and < 1/1000), very rare (<1/10000) and unknown frequency (cannot estimate frequency from available data).
Within each frequency group, side effects are listed in order of decreasing severity.
Side effects are given with a distribution by frequency and system-organ classes.
Violations of laboratory and instrumental parameters
: often - ECG abnormalities, increased levels of prolactin1, increased activity of microsomal liver enzymes, increased transaminase activity, increase or decrease in body weight; uncommon – prolongation of the QT interval on the electrocardiogram.
Cardiovascular disorders
: often – atrioventricular block, tachycardia; uncommon – His bundle block, atrial fibrillation, bradycardia, sinus bradycardia, palpitations.
Hematological and lymphatic disorders
: often – anemia;
uncommon –
thrombocytopenia, neutropenia;
very rarely -
agranulocytosis.
Nervous system disorders
: very often – parkinsonism2, akathisia2, headache; often – dizziness, sedation, drowsiness, tremor, dystonia2, tardive dyskinesia, dyskinesia2; uncommon – convulsions, fainting, postural dizziness, hypoesthesia, paresthesia, lethargy, hypersomnia.
Visual disorders
: often – blurred vision, conjunctivitis; rarely – floppy iris syndrome (intraoperative)4; with unknown frequency - retinal artery occlusion.
Disorders of the hearing organ and labyrinth
: often – vertigo; infrequently – ear pain.
Respiratory, thoracic and mediastinal disorders
: often - shortness of breath, cough, sinus congestion, pharyngolaryngeal pain; rarely – sleep apnea syndrome.
Gastrointestinal disorders
: often - vomiting, diarrhea, constipation, nausea, abdominal pain, dyspepsia, toothache, dry mouth, stomach discomfort, gastritis;
rarely –
mechanical intestinal obstruction, pancreatitis; very rarely - intestinal obstruction.
Renal and urinary tract disorders
: often – urinary incontinence; infrequently – urinary retention.
Skin and subcutaneous tissue disorders
: often – rash, eczema; Uncommon: Quincke's edema, itching, acne, alopecia, dry skin.
Musculoskeletal and connective tissue disorders
: often – arthralgia, back pain, pain in the limbs, myalgia; uncommon – muscle weakness, pain in the neck, pain in the buttocks, musculoskeletal pain in the chest.
Endocrine system disorders
: rarely
-
impaired secretion of antidiuretic hormone.
Metabolic and nutritional disorders
: often – hyperglycemia;
uncommon –
diabetes mellitus3, increased appetite, decreased appetite; rarely - hypoglycemia; very rarely - diabetic ketoacidosis; with unknown frequency – water intoxication.
Infections
: very often - upper respiratory tract infections; often – pneumonia, influenza, lower respiratory tract infections, bronchitis, urinary tract infections, ear infections, sinusitis, viral infections; uncommon – cystitis, gastroenteritis, infections, localized infections, subcutaneous abscess.
Injuries, poisonings and complications associated with the drug administration procedure
: often – fall; uncommon – pain during the drug administration procedure.
Vascular disorders:
often – hypertension, hypotension; infrequently – orthostatic hypotension.
General disorders and disorders in the area of drug administration:
often – pyrexia, peripheral edema, chest pain, fatigue, pain, pain in the area of drug administration, asthenia, flu-like condition; infrequently - compaction in the area of drug administration, compaction, reactions in the area of drug administration, discomfort in the chest, slowness, poor health; rarely – hypothermia.
Immune system disorders
: uncommon – hypersensitivity; with unknown frequency - anaphylactic reactions.
Hepatobiliary disorders
: rarely
–
jaundice.
Disorders of the reproductive system and mammary glands
: often – amenorrhea, erectile dysfunction, galactorrhea;
uncommon – sexual dysfunction, gynecomastia; with unknown frequency –
priapism.
Mental disorders
: very often – depression, insomnia, anxiety; often – agitation, sleep disorders; infrequently – mania, decreased libido, nervousness.
1– hyperprolactinemia in some cases can lead to gynecomastia, menstrual irregularities, amenorrhea and galactorrhea.
