Risperidone is one of the popular and effective antipsychotic drugs (neuroleptics). The active substance itself is a benzisoxazole derivative. This medicine was first approved for use in 1993. Used in psychiatric and neurological practice. It is usually prescribed for all kinds of productive disorders, for example, hallucinations, delusions, etc. In addition, the drug reduces irritability and auto-aggression.
One of the advantages of Risperidone is that with its use the rate of elimination of mental and psychosomatic symptoms is twice as high as with the use of typical antipsychotics. In addition, this treatment appears to be safer.
Method of action
After oral administration, the drug is completely absorbed in the gastrointestinal tract and quickly distributed throughout the body. In this case, food does not affect the process in any way. Thanks to this, it is possible to achieve the optimal concentration of the substance in the plasma - it corresponds to the dose used.
A week after taking the drug, it is almost completely eliminated from the body - 70% is excreted in urine, 14% in feces. However, it is worth considering that in some situations slow elimination may occur. Most often this is due to poor functioning of the liver and kidneys (in old age, with renal failure, etc.).
Special translations
Translator/editor: Marina Lelyukhina
Original: https://spectrumnews.org/news/risperidone-use-in-children-with-autism-carries-heavy-risks/
Our public VKontakte: https://vk.com/public57544087
Our Facebook group: https://www.facebook.com/specialtranslations If you liked the material, help those who need help:
Copying the full text for distribution on social networks and forums is possible only by citing publications from the official pages of Special Translations or through a link to the site. When quoting text on other sites, place the full translation header at the beginning of the text
____
Risperidone, the first drug approved for children with autism and the most widely used, improves some of the child's behavior but may have severe side effects, according to anecdotal evidence from experience with its use.
The medicine is effective in correcting mood swings and aggression that can occur with autism. “The drug is great for tantrums, aggression, self-injurious behavior,” says Lawrence Scahill, a professor of pediatrics at the Marcus Autism Center at Emory University in Atlanta, who conducted clinical trials of risperidone. The changes can be dramatic and usually appear within a week, she says. It also helps with hyperactivity and repetitive behavior, although the Food and Drug Administration has not approved its use for these purposes.
Because of this effect, risperidone allows children with autism to receive social services, participate in educational programs and receive behavioral interventions, the expert says.
"If a child can't sit still in a speech therapy session and attacks the teachers, it won't be helpful," says Christopher McDougle, director of the Lurie Center for Autism at MassGeneral Hospital for Children in Boston, who has studied the use of risperidone in children and adults. .
But in addition, risperidone has significant disadvantages and limitations. Not all people respond to it; when the medication is stopped, symptoms often return and the medication fails to improve any of the core symptoms of autism.
In other words, risperidone is not a “cure for autism,” says Benedetto Vitiello, director of the Child and Adolescent Treatment and Preventive Intervention Research Branch at the National Institute of Mental Health. “It doesn't really affect the key symptoms of autism.”
But more concerning are the side effects, the most significant of which is weight gain due to an increased appetite. Children taking risperidone gained an average of 2.5 kilograms over 8 weeks of taking the medication. In addition, the drug may cause drowsiness, hormonal changes, and in rare cases, involuntary movements. If we prescribe risperidone to a child with ASD, Scahill says, “I will use the lowest dosage possible and monitor closely to see if the medication can be discontinued.”
Reduced irritability:
Although numerous studies have noted risks associated with the use of risperidone, efforts to find a safer alternative have so far been unsuccessful.
Aripiprazole, the only drug also approved to treat irritability in autism, has similar side effects (the FDA approved it for the treatment of children with autism in 2009). And a clinical trial designed to study the safety and effectiveness of minimally low doses of risperidone last year led to dismal results.
Risperidone is an antipsychotic that blocks the brain's dopamine and serotonin receptors. It was originally developed to treat schizophrenia. The American Food and Drug Association (FDA) approved the use of risperidone in the treatment of schizophrenia in 1993.
Older drugs, among which haloperidol is most often mentioned, were also based on reducing dopamine activity. Before risperidone came onto the market, these drugs were often prescribed to treat severe behaviors in some people with ASD, such as severe temper tantrums and self-injury, symptoms often lumped together under the umbrella of “irritability.”
After risperidone was approved for the treatment of schizophrenia, scientists began exploring its use in autism. “The idea was that maybe we could find a way to reduce the number of meltdowns in a child, to give him an extra 1-2 seconds before he loses his temper, becomes aggressive or hurts himself,” says Scahill.
A 2002 randomized clinical trial of children with autism aged 5-17 years found that 57% experienced a reduction in tantrums, aggression and self-injury after taking risperidone, compared with 14% in children receiving placebo. Among children who responded well to the drug, about 70 percent showed improvement 6 months after starting the drug.
In October 2006, the FDA approved the use of risperidone for the treatment of irritability in children with ASD aged 5–16.
In January, scientists published a new analysis of 2,002 cases in which participants were classified by the type of aggressive behavior they exhibited—aggressive outbursts in response to provocation and unmotivated aggression, for example. Risperidone significantly improved the situation in both groups.
The challenge is how to use medication to maintain improvements and minimize risks.
Last year, scientists at Janssen Research and Development—a sister company of Janssen Pharmaceuticals, Inc., which makes risperidone under the market name Risperdal—published the results of a study designed to see whether a low dose of risperidone could reduce irritability in children with ASD.
The researchers randomly assigned children with autism into 3 groups. Each day for six weeks, one third of the children received a standard dose of risperidone, one third received a dose below the FDA recommended minimum, and the remaining third received a placebo.
The lowest dose reduced a number of the drug's side effects, including drowsiness and increased appetite, which were mentioned in a 2013 article. Unfortunately, this dose was no more effective than placebo in reducing irritability.
“It's a trade-off between the improvements you're looking for (and) the risks for each individual patient,” says Gahan Pandina, CEO of Janssen Research and Development.
Informed decision:
The researchers also found that after six weeks of taking a standard dose of risperidone, children had higher insulin levels and levels of insulin resistance than children receiving a placebo. Weight gain and resulting metabolic disorders are a side effect of almost all antipsychotic drugs, but rapid weight gain is especially undesirable in children.
"It's a cumulative effect," says Jeremy Veenstra-VanderWeele, medical director of the Treatment and Research Institute for Autism Spectrum Disorders at Vanderbilt University in Nashville, Tennessee. “It is possible to change the physical condition and distribution of adipose tissue for life.”
Risperidone may also cause loss of energy and drowsiness. In a 2011 publication, Shafali Jeste and colleagues examined the medical records of 70 children with autism treated with risperidone. It was found that drowsiness was mentioned less often in the records than weight gain, but was more often the reason why parents stopped taking the drug.