2- extrapyramidal disorders may manifest as: parkinsonism (hypersalivation, musculoskeletal stiffness, parkinsonism, drooling, cogwheel rigidity, bradykinesia, hypokinesia, mask-like face, muscle tension, akinesia, nuchal rigidity, muscle rigidity, parkinsonian gait , glabellar reflex disorders), akathisia (akathisia, restlessness, hyperkinesia and restless legs syndrome), tremor, dyskinesia (dyskenesia, muscle twitching, choreoathetosis, athetosis and myoclonus), dystonia.
Dystonia includes dystonia, muscle spasms, hypertension, torticollis, involuntary muscle contractions, muscle contracture, blepharospasm, eye movements, tongue paralysis, facial spasm, laryngospasm, myotonia, opisthotonus, oropharyngeal spasm, pleurototonus, tongue spasm, and trismus. Tremors include tremor and parkinsonian resting tremor. It should also be noted that there is a wider range of symptoms that are not always of extrapyramidal origin.
3- In placebo-controlled studies, diabetes mellitus occurred in 0.18% of patients receiving risperidone compared with 0.11% of patients in the placebo group. The overall incidence of diabetes mellitus across all clinical trials was 0.43% of all patients taking risperidone.
4– was observed only in the post-marketing period.
The following additionally lists side effects observed during clinical studies of oral dosage forms of risperidone, but which did not appear with the use of a long-acting injectable form of risperidone - Rispolept Consta®. Side effects are given with distribution by system-organ classes:
Laboratory abnormalities:
increase in body temperature, increase in the number of esonophils, increase in the number of leukocytes, decrease in hemoglobin level, increase in the level of creatine phosphokinase, decrease in body temperature.
Infections
: tonsillitis, inflammation of subcutaneous fat, otitis media, eye infections, akarodermatitis, respiratory tract infections, onychomycosis, chronic otitis media.
From the blood and lymphatic system:
granulocytopenia.
From the immune system:
hypersensitivity to the drug.
Metabolic and nutritional disorders:
anorexia, polydipsia.
Mental disorders:
confusion, lethargy, anorgasmia, affective flattening.
From the nervous system:
lack of response to stimuli, loss of consciousness, neuroleptic malignant syndrome, diabetic coma, stroke, depression of consciousness, cerebral ischemia, cerebrovascular disorders, transient ischemic attack, dysarthria, impaired attention, impaired balance, impaired speech, impaired coordination, impaired movement.
Ophthalmological disorders
: ocular hyperemia, discharge from the eyes, swelling of the area around the eyes, dry eyes, increased lacrimation, photophobia, decreased visual acuity, involuntary rotation of the eyeballs, glaucoma.
From the ear and labyrinth:
tinnitus.
Vascular disorders
: tides.
Respiratory, thoracic and mediastinal disorders:
wheezing, aspiration pneumonia, pulmonary congestion, respiratory distress, wheezing, nosebleeds, nasal congestion, hyperventilation, dysphonia.
From the gastrointestinal tract:
dysphagia, fecal incontinence, fecaloma, lip swelling, cheilitis.
For the skin and subcutaneous tissues:
skin lesions, skin disorders, discoloration of the skin, seborrheic dermatitis, hyperkeratosis, dandruff, erythema.
From the musculoskeletal system and connective tissue
: rhabdomyolysis, joint swelling, postural abnormalities, joint stiffness.
From the kidneys and urinary tract:
enuresis, dysuria, pollakiuria.
From the reproductive system and mammary glands
: erectile dysfunction, vaginal discharge, menstrual irregularities.
General disorders and phenomena caused by administration of the drug:
General swelling, facial swelling, gait disturbance, thirst, chills, cold extremities, Johnson & Johnson syndrome (Unknown)
JANSSEN, pharmaceutical division of Johnson&Johnson LLC
121614 Moscow, st. Krylatskaya, 17, bldg. 2 Tel. Fax
Indications
Risperidone was originally used to treat schizophrenia and psychosis. But as experience has accumulated about the action of this medicine, the indications for its use have expanded. Currently indicated for the following diseases and conditions:
- Schizophrenia
- Schizotypal disorder
- Organic mental disorders
- Schizoid personality disorder
- Manic and hypomanic states in bipolar affective disorder, schizoaffective disorder
- Psychoses
- Delusions: paranoia, paranoid, paraphrenia
- Depressive and anxiety states, neuroses and neurosis-like conditions
- Aggressiveness
- Autism
The drug is recommended for adults and children over 13 years of age. At a younger age, it is prescribed by the doctor’s decision in cases where the potential risk of side and negative effects of the drug is less than the risk of worsening the condition as the disease progresses.
Use during pregnancy and breastfeeding
There are no absolute contraindications to taking risperidone during pregnancy and lactation. However, due to the lack of special research in this direction, use during pregnancy is allowed only in cases described by the following formulation: when the benefits of taking it for the pregnant woman outweigh the potential risks to the fetus.
Due to the fact that risperidone passes into breast milk, it is recommended to transfer the child to artificial nutrition for the period of treatment of a breastfeeding mother.
Rispolept oral solution 1mg/ml 30ml fl
Use in elderly patients with dementia. Increased Mortality in Elderly Patients with Dementia Elderly patients with dementia treated with atypical antipsychotics experienced increased mortality compared with placebo in studies of atypical antipsychotics, including risperidone. When using risperidone in this population, the incidence of death was 4.0% for patients taking risperidone compared with 3.1% for placebo. The mean age of patients who died was 86 years (range, 67–100 years). Data collected from two large observational studies show that older patients with dementia treated with typical antipsychotic medications also have a slightly increased risk of death compared with patients not treated. At present, insufficient data have been collected to accurately assess this risk. The reason for the increase in this risk is also unknown. Also unknown is the extent to which the increased mortality may be attributable to antipsychotic drugs rather than to the characteristics of this patient population.
Concomitant use with furosemide In elderly patients with dementia, increased mortality was observed with concomitant use of furosemide and oral risperidone (7.3%, mean age 89 years, range 75-97 years) compared with the group receiving risperidone alone (3.1%, mean age 84 years, range 70-96 years) and the furosemide-only group (4.1%, mean age 80 years, range 67-90 years). An increase in mortality in patients taking risperidone with furosemide was observed in 2 of 4 clinical studies. Concomitant use of risperidone with other diuretics (mainly low-dose thiazide diuretics) was not associated with an increase in mortality.
No pathophysiological mechanisms have been established to explain this observation. However, special care should be taken when prescribing the drug in such cases. Before prescribing, the risk/benefit ratio must be carefully assessed. There was no increase in mortality in patients taking other diuretics concomitantly with risperidone. Regardless of treatment, dehydration is a common risk factor for mortality and should be carefully monitored in older patients with dementia.
In elderly patients with dementia, an increase in cerebrovascular adverse events (acute and transient cerebrovascular accidents), including patient deaths (mean age 85 years, range 73-97 years) was observed with risperidone compared with placebo.
Cardiovascular effects.
In placebo-controlled clinical trials, an approximately 3-fold increased risk of cerebrovascular side effects was observed in patients with dementia taking certain atypical antipsychotic drugs. Pooled data from 6 placebo-controlled studies involving primarily elderly patients with dementia (age >65 years) demonstrate that cerebrovascular adverse events (serious and non-serious) occurred in 3.3% (33/1009) of patients treated with risperidone and in 1.2% (8/712) of placebo patients. The risk ratio was 2.96 (1.34, 7.50) with a 95% confidence interval. The mechanism by which this risk increases is unknown. An increased risk cannot be excluded for other antipsychotic drugs, as well as for other patient populations. Rispolept® should be used with caution in patients with risk factors for stroke.
The risk of cerebrovascular side effects is much higher in patients with mixed or vascular dementia compared to patients with Alzheimer's dementia.
Therefore, patients with any type of dementia other than Alzheimer's should not take risperidone.