"Until the situation becomes critical, parents are willing to tolerate weight gain if the child's behavior improves," says Jeste, an assistant professor of psychiatry and neuroscience at the Center for Autism Research and Treatment at the University of California, Los Angeles. Drowsiness, which can interfere with school and professional activities, is a much stronger argument against.
Risperidone may also cause a less common side effect such as tardive dyskinesia, or involuntary repetitive movements.
Like many other antipsychotic drugs, it also increases levels of the hormone prolactin, a hormone secreted by the pituitary gland. High levels of the hormone can cause breast development in boys and lactation in girls, as well as menstrual problems and sexual dysfunction.
"When prolactin levels rise, your body starts to think it's pregnant," says McDougle.
However, scientists warn that high levels of prolactin do not always cause clinically described symptoms and it is not entirely clear whether increased levels of the hormone itself cause health problems. Much more research is needed into the effects of chronically elevated prolactin levels, especially in children and adolescents.
The side effects of risperidone have been the subject of several lawsuits. In late 2013, 500 plaintiffs filed lawsuits alleging the drug harmed their health, according to Johnson & Johnson's annual report. Johnson & Johnson, a subsidiary of which is Janssen Pharmaceuticals.
“Janssen intends to defend the company against the allegations made in these lawsuits,” said Greg Panico, a spokesman for Janssen Research and Development. .
Last fall, Johnson & Johnson agreed to pay more than $2.2 billion to resolve allegations that it mismarketed Risperdal and two other drugs.
In the final analysis, the researchers said that risperidone should be prescribed with caution, only to children with the most severe symptoms and only after other treatments have failed.
“Could someone be harmed if intervention is not prescribed? And if the answer is yes, I will think about the possibility of prescribing risperidone,” says Veenstra-VanderWeele says. “If the answer is no, I’ll think about what more can be done.”
Disclosure: The author owns shares of Johnson & Johnson, of which Janssen Pharmaceuticals is a subsidiary.
Bibliography:
1: Shea S. et al. Pediatrics 114, e634-641 (2004)
2: McDougle CJ et al. Am. J. Psychiatry 162, 1142-1148 (2005)
3: McCracken JT et al. N.Eng. J. Med. 347, 314-321 (2002)
4: Carroll D. et al. Child Adolesc. Psychiatr. Clin. N. Am. 23, 57-72 (2014)
5: Kent JM et al. J. Autism Dev. Discord. 43, 1773-1783 (2013)
6: Lemmon ME et al. J. Child Neurol. 26, 428-432 (2011)
Indications for use
There are a number of ailments for which Risperidone tablets are particularly effective. The main ones include:
- Schizophrenia (acute or chronic). The active substance makes it possible to carry out treatment at a symptomatic level.
- Psychotic states. Helps eliminate emotional detachment, delusions, poor speech, etc.
- Depression, if accompanied by anxiety.
- Behavioral disorders, including outbursts of anger, severe agitation.
- Dementia if aggressiveness is present.
- Bipolar disorders. Treatment of mania.
- Autism in children and adolescents. The tool allows you to fight auto-aggression.
The drug is also used in the treatment of relapses if a person is diagnosed with chronic schizophrenia and acute psychotic states periodically appear.
Long-term prospects for long-term treatment with risperidone in children with schizophrenia
Risperidone, introduced into practice about 10 years ago, has a unique activity, acting on both D2-dopamine and 5HT2A-serotonin receptors. In relation to α1-adrenergic, H2-histamine and M1-muscarinic receptors, it has less affinity than traditional antipsychotics, however, with its use, a number of side effects may occur, such as nausea, increased appetite, excess weight, galactorrhea, etc. . Currently, risperidone is successfully used in the treatment of acute and chronic forms of schizophrenia in adult patients. The effectiveness of risperidone in the treatment of negative symptoms of schizophrenia and forms resistant to traditional therapy is especially strongly emphasized [3,4,7–11]. An analysis of modern publications shows that risperidone is still little used in domestic child psychiatry. At the same time, foreign psychiatrists use this drug in children to treat hyperactivity, impulsivity, aggression, stereotypic behavior, and obsessive-compulsive disorders. A number of foreign psychiatrists suggest that atypical antipsychotics, in particular risperidone, can play an important role in the treatment of severe autistic disorders [11,17–20]. The relative safety of risperidone (the absence of toxic and mutagenic effects in the experiment, the absence of complications when taking average daily doses by middle-aged people), as well as the noted facts of overcoming therapeutic resistance in the treatment of severe forms of endogenous diseases in adults [4,19] make risperidone attractive for its use in children's practice. In order to study the clinical effectiveness of risperidone in the treatment of intractable, early-onset forms of childhood schizophrenia, children aged 3–12 years (average age 6.4±2.3 years) were treated with risperidone in 1999–2006. under supervision at the Department of Early Childhood at the Mental Health Research Center of the Russian Academy of Medical Sciences. This report is devoted to the follow-up of these results. The criteria for inclusion in the observation group were severe negative and positive mental disorders, disease duration of more than a year, and resistance to previous therapy. Cases with psychoorganic syndrome, mental retardation, convulsive attacks, and severe somatic diseases were not included in the study. Before treatment began, all children were examined by a psychiatrist, neurologist, pediatrician, and psychologist. EEG, ECG, clinical tests of blood, urine, etc. were carried out. In addition, control examinations of the somatic condition of patients, monitoring of biochemical parameters and ECG were regularly prescribed over time. For the use of risperidone in children, informed consent was obtained from parents with the condition of regular visits to the doctor, as well as parents recording the mental and physical state of the child according to a certain scheme. In addition, permission from the ethics committee of the Scientific Center for Mental Health of the Russian Academy of Medical Sciences was obtained for the use of risperidone in children. To assess the effectiveness of risperidone, standardized scales adapted to childhood were used: positive and negative symptoms (PANSS), clinical global impression (CGI). They noted the severity of clinical manifestations, their dynamics, as well as the degree of improvement or deterioration of the condition using a seven-point system [5]. Possible complications and side effects of therapy were recorded using the UKU scale [5,6]. The drug was considered effective if it reduced the total mental state score on the PANSS scale by 20% or more. To avoid possible adverse reactions of risperidone when used in children, we calculated a safe dosage range for the drug, based on theoretical developments of the so-called therapeutic “small dose effect”, known in medicine. The phenomenon of specific cascade responses in the body caused by “small doses” allows one to achieve the same treatment results as when taking a standard macrodose, and at the same time helps to avoid unnecessary complications [7]. Children aged 3–4 years were prescribed 1/20 of the average dose for adults, children 4–5 years old – 1/10 of the dose. Taking into account the rate at which risperidone is eliminated from the body (36 hours or more), in a number of cases, the technique of fractional (every other day) administration of the drug was used. In some cases, the maximum dose of risperidone did not exceed 0.5–0.6 mg/day, which corresponded to the recommendations of leading foreign child psychiatrists [1,11,20,21]. Treatment of patients was not limited to risperidone monotherapy, but in some cases was combined with the use of other antipsychotics - etaprazine, neuleptil and haloperidol in small doses (1/5–1/4 of the average therapeutic dose per day). These drugs were used during the period of exacerbation of psychotic symptoms for a short period of time (1–1.5 weeks). In several cases, the EEG revealed slow-wave peak-like paroxysmal activity, which was clinically expressed by dysphoric outbursts, increased impulsivity and aggressiveness. To mitigate these disorders, sodium valproate (50–150 mg per day) was occasionally added to the main risperidone therapy in small doses. In addition, basic treatment with risperidone was combined with the use of biotics - cerebrolysin, cerebramin, cortexin, biolan, deltaran, etc. The first clinical observation of the effectiveness of risperidone was carried out from 1999 to 2002. The second stage of studying the effectiveness of risperidone was carried out from 2002 to 2006. in 49 children aged 4–12 years (38 boys and 11 girls), also with early onset schizophrenia. The diagnosis of the disease in both groups was qualified according to ICD-10 and DSM-4 as an undifferentiated form of schizophrenia, childhood type. The average age of children in both groups was 5.5±1.2 years, the average duration of the disease was 4.5±1.7 years. When selecting patients for treatment with risperidone, their condition was determined by severe mental disorders within the framework of malignant childhood schizophrenia, or according to ICD 10 - F20.8xx3. The clinical characteristics of the cases included in the observation cohort were determined by catatonic-regressive attacks, accompanied by a suspension of mental development, pronounced autistic manifestations, undulating psychotic symptoms, mutism with the subsequent formation of a specific oligophrenia-like defect. Against this background, repeated exacerbations of the disease were noted in the form of increased affective, catatonic disorders, rudiments of delirium and hallucinations. According to the PANSS scale, the level of violations was determined in the range of 102–165 points (average - 132 points). According to the CGI scale, the severity of the disease was assessed at 6–7 points. From the first two months of treatment, all observed children showed a clear positive effect with a significant reduction in productive and negative symptoms. Impulsive catatonic agitation disappeared from productive symptoms, and the severity of delusional and hallucinatory symptoms decreased. By the end of the first year, the smoothing out of the negative manifestations of the disease was especially noticeable. At the same time, positive symptoms persisted in a number of cases, but were mild and transient in nature. Against this background, seasonal exacerbations of the disease were observed, which, however, did not reach the severity of the manifestations of the primary episodes. Autistic symptoms in patients were noticeably reduced. Their interaction with surrounding children and adults has improved significantly. During communication, eye contact appeared, negativism, manifestations of pseudo-deafness and pseudo-blindness decreased. Social behavior also became more adequate. Emotional contact with loved ones and acquaintances was especially positive. Elements of empathy, a sense of tact, and humor appeared in the children’s behavior, i.e. emotional resonance is adequate to the mood of others. There has been progress in cognitive development - primarily an increase in vocabulary in impressive speech, as well as in some cases expressive speech in the form of individual words and phrases that children used for communicative purposes to express their feelings and exchange impressions with loved ones. Several children's cognitive interests (playing, everyday) expanded. In all cases, an improvement in mood and somatovegetative status was revealed - turgor and skin color normalized, and shine in the eyes and hair appeared. However, the further dynamics of the disease in the cohort of patients turned out to be different, and according to the effectiveness of risperidone therapy, the cohort was divided into two groups. In 17 (35%) patients assigned to the group of malignant schizophrenia, the mental state at follow-up remained relatively severe, and therefore they did not enter general educational institutions on time and were not systematically educated at home. But at the same time, the children were adapted to the microsocial conditions of the family, mastered the basic hygienic skills of self-care, and simple elementary phrasal speech. Some of them were able to learn to read and write on a computer, made attempts to compose simple stories of everyday content, kept a diary, and completed some training programs, although in general cognitive activity remained dissociated, specific thinking disorders persisted (diversity, slippages, cliffs), and the level of lag in intellectual development reached severe mental retardation. In the other 32 patients (65%), who also suffered catatonic-regressive attacks in early childhood, the dynamics during long-term therapy with risperidone turned out to be different. In children, the progression of the disease noticeably changed and became smaller. During therapy with risperidone, the children developed quite developed speech. At the age of 7–8, these children were able to enter correctional schools, where they studied according to a mass program, but in gentle conditions; 6 patients, while undergoing treatment, entered universities. The mental state of these patients still retains mild autistic, affective and catatonic manifestations in speech, motor skills, behavior, as well as individual schizotypal stigmas at the level of emotions, the autonomic nervous system, and individual motor disorders. The condition of 9 children and adolescents is currently determined by neurosis-like and psychopath-like symptoms, episodic, often seasonal, affective fluctuations, against the background of which fragmentary productive symptoms are noted. At the same time, during treatment with risperidone, all these children show a fairly high performance capacity, concentration of attention, thinking, and criticism of their condition. Some of these children developed new interests, hobbies (one for music, another for organizing a business and making money), a tendency to develop and enrich their personality, and expand the range of age-related interests. On the PANSS scale, a year after the start of therapy, there was a decrease in the group average total score from 132 to 84 points (ranging from 90 to 42 points), after 6 years - to 64 points in the subgroup with an unfavorable course and to 48 points in the subgroup with a favorable course . Figure 1 shows the dynamics of the decrease in the average total score of the PANSS scale, reflecting a decrease in the severity of the mental state of patients during treatment (p<0.005). The average percentage improvement for the group during follow-up from 1999 to 2006 was 52%. The severity of the condition on the CGI scale decreased to 4 points. According to the UKU scale, the severity of complications did not exceed 1–2 points. During treatment with risperidone, some of the patients, at the initiative of their parents, took a long (up to 3–6 months) break from taking the drug. After a break of 4–5 months, they experienced a return of previous psychotic symptoms to a lesser extent than before treatment, however, the patients lost their previous working capacity and activity, and interrupted the educational process at school and college. Resumption of risperidone treatment again improved their mental status. These facts led to the conclusion that interrupting treatment with risperidone was inappropriate. But due to the need for long-term use of the drug, certain measures were taken to prevent therapeutic addiction directly to risperidone. For this purpose, breaks were taken in treatment for 1–2 summer months or an intermittent course of treatment was prescribed in the form of 0.1–0.2 mg 2–3 times a week, which was pharmacokinetically justified, because up to 75% of the drug and its derivatives remained in circulation in the patient’s blood for 2–3 days. In conclusion, it should be added that during long-term therapy with risperidone, no serious side effects clearly associated with taking the drug were recorded. According to the UKU scale, only minor autonomic abnormalities were noted, mainly at the beginning of therapy (low-grade fever, sleep disturbances, headaches, discomfort in the epigastric region); in isolated cases, allergic manifestations in the form of skin rash and slight weight gain were noted, which disappeared on their own and did not lead to discontinuation of the drug. Thus, summing up the results of long-term therapy with risperidone, we can conclude that treatment with small doses of the drug is effective and safe in childhood. It was after a one-year period of treatment that improvements in cognitive functions, attention, performance, thinking, speech, and social behavior became noticeable. Treatment with risperidone, apparently, not only alleviated the current condition of patients, but also generally influenced the pathogenesis of the disease. This hypothesis is confirmed by the fact that to date, in the vast majority of patients, the disease has acquired a regressive course, regardless of their initial condition. Since the reduction of psychopathological manifestations was achieved in the process of complex treatment, it is advisable to recommend a combination of risperidone with small doses of other antipsychotics and courses of biotics. Observation shows that a gentle treatment regimen and an individual approach to the choice of therapeutic dosages, refusal to force maximum daily dosages allows the use of risperidone for a long time without the risk of complications for the physical health of children.
Literature 1. Arena D., Rosenbaum D. Pharmacotherapy of mental disorders. Per. from English – M.: BINOM, 2004. – P. 61–63. 2. Beznos S.A., Shaposhnikov N.N., Ryazanova E.A. Experience in using the drug “rispolept” based on materials from the children’s department of the Psychiatric Hospital of Krasnodar / Materials of the Second Scientific and Practical Conference of Psychiatrists and Narcologists of the Southern Federal District, June 21–23, 2006, Rostov-on-Don. – pp. 29–32. 3. Vovin R.Ya., Mazo G.E., Ivanov M.V., Kosterin D.N. The use of rispolept to relieve exacerbations of schizophrenia // Psychiatry and psychopharmacotherapy. – 2000. – App. No. 2. – pp. 6–8. 4. Kalinin V.V. // Social and clinical psychiatry. – 1999, No. 1. – P. 97–105. 5. Kozlovskaya G.V., Kalinina M.A., Goryunova A.V., Proselkova M.E. Experience of using rispolept in the treatment of early childhood autism and schizophrenia in children // Psychiatry and psychopharmacotherapy. – 2000. – App. No. 2. – pp. 10–12. 6. Kozlovskaya G.V., Kalinina M.A. The effectiveness of rispolept in children in the prolonged (for 2 years) treatment of schizophrenia and early childhood autism // Psychiatry and psychopharmacotherapy. – 2003. – App. No. 1. – pp. 10–13. 7. Kozlovskaya G.V. Kalinina M.A. Goryunova et al. Psychopharmacology in micropsychiatry // Psychiatry and psychopharmacotherapy. – 2006, No. 5, vol. 7. – P. 256–259. 8. Kolyutskaya E.V., Dorozhenok I.Yu., Ilyina N.A. // Social and clinical psychiatry. – 1998, No. 4. – P. 88–91. 9. D.N. Kosterin, G.E. Mazo, M.V. Ivanov // Social and clinical psychiatry. – 2000, No. 1. – P. 46–47. 10. Mosolov S.N., Kalinin V.V., Eremin A.V. et al. Comparative randomized study of the effectiveness and tolerance of risperidone and haloperidol in the relief of acute conditions in patients with schizophrenia and schizoaffective psychosis // Psychiatry and psychopharmacotherapy. – 2000. – App. No. 2. – pp. 3–6. 11. Shinaev N.N., Akzhiginov R.G., Volkova N.P. The use of the atypical antipsychotic rispolept in the clinic of borderline mental disorders // Psychiatry and psychopharmacotherapy. – 2000. – App. No. 2. – pp. 8–10. 12. Guide to Clinical Child and Adolescent Psychiatry: Trans. from English / Ed. K.S. Robson. – M.: Medicine, 1999. – P. 227–255. 13. Armenteros JL, Whitaker AH, Welikson M., et al., Risperidon in ado-lescents with schizophrenia: an open pilot study // J Am Acad Child Adolesc Psy-chiatry 36: 5, 694–700, May, 1997. 14. Carlsson A., Waters N., Carlsson ML, Neurotransmitter interactions in schizophrenia–therapeutic implications // Biol. Psychiatry. – 1999; 46:1388–1395. 15. Crismon M. L, Dorson PG Schizophrenia in: Dipiro JT, Talbert RL, and Yee GC eds. Pharmacotherapy: A Pathophysiologic Approach. New York, NY: McGraw–Hil/Appleton and Lange; 1999. 16. Falkai P, Wobrock T, et al. Guidelines for biological treatment of schizophrenia. Part 1 // World J Biological Psychiatry 6(3), 132–144, 2005. 17. Findling RL, Maxwell K., Wiznizer M. An open clinical trial of risperi-don monotherapy in young children with autistic disorders // Psychopharmacol Bull 33: 1, 155–9, 1997. 18. Kapur S., Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2receptor occupancy of clozapine, risperidon and olanzapine in schizophrenia // Am J Psychiatry 158: 286–293, 1999. 19. Kerwin RW Role of atypical antipsychotic in schizophrenia // Scizophr Bull: 25; 281–282, 2001. 20. Posey DJ, Walsh KH, Wilson GA, et al. Risperidon in the treatment of two very young children with autism // J Child Adolesc Psychopharmacol, 1999, 9, 273–276. 21. Stahl SM Psychopharmacology of Antipsychotics, 1999, M Dunits, London Reprinted, 2000, USA. – 77, 109–119. 22. Tasman A., Kay J., Lieberman JA Psychiatry, Second Ed, v. 1, Jwilly a Sons, LTD, 2004: 770–773. The article was published in the journal “Issues of Mental Health of Children and Adolescents”. – 2006 (6), No. 2, pp. 56–62.
Contraindications and restrictions
The use of Risperidone is completely prohibited in the following cases:
- if the patient has paroxysmal epilepsy;
- with Parkinson's disease;
- during breastfeeding;
- in the presence of hypersensitivity to the drug itself.
As for pregnancy, the use of the medicine during this period is not recommended. However, if its use causes less harm to the fetus than refusing therapy with this substance, the attending physician has the right to prescribe such a drug. However, the treatment itself must be supervised by a specialist.
There are also a number of diseases, in the presence of which the pills should be taken carefully with strict control of the dose and the body’s reaction. Their list includes the following:
- diseases of the cardiovascular system;
- cerebrovascular accident;
- dehydration.
In addition, such an active substance is not recommended to be combined with other drugs that affect the central nervous system. If such therapy turns out to be mandatory, it is necessary to correctly determine the dose of each of the drugs used.
Risperidone Organika
Interactions related to the pharmacodynamics of the drug
Drugs that prolong the QT interval
As with other antipsychotic drugs, caution should be exercised when co-prescribing Risperidone Organic with drugs that prolong the QT interval, for example, with antiarrhythmic drugs (quinidine, disopyramide, procainamide, propafenone, amiodarone, sotalol, etc.). tricyclic antidepressants (amitriptyline, etc.), tetracyclic antidepressants (maprotiline, etc.), some antihistamines, other antipsychotics, some antimalarials (quinine, mefloquine, etc.), drugs that cause electrolyte imbalance (hypokalemia, hypomagnesemia), bradycardia or inhibiting the hepatic metabolism of risperidone.
Centrally acting drugs and alcohol
Risperidone Organic should be used with caution in combination with other centrally acting drugs and substances, especially alcohol, opiates, antihistamines and benzodiazepines due to the increased risk of sedation.
Levodopa and dopamine receptor agonists
Risperidone Organic may reduce the effectiveness of levodopa and other dopamine receptor agonists. If this combination is necessary, especially in end-stage Parkinson's disease, the lowest effective dose of each drug should be prescribed.
Antihypertensive drugs
When Risperidone Organic is used in combination with antihypertensive drugs, clinically significant hypotension is possible.
Paliperidone
It is not recommended to use Risperidone Organica and paliperidone at the same time, since paliperidone is an active metabolite of risperidone. The combined use of paliperidone and Risperidone Organic may lead to an increase in the concentration of the active antipsychotic fraction.
Interactions related to drug pharmacokinetics
Eating does not affect the absorption of risperidone. Risperidone Organic is primarily metabolized by the CYP2D6 isoenzyme and to a lesser extent by the CYP3A4 isoenzyme. Risperidone Organic and its active metabolite 9-hydroxyrisperidone are substrates of P-glycoprotein (P-gp). Drugs that affect the activity of the CYP2D6 isoenzyme and drugs that significantly inhibit or induce the activity of the CYP3A4 isoenzyme and/or P-gp may affect the pharmacokinetics of the active antipsychotic fraction of Risperidone Organic.
CYP2D6 isoenzyme inhibitors
With simultaneous use of Risperidone Organic and potent inhibitors of the CYP2D6 isoenzyme (for example, paroxetine, quinidine), the plasma concentration of risperidone and, to a lesser extent, the active antipsychotic fraction may increase. Higher doses of a potent CYP2D6 inhibitor may increase the concentration of the active antipsychotic fraction of Risperidone Organic. When initiating or discontinuing therapy with a combination of Risperidone Organic and paroxetine, quinidine or another strong CYP2D6 inhibitor, especially in high doses, the dose of Risperidone Organic should be adjusted.
CYP3A4 and/or P-gp inhibitors
The combined use of Risperidone Organic and potent inhibitors of the CYP3A4 isoenzyme and/or P-gp (for example, itraconazole) can significantly increase the concentration of the active antipsychotic fraction of Risperidone Organic in plasma. When initiating or discontinuing therapy with a combination of Risperidone Organic and itraconazole or another strong inhibitor of the isoenzyme CYP3A4 and/or P-gp, the dose of Risperidone Organic should be adjusted.
Inducers of the isoenzyme CYP3A4 and/or P-gp
Concomitant use of Risperidone Organic with a strong inducer of the CYP3A4 isoenzyme and/or P-gp (for example, carbamazepine) may reduce the concentration of the active antipsychotic fraction of Risperidone Organic in plasma. When initiating or discontinuing therapy with a combination of Risperidone Organic and carbamazepine or another strong inducer of the isoenzyme CYP3A4 and/or P-gp, the dose of Risperidone Organic should be adjusted.
The effect of CYP3A4 isoenzyme inducers manifests itself over time, so it may take up to 2 weeks after starting treatment to achieve maximum effect. Accordingly, when discontinuing an inducer of the CYP3A4 isoenzyme, it may take up to 2 weeks until the effect disappears.
Drugs with high binding to plasma proteins
When Risperidone Organic is used together with drugs that are highly bound to plasma proteins, no clinically significant increase in drug concentration is observed.
Children
Drug interaction studies were conducted only in adult patients. The relevance of these studies in children is unknown.
The combined use of psychostimulants (for example, methylphenidate) and Risperidone Organic in children does not change the pharmacokinetic parameters and effectiveness of risperidone.
Effect of other drugs on the pharmacokinetics of risperidone
Antibacterial drugs
Erythromycin, a moderate inhibitor of CYP3A4 and P-gp, does not affect the pharmacokinetics of Risperidone Organic and the active antipsychotic fraction.
Rifampin, a potent inducer of the CYP3A4 and P-gp isoenzymes, causes a decrease in the concentration of the active antipsychotic fraction in plasma.
Anticholinesterase drugs
Donepezil and galantamine, which are substrates of the CYP2D6 and CYP3A4 isoenzymes, do not have a clinically significant effect on the pharmacokinetics of Risperidone Organic and the active antipsychotic fraction.
Antiepileptic drugs
Carbamazepine, a powerful inducer of the CYP3A4 and P-gp isoenzymes, reduces the concentration of the active antipsychotic fraction of Risperidone Organic in plasma. Similar effects were observed with the use of phenytoin and phenobarbital, which are also inducers of the CYP3A4 isoenzyme and P-gp.
Topiramate moderately reduces the bioavailability of Risperidone Organic, but not the active antipsychotic fraction. This interaction is not considered clinically significant.
Antifungal drugs
Itraconazole, a potent inhibitor of the CYP3A4 and P-gp isoenzyme, at a dose of 200 mg/day increases the concentration of the active antipsychotic fraction in plasma by approximately 70% when using Risperidone Organic at a dose of 2 to 8 mg/day.
Ketoconazole, a potent inhibitor of the CYP3A4 and P-gp isoenzyme, at a dose of 200 mg/day increases the concentration of Risperidone Organic in plasma and reduces the concentration of 9-hydroxyrisperidone in plasma.
Neuroleptics
Phenothiazines may increase plasma concentrations of Risperidone Organic, but not the active antipsychotic fraction.
Antiviral drugs
Protease inhibitors
: No official research data available. Because ritonavir is a strong inhibitor of CYP3A4 and a weak inhibitor of CYP2D6, ritonavir and ritonavir-boosted protease inhibitors may lead to increased concentrations of the active antipsychotic fraction of Risperidone Organic.
Beta blockers
Some beta-blockers may increase plasma concentrations of Risperidone Organic, but not the active antipsychotic fraction.
Calcium channel blockers
Verapamil, a moderate inhibitor of the CYP3A4 isoenzyme and P-gp, increases the concentration of Risperidone Organic and the active antipsychotic fraction in plasma.
Gastrointestinal drugs
H2 receptor antagonists:
Cimetidine and ranitidine, which are weak inhibitors of the CYP3A4 and CYP2D6 isoenzymes, increase the bioavailability of Risperidone Organic, but minimally affect the concentration of the active antipsychotic fraction.
Serotonin reuptake inhibitors and tricyclic antidepressants
Fluoxetine, a potent inhibitor of the CYP2D6 isoenzyme, increases the plasma concentration of Risperidone Organic, but has a lesser effect on the concentration of the active antipsychotic fraction.
Paroxetine, a potent inhibitor of the CYP2D6 isoenzyme, increases the concentration of Risperidone Organic in plasma, but in doses up to 20 mg/day it has a lesser effect on the concentration of the active antipsychotic fraction. However, higher doses of paroxetine may increase the concentration of the active antipsychotic fraction of Risperidone Organic.
Tricyclic antidepressants may increase the plasma concentration of Risperidone Organic, but do not affect the concentration of the active antipsychotic fraction.
Amitriptyline does not affect the pharmacokinetics of Risperidone Organic or the active antipsychotic fraction.
Sertraline is a weak inhibitor of the CYP2D6 isoenzyme, and fluvoxamine is a weak inhibitor of the CYP3A4 isoenzyme. At doses up to 100 mg/day, sertraline and fluvoxamine do not have a clinically significant effect on the concentration of the active antipsychotic fraction of Risperidone Organic. However, the use of sertraline or fluvoxamine in doses above 100 mg/day may lead to an increase in the concentration of the active antipsychotic fraction of Risperidone Organic.
Effect of risperidone on the pharmacokinetics of other drugs
Antiepileptic drugs
Risperidone Organic does not have a clinically significant effect on the pharmacokinetics of valproic acid or topiramate.
Neuroleptics
Aripiprazole, a substrate of CYP3A4 and CYP2D6 isoenzymes:
Risperidone Organic does not have a clinically significant effect on the pharmacokinetics of aripiprazole and its active metabolite, dehydroaripiprazole.
Cardiac glycosides
Risperidone Organic does not have a clinically significant effect on the pharmacokinetics of digoxin.
Risperidone Organic does not have a clinically significant effect on the pharmacokinetics of lithium preparations.
Concomitant use with furosemide
See the Cautions section for information regarding increased mortality in elderly patients with dementia taking furosemide concomitantly.
Side effects
Although the drug is considered safe, in some cases certain side effects may occur. These include:
- Drowsiness, fatigue, decreased concentration, tremors or seizures.
- Abdominal pain, nausea, vomiting, constipation, sudden changes in body weight - decrease or increase.
- Pressure surges.
- Decreased libido and sexual dysfunction.
- Autoimmune disorders.
- Skin rash, dry skin, itching.
- Allergic rhinitis.
- Deterioration of vision.
Most often, such symptoms are temporary and disappear after finishing the course of taking the pills. However, if any side effects are detected, you must inform your doctor about it.
In the modern world, neuropsychiatric disorders are among the most widespread diseases. According to WHO estimates, about 450 million people experience them throughout their lives [1], so the proportion of people in the general population receiving antipsychotic drugs is extremely high [2]. The most common reason for prescribing antipsychotics is schizophrenia, although they are widely used for other psychiatric conditions (eg, bipolar disorder, Alzheimer's disease, etc.).
The prevalence of schizophrenia is approximately 1% [3]. The disease usually begins in late adolescence or young adulthood and has a chronic course, characterized by acute attacks with varying periods of remission in between. In half of patients, the disease continues throughout life and requires constant use of medications [4].
Acute episodes of schizophrenia are more often manifested by positive clinical symptoms - delusions, hallucinations, disturbances in thinking and speech, mood changes, and often catatonic phenomena. Patients with chronic forms of the disease also develop negative symptoms - lack of drive and initiative, social withdrawal and flattening of emotional expressiveness. Along with positive and negative symptoms, the primary signs of schizophrenia also include impaired cognitive function. Cognitive deficit is considered not only as an integrative indicator of the volume and severity of procedural psychopathological manifestations, but also as a possible target for therapeutic intervention [5]. It is assumed that the study of cognitive functions can contribute to the selection of the most adequate treatment options already at the initial stage of the disease and determine the direction of further treatment and rehabilitation measures.
Schizophrenia entails disability and high mortality - approximately 2 times higher than in the general population. In particular, mortality from cardiovascular diseases in patients with schizophrenia is 2–3 times higher than that in the general population. About 10% of patients with schizophrenia commit suicide. Suicidal thoughts occur in 40% of patients, and suicide attempts are observed in 23% [6]. Annual direct and indirect costs associated with schizophrenia in the early 1990s. in the USA amounted to 32.5–65 billion dollars, the economic consequences of completed suicide attempts amounted to about 7 billion [7, 8]. In 1990, schizophrenia accounted for 2.5% of all US health care expenditures and 22% of mental health expenditures [7]. In economically developed countries, schizophrenia is one of the main causes of hospitalization.
Effective antipsychotic drugs were introduced into medical practice in the middle of the last century, but the search for new drugs for the treatment of schizophrenia does not stop. This is due to resistance to traditional (“typical”) antipsychotics, which is observed in approximately 20–30% of newly diagnosed individuals, the lack of a pronounced effect on negative symptoms in traditional antipsychotics, and their severe, including irreversible, side effects, in particular tardive dyskinesia. The latter develops in 5–45% of patients with schizophrenia treated with typical antipsychotics [9].
A new era of antipsychotic therapy began with the development of the first atypical drug, clozapine, patented in 1960. Its “atypicality” consisted primarily in the almost complete absence of extrapyramidal side effects. Other important advantages of clozapine over its predecessors are its positive effect on negative symptoms and effectiveness against positive symptoms in severe patients who respond poorly to treatment with traditional antipsychotics [10]. However, in the mid-1970s. the use of clozapine was suspended in several countries because it was associated with a significant increase in the risk of agranulocytosis and death. Experience with clozapine in the 1970s–80s. in other countries (Germany, China, Scandinavian countries) showed that with careful monitoring and timely discontinuation of the drug if signs of blood lesions are detected, its safety increases significantly [11]. These observations served as the basis for the return of clozapine to the UK market in 1989 and the US in 1990. In the 1990s. New atypical antipsychotics were also developed, one of which was risperidone. Currently, atypical antipsychotics are considered first-line drugs for the treatment of various psychotic disorders, including schizophrenia.
Pharmacodynamic and pharmacokinetic properties of risperidone
Risperidone is a benzisoxazole derivative and differs in chemical structure from clozapine and olanzapine, which belong to the group of dibenzodiazepines. It has high affinity for serotonin (5-HT2), dopamine (D2) and alpha1-adrenergic receptors [12]. The drug also binds to H1-histamine receptors, but its affinity for them is much lower than that of most other atypical antipsychotics, which is explained by the weak sedative potential of risperidone. The antipsychotic effect of the drug is associated with blockade of D2 receptors of the mesolimbic and mesocortical systems. The antagonistic effect on 5-HT2 receptors causes a decrease in the severity of negative symptoms of schizophrenia.
In experimental studies, risperidone was 1.5–3.5 times superior to haloperidol in D2-blocking activity, but caused catalepsy much less frequently [13]. It was also superior to clozapine, olanzapine and quetiapine in terms of its affinity for dopaminergic receptors. In a human study of nine patients receiving 7–14-day courses of risperidone at fixed daily doses of 2 to 6 mg, it exhibited affinity for D2 receptors similar to traditional antipsychotics, but greater than that of clozapine [14].
Risperidone has a stronger effect on 5-HT2 receptors than traditional antipsychotics. However, its affinity for 5-HT2 receptors is lower than that of most atypical antipsychotics, which may determine the significantly smaller effect of risperidone on the body weight of patients. On the one hand, the drug’s balanced antagonism of central serotonergic and dopaminergic receptors (5-HT2alpha/D2 affinity ratio is approximately 20) significantly reduces the risk of extrapyramidal symptoms compared to traditional antipsychotics [15, 16]. This is facilitated by the blocking of alpha1-adrenergic type A receptors by risperidone [17]. On the other hand, the α1-adrenergic blocking effect of risperidone may cause the development of orthostatic hypotension.
Risperidone is well absorbed after oral administration, reaching peak concentrations within 1–2 hours. Food does not affect the bioavailability of the drug. Biotransformation of risperidone occurs in the liver with the participation of isoenzymes of the cytochrome P450 system, mainly CYP 2D6, and leads to the formation of 9-hydroxyrisperidone, which is similar in activity to the drug itself.
The concentrations of active substances (risperidone and metabolite) do not differ between fast and slow inactivators. The half-life of the active antipsychotic fraction is approximately 24 hours. In the dose range from 0.5 to 25 mg, the kinetics of risperidone is linear, i.e. its concentrations in the blood increase in proportion to increasing doses. The drug is excreted from the body mainly (70%) with urine and partially with bile. Its clearance is reduced in individuals with severe hepatic or renal impairment. Risperidone blood concentrations may also increase when administered concomitantly with clozapine, paroxetine and fluoxetine [18, 19]. In contrast, carbamazepine accelerates the clearance of risperidone [20]. The metabolism of risperidone may be altered by drugs that affect not only CYP 2D6 but also CYP 3A4, but the clinical consequences of these drug interactions are minimal [21].
Risperidone is safe in a fairly wide range of concentrations – from 25 to 150 µg/l, which allows it to be considered a drug with an extended therapeutic index [22]. The favorable benefit/risk ratio determines the widespread use of risperidone in medical practice. In particular, it is the most commonly prescribed antipsychotic in North America [12].
In addition to the treatment of schizophrenia and other psychotic conditions, risperidone is also indicated for the prevention of exacerbations in patients with schizophrenia. It is also used:
- for behavioral disorders in patients with dementia with symptoms of aggressiveness (outbursts of anger, physical violence), mental disorders (agitation, delirium) or psychotic symptoms;
- as an adjuvant drug in the treatment of mania in bipolar disorders;
- as an auxiliary drug in the treatment of behavioral disorders in adults and adolescents over 15 years of age with a reduced intellectual level or mental retardation in cases where destructive behavior (aggression, impulsivity, auto-aggression) prevails in the clinical picture of the disease.
Effectiveness of risperidone in schizophrenia
The antipsychotic efficacy of risperidone was proven in 4-, 6- and 8-week placebo-controlled studies [12]. Risperidone has also been actively studied in comparative clinical studies with typical antipsychotics. In most of them, the comparator drug was haloperidol. Thus, in an 8-week double-blind randomized study, the Canadian Risperidone Study (145 patients diagnosed with chronic schizophrenia), risperidone in different daily doses (2, 6 and 10 mg) was compared with placebo and haloperidol (20 mg). The most effective and safe dose was 6 mg/day [23], in which risperidone caused an almost 20% decrease in scores on the Positive and Negative Syndrome Scale (PANSS) in 72.7% of patients, which is 25% less. % exceeded the effectiveness of haloperidol (47.6%), but the difference did not reach statistical significance. Both drugs had an equal effect on positive symptoms, and risperidone was significantly superior to haloperidol in its effect on negative symptoms. A significantly greater benefit of risperidone compared with haloperidol was also found when assessed on the general psychopathology subscale.
It should be emphasized that in the early stages of the study, the proportion of patients who dropped out due to treatment failure was 63% in the placebo group, 52% in the haloperidol group, and only 4.5% in the risperidone group.
The risk of developing parkinsonism with risperidone at a daily dose of 6 mg was comparable to that of placebo and significantly lower than the risk with haloperidol. Risperidone, in contrast to haloperidol and placebo, reduced the incidence of dyskinesia.
In a similar study conducted in the USA, risperidone was superior to haloperidol and placebo in terms of effectiveness in relation to positive and negative symptoms, and was also significantly less likely than haloperidol to cause extrapyramidal disorders [24]. A large European study failed to demonstrate a clear advantage of risperidone over haloperidol in terms of efficacy in patients with schizophrenia, but, as in North American studies, it was demonstrated to be superior in reducing the risk of extrapyramidal symptoms [25].
A pooled analysis of the results of eight comparative studies of risperidone with traditional antipsychotics showed that it is superior to comparator drugs in terms of their effect on negative symptoms [26]. The difference in PANSS scores was 0.74 and was borderline significant (p = 0.058).
Overall, in a meta-analysis of 11 randomized controlled trials (2513 patients), clinical improvement (20% reduction in baseline Brief Psychiatric Rating Scale (BPRS) and PANSS scores was observed in a greater proportion of patients treated with risperidone than with treatment with typical antipsychotics, but the absolute difference was small (57 versus 52%) [26].Patients receiving risperidone were less likely to need prescription correctors of extrapyramidal symptoms (22.9 and 38.4%, respectively), although the frequency of use of the latter increased with increasing doses of risperidone (more than 8 mg): Patients receiving risperidone were less likely to drop out of studies than patients in the comparison group.
Similar advantages of risperidone over haloperidol were demonstrated in another meta-analysis [27].
Another meta-analysis, which included 12 double-blind randomized studies lasting no more than 8 weeks (1056 patients receiving risperidone and 703 patients receiving other antipsychotics, including 473 - haloperidol), demonstrated higher effectiveness of risperidone compared not only with haloperidol, but also with other antipsychotic drugs [28]. Thus, a decrease in initial PANSS scores by 20% was observed in 65.9% of patients receiving risperidone, in 54.3% - haloperidol and in 54.9% - other antipsychotic drugs, by 30% - in 54.0; 46.6 and 46.5%, by 40% or more – in 43.8; 33.7 and 34.4% respectively. Overall, treatment-induced decreases in baseline PANSS scores were significantly more common in the risperidone group (–24.7) than in the other antipsychotic group (–19.8; p < 0.01), including haloperidol (–19.8; p < 0.01). 0.05). In addition, risperidone was superior to comparators in improving positive symptoms: -7.8 versus -6.3 for other antipsychotics (p < 0.01) and -7.1 for haloperidol.
A meta-analysis that specifically assessed the effect of risperidone on negative symptoms of schizophrenia also revealed its advantages over traditional antipsychotic drugs: the use of risperidone in a daily dose of 4-8 mg increased the likelihood of improvement in negative symptoms compared with the use of haloperidol, perphenazine and zuclopenthixol by 1.43 times [29]. In general, risperidone is approximately 25% more effective than traditional antipsychotics in affecting positive symptoms of schizophrenia and 60% more effective in affecting negative symptoms [27].
In addition, risperidone is superior to traditional antipsychotics in eliminating symptoms of schizophrenia such as hostility and aggression [30], and also eliminates symptoms of anxiety and depression faster and significantly better than haloperidol [31]. The advantages of risperidone over haloperidol also include a more rapid development of antipsychotic action [17]. The fundamental difference between risperidone and traditional antipsychotics is its beneficial effect on the cognitive functions of patients with schizophrenia [32].
The superiority of risperidone over traditional antipsychotics was also confirmed by the results of a large (684 patients) 2-year prospective study in real medical practice [33]. Patients treated with risperidone experienced more favorable clinical outcomes (reduction in psychiatric symptoms and side effects) compared with patients treated with traditional antipsychotics, as well as improvements in health-related quality of life (HRQOL).
Clinical studies have compared risperidone with other atypical antipsychotics. The largest number of studies have been devoted to the comparative evaluation of risperidone and olanzapine. They found conflicting results. A targeted meta-analysis failed to identify a significant difference in the effectiveness of these drugs in patients with schizophrenia [34].
Other meta-analyses have demonstrated equal efficacy in schizophrenia for risperidone, olanzapine and amisulpride [35], as well as olanzapine, quetiapine and risperidone [36].
Another meta-analysis showed that in schizophrenia, sertindole and quetiapine show equal efficacy to haloperidol, while risperidone and olanzapine are superior [37]. As for negative symptoms, this meta-analysis was able to demonstrate the advantages of risperidone and olanzapine over haloperidol, sertindole showed equal effectiveness with a typical antipsychotic, and quetiapine was even slightly inferior to it. In a direct comparative multicenter study CAFE (Comparison of Atypicals in First Episode Psychosis) of risperidone with olanzapine and quetiapine in patients with a first episode of schizophrenia, the effectiveness of these drugs was equal [38]. Short-term (8 weeks) [39] and long-term (1 year) studies [40] also showed equal efficacy of risperidone with ziprasidone in patients with schizophrenia and schizoaffective disorders.
The results of a number of studies confirm the effectiveness of risperidone in schizophrenia resistant to traditional antipsychotics [41–44]. In a comparative study with clozapine in patients with treatment-refractory schizophrenia, risperidone showed equal efficacy to the comparator drug, but was significantly less likely to cause weight gain and sedation [44].
Effectiveness of risperidone in other mental disorders
A meta-analysis of six clinical trials (1343 patients) demonstrated equal effectiveness of risperidone with haloperidol in reducing manic symptoms when used as monotherapy or as an adjuvant to lithium or an anticonvulsant [45]. Risperidone monotherapy for manic episodes in patients with bipolar disorder is effective for a long time and is well tolerated by patients [46].
Risperidone has found fairly widespread use for behavioral disorders in both adults and children. In a randomized trial of 37 adult patients, it was significantly superior to placebo in improving pathological behavior [47]. The results of studies in children allow us to consider risperidone as a useful drug in pediatrics for the correction of behavioral disorders and psychotic symptoms associated with a variety of psychiatric diseases. Risperidone has been proven effective in the treatment of behavioral disorders in children with autism [48–51]. The drug is effective and safe for the treatment of destructive behavior in children with reduced intelligence, including long-term use [52]. In children with destructive behavior, risperidone can significantly reduce aggressiveness and the severity of psychotic symptoms.
A number of studies have shown the effectiveness of risperidone in low doses (1–2 mg/day) in patients with dementia [53–56]. Its advantage over other powerful neuroleptics in these patients is a lower risk of extrapyramidal disorders, as well as sedative and anticholinergic side effects, especially dangerous for the elderly [11]. The large CATIE–AD (Clinical Antipsychotic Trials in Intervention Effectiveness–Alzheimer's Disease Study), an initiative of the US National Institute of Mental Health, identified three atypical antipsychotic medications that were associated with the highest efficacy and significant improvement in neuropsychotic symptoms in patients with Alzheimer's disease. symptoms compared to placebo [57]. They were risperidone, olanzapine and quetiapine. Preliminary analysis of the first of three phases of this study failed to show superiority of any of these drugs in terms of efficacy, but their use was associated with varying rates of individual side effects. It should be noted that the study design involved the evaluation of drugs in real-world medical practice and was free from many of the limitations of randomized clinical trials.
Unfortunately, a recent analysis of 17 placebo-controlled, 10-week studies involving 5106 patients found that olanzapine, aripiprazole, quetiapine, and risperidone for dementia-related psychosis were associated with increased mortality in older patients (4.5 vs. 2.6). % in the placebo group) [58]. Most deaths were due to cardiovascular (heart failure, sudden death) or infectious (pneumonia, etc.) causes. The risk of deaths increased by 1.6–1.7 times during treatment with atypical antipsychotics of all classes, so it is considered as their group property. This was the basis for the inclusion in the instructions for use of atypical antipsychotics with warnings regarding their use in elderly patients with psychoses associated with dementia.
Risperidone, in addition to antidepressants, is used as an augmentative agent in patients with resistant depression [59]. Risperidone itself is believed to have antidepressant properties, which may be due to its effect on beta1-adrenergic receptors [22].
Although risperidone, like clozapine, can cause obsessive-compulsive symptoms (possibly due to serotonergic activity), its addition to selective serotonin reuptake inhibitors has increased the effectiveness of treatment for obsessive-compulsive disorder [60, 61]. The results of a small study suggest that risperidone