Physicians should assess the risk/benefit ratio of using Rispolept® in elderly patients with dementia, taking into account the precursors of stroke risk individually for each patient. Patients and caregivers should be cautioned to immediately report signs and symptoms of cardiovascular events, such as sudden weakness or stiffness/numbness in the face, legs, arms, as well as difficulty speaking and vision problems. All possible treatment options should be considered, including discontinuation of risperidone.
Rispolept® should only be used for the short-term treatment of persistent aggression in patients with moderate to severe Alzheimer's dementia, as an adjunct to non-pharmacological treatments when they are ineffective or of limited effectiveness, and when there is a risk of harm to the patient himself or herself to other persons.
Patients' condition and the need for continued risperidone therapy should be continually assessed.
Orthostatic hypotension.
Risperidone has alpha-blocking activity and may therefore cause orthostatic hypotension in some patients, especially during initial dose titration. Clinically significant hypotension has been observed in the post-marketing period when used concomitantly with antihypertensive drugs. Rispolept® should be used with caution in patients with known cardiovascular disease (eg, heart failure, myocardial infarction, cardiac conduction disorders, dehydration, hypovolemia or cerebrovascular disease). Appropriate dose adjustment is also necessary. It is recommended to evaluate the possibility of dose reduction if hypotension occurs.
Tardive dyskinesia and extrapyramidal disorders.
Drugs with dopamine receptor antagonist properties can cause tardive dyskinesia, which is characterized by rhythmic involuntary movements, mainly of the tongue and/or facial muscles. The occurrence of extrapyramidal symptoms is a risk factor for the development of tardive dyskinesia. If a patient experiences objective or subjective symptoms indicating tardive dyskinesia, the advisability of discontinuing all antipsychotic drugs, including Rispolept®, oral solution, should be considered.
Neuroleptic malignant syndrome (NMS).
Antipsychotics, including risperidone, may cause neuroleptic malignant syndrome (NMS), which is characterized by hyperthermia, muscle rigidity, instability of autonomic nervous system function, depression of consciousness, and increased serum concentrations of creatine phosphokinase. Myoglobinuria (rhabdomyolysis) and acute renal failure may also occur in patients with NMS. If a patient experiences objective or subjective symptoms of NMS, all antipsychotic drugs, including Rispolept®, must be immediately discontinued.
Parkinson's disease and dementia with Lewy bodies.
Antipsychotic medications, including Rispolept®, should be prescribed with caution to patients with Parkinson's disease or dementia with Lewy bodies. Both groups of patients have an increased risk of developing neuroleptic malignant syndrome and increased sensitivity to antipsychotic drugs (including dullness of pain sensitivity, confusion, postural instability with frequent falls and extrapyramidal symptoms). Parkinson's disease may worsen when taking risperidone.
Hyperglycemia and diabetes mellitus.
Hyperglycemia, diabetes mellitus, and exacerbation of existing diabetes mellitus were observed during treatment with Rispolept®. It is likely that weight gain prior to treatment is also a predisposing factor. Very rarely, ketoacidosis and rarely, diabetic coma can occur. All patients should be clinically monitored for symptoms of hyperglycemia (such as polydipsia, polyuria, polyphagia and weakness). Patients with diabetes mellitus should be regularly monitored for worsening glucose control.
Increase in body weight.
During treatment with Rispolept®, a significant increase in body weight was observed.
It is necessary to monitor patients' body weight.
Hyperprolactinemia.
Based on the results of tissue culture studies, it has been suggested that the growth of breast tumor cells may be stimulated by prolactin. Although clinical and epidemiological studies have not shown a clear association between hyperprolactinemia and antipsychotic drug use, caution should be exercised when prescribing risperidone to patients with a history of this. Rispolept® should be used with caution in patients with existing hyperprolactinemia and in patients with possible prolactin-dependent tumors.
Prolongation of the QT interval.
QT prolongation has been observed very rarely during post-marketing surveillance. As with other antipsychotics, caution should be exercised when prescribing Rispolept® to patients with known cardiovascular diseases, family history of QT interval prolongation, bradycardia, electrolyte imbalance (hypokalemia, hypomagnesemia), as this may increase the risk of an arrhythmogenic effect; and when used together with drugs that prolong the QT interval.
Cramps.
Rispolept® should be used with caution in patients with a history of seizures or other medical conditions that may lower the seizure threshold.
Priapism.
Priapism may occur with risperidone due to alpha-blocking effects.
Regulation of body temperature.
Antipsychotic drugs are associated with such undesirable effects as disruption of the body's ability to regulate temperature. Caution should be exercised when prescribing Rispolept® to patients with conditions that may contribute to an increase in core body temperature, such as intense physical activity, dehydration, exposure to high external temperatures, or concomitant use of drugs with anticholinergic activity.
Venous thromboembolism.
Cases of venous thromboembolism have been reported with the use of antipsychotic drugs.
Since patients taking antipsychotic drugs are often at risk of developing venous thromboembolism, all possible risk factors should be identified before and during treatment with Rispolept®, and preventive measures should be taken.
Children and teenagers.
Before prescribing Rispolept® to children or adolescents with mental retardation, their condition must be carefully assessed for the presence of physical or social causes of aggressive behavior, such as pain or inadequate demands of the social environment.
The sedative effect of risperidone should be carefully monitored in this population due to the possible effect on learning ability. Changing the timing of risperidone administration may improve control of the effects of sedation on attention in adolescents and children.
Risperidone use was associated with mean increases in body weight and body mass index.
Height changes in longitudinal studies were within expected age-related norms. The effects of long-term use of risperidone on sexual development and growth have not been fully studied.
Due to the possible impact of prolonged hyperprolactinemia on growth and sexual development in children and adolescents, regular clinical assessment of hormonal status should be carried out, including measurement of height, weight, monitoring of sexual development, menstrual cycle and other possible prolactin-dependent effects.
During treatment with risperidone, regular monitoring for the presence of extrapyramidal symptoms and other movement disorders should be carried out.
Effects on the ability to drive vehicles and machines Rispolept®, oral solution, may have a small or moderate effect on the ability to drive vehicles and machines. Patients should be advised to refrain from driving a car and operating machinery until their individual sensitivity to the drug is determined.
Side effects
The drug is usually well tolerated. According to clinic doctors, it is one of the well-tolerated antipsychotics. In approximately 10% of cases, the following side effects are possible.
- Increased muscle tone, restlessness, trembling, feeling of stiffness, “extrapyramidal syndrome,” parkinsonism. To prevent these phenomena, correctors may be additionally prescribed: trihexyphenidyl (Cyclodol), biperiden (Akineton, Mendylex, Bezac), amantadine (PK-Merz), etc.
- Headache.
- Insomnia.
Rare but unpleasant side effects include metabolic (metabolic) disorders: weight gain and the development of diabetes. As a rule, such effects develop in persons predisposed to this.
Therefore, to avoid them, it is recommended to conduct an examination with blood tests before starting therapy to exclude an increase in glucose and prolactin in the blood.
Rispolept®
Use in elderly patients with dementia
Increased mortality in older patients with dementia
Elderly patients with dementia treated with atypical antipsychotics experienced increased mortality compared with placebo in studies of atypical antipsychotics, including risperidone. When using risperidone in this population, the incidence of death was 4.0% for patients taking risperidone compared with 3.1% for placebo. The mean age of patients who died was 86 years (range, 67–100 years). Data collected from two large observational studies show that older patients with dementia treated with typical antipsychotic medications also have a slightly increased risk of death compared with patients not treated. At present, insufficient data have been collected to accurately assess this risk. The reason for the increase in this risk is also unknown. Also unknown is the extent to which the increased mortality may be attributable to antipsychotic drugs rather than to the characteristics of this patient population.
Combined use with furosemide
In elderly patients with dementia, there was an increased mortality rate when taking furosemide and oral risperidone concomitantly (7.3%, mean age 89 years, range 75-97 years) compared with the risperidone alone group (3.1%, mean age 84 years , range 70-96 years) and the furosemide-only group (4.1%, mean age 80 years, range 67-90 years). An increase in mortality in patients taking risperidone with furosemide was observed in 2 of 4 clinical studies. Concomitant use of risperidone with other diuretics (mainly low-dose thiazide diuretics) was not associated with an increase in mortality.
No pathophysiological mechanisms have been established to explain this observation. However, special care should be taken when prescribing the drug in such cases. Before prescribing, the risk/benefit ratio must be carefully assessed. There was no increase in mortality in patients taking other diuretics concomitantly with risperidone. Regardless of treatment, dehydration is a common risk factor for mortality and should be carefully monitored in older patients with dementia.
In elderly patients with dementia, an increase in cerebrovascular adverse events (acute and transient cerebrovascular accidents), including patient deaths (mean age 85 years, range 73-97 years) was observed with risperidone compared with placebo.
Cardiovascular effects
In placebo-controlled clinical trials, an approximately 3-fold increased risk of cerebrovascular side effects was observed in patients with dementia taking certain atypical antipsychotic drugs. Pooled data from 6 placebo-controlled studies involving primarily elderly patients with dementia (age >65 years) demonstrate that cerebrovascular adverse events (serious and non-serious) occurred in 3.3% (33/1009) of patients treated with risperidone. and in 1.2% (8/712) of patients receiving placebo. The risk ratio was 2.96 (1.34, 7.50) with a 95% confidence interval. The mechanism by which this risk increases is unknown. An increased risk cannot be excluded for other antipsychotic drugs, as well as for other patient populations. Rispolept® should be used with caution in patients with risk factors for stroke.
The risk of cerebrovascular side effects is much higher in patients with mixed or vascular dementia compared to patients with Alzheimer's dementia. Therefore, patients with any type of dementia other than Alzheimer's should not take risperidone.
Physicians should assess the risk/benefit ratio of using Rispolept® in elderly patients with dementia, taking into account the precursors of stroke risk individually for each patient. Patients and caregivers should be cautioned to immediately report signs and symptoms of cardiovascular events, such as sudden weakness or stiffness/numbness in the face, legs, arms, as well as difficulty speaking and vision problems. All possible treatment options should be considered, including discontinuation of risperidone.
Rispolept® should only be used for the short-term treatment of persistent aggression in patients with moderate to severe Alzheimer's dementia, as an adjunct to non-pharmacological treatments when they are ineffective or of limited effectiveness, and when there is a risk of harm to the patient himself or herself to other persons.
Patients' condition and the need for continued risperidone therapy should be continually assessed.
Orthostatic hypotension
Risperidone has alpha-blocking activity and may therefore cause orthostatic hypotension in some patients, especially during initial dose titration. Clinically significant hypotension has been observed in the post-marketing period when used concomitantly with antihypertensive drugs. Rispolept® should be used with caution in patients with known cardiovascular disease (eg, heart failure, myocardial infarction, cardiac conduction disorders, dehydration, hypovolemia or cerebrovascular disease). Appropriate dose adjustment is also necessary. It is recommended to evaluate the possibility of dose reduction if hypotension occurs.
Tardive dyskinesia and extrapyramidal disorders
Drugs with dopamine receptor antagonist properties can cause tardive dyskinesia, which is characterized by rhythmic involuntary movements, mainly of the tongue and/or facial muscles. The occurrence of extrapyramidal symptoms is a risk factor for the development of tardive dyskinesia. If a patient experiences objective or subjective symptoms indicating tardive dyskinesia, the advisability of discontinuing all antipsychotic drugs, including Rispolept®, should be considered.
Neuroleptic malignant syndrome (NMS)
Antipsychotics, including risperidone, may cause neuroleptic malignant syndrome (NMS), which is characterized by hyperthermia, muscle rigidity, instability of autonomic nervous system function, depression of consciousness, and increased serum concentrations of creatine phosphokinase. Myoglobinuria (rhabdomyolysis) and acute renal failure may also occur in patients with NMS. If a patient experiences objective or subjective symptoms of NMS, all antipsychotic drugs, including Rispolept®, must be immediately discontinued.
Parkinson's disease and dementia with Lewy bodies
Antipsychotic medications, including Rispolept®, should be prescribed with caution to patients with Parkinson's disease or dementia with Lewy bodies. Both groups of patients have an increased risk of developing neuroleptic malignant syndrome and increased sensitivity to antipsychotic drugs (including dullness of pain sensitivity, confusion, postural instability with frequent falls and extrapyramidal symptoms). Parkinson's disease may worsen when taking risperidone.
Hyperglycemia and diabetes mellitus
Hyperglycemia, diabetes mellitus, and exacerbation of existing diabetes mellitus were observed during treatment with Rispolept®. It is likely that weight gain prior to treatment is also a predisposing factor. Very rarely, ketoacidosis and rarely, diabetic coma can occur. All patients should be clinically monitored for symptoms of hyperglycemia (such as polydipsia, polyuria, polyphagia and weakness). Patients with diabetes mellitus should be regularly monitored for worsening glucose control.
Weight gain
During treatment with Rispolept®, a significant increase in body weight was observed. It is necessary to monitor patients' body weight.
Hyperprolactinemia
Based on the results of tissue culture studies, it has been suggested that the growth of breast tumor cells may be stimulated by prolactin. Although clinical and epidemiological studies have not shown a clear association between hyperprolactinemia and antipsychotic drug use, caution should be exercised when prescribing risperidone to patients with a history of this. Rispolept® should be used with caution in patients with existing hyperprolactinemia and in patients with possible prolactin-dependent tumors.
Prolongation of the QT interval
QT prolongation has been observed very rarely during post-marketing surveillance. As with other antipsychotics, caution should be exercised when prescribing Rispolept® to patients with known cardiovascular diseases, family history of QT interval prolongation, bradycardia, electrolyte imbalance (hypokalemia, hypomagnesemia), as this may increase the risk of an arrhythmogenic effect; and when used together with drugs that prolong the QT interval.
Convulsions
Rispolept® should be used with caution in patients with a history of seizures or other medical conditions that may lower the seizure threshold.
Priapism
Priapism may occur with risperidone due to alpha-blocking effects.
Body temperature regulation
Antipsychotic drugs are associated with such undesirable effects as disruption of the body's ability to regulate temperature. Caution should be exercised when prescribing Rispolept® to patients with conditions that may contribute to an increase in core body temperature, such as intense physical activity, dehydration, exposure to high external temperatures, or concomitant use of drugs with anticholinergic activity.
Venous thromboembolism
Cases of venous thromboembolism have been reported with the use of antipsychotic drugs. Since patients taking antipsychotic drugs are often at risk of developing venous thromboembolism, all possible risk factors should be identified before and during treatment with Rispolept®, and preventive measures should be taken.
Children and teenagers
Before prescribing Rispolept® Quicklet to children or adolescents with mental retardation, their condition must be carefully assessed for the presence of physical or social causes of aggressive behavior, such as pain or inadequate demands of the social environment.
The sedative effect of risperidone should be carefully monitored in this population due to the possible effect on learning ability. Changing the timing of risperidone administration may improve control of the effects of sedation on attention in adolescents and children. Risperidone use was associated with mean increases in body weight and body mass index. Height changes in longitudinal studies were within expected age-related norms. The effects of long-term use of risperidone on sexual development and growth have not been fully studied.
Due to the possible impact of prolonged hyperprolactinemia on growth and sexual development in children and adolescents, regular clinical assessment of hormonal status should be carried out, including measurement of height, weight, monitoring of sexual development, menstrual cycle and other possible prolactin-dependent effects.
During treatment with risperidone, regular monitoring for the presence of extrapyramidal symptoms and other movement disorders should be carried out.
Excipients
The drug Rispolept®, film-coated tablets, contains lactose. Patients with rare hereditary diseases associated with galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption should not be prescribed Rispolept®, film-coated tablets.
Tablets of the drug dosage 2 mg contain sunset yellow dye (E110), which can cause allergic reactions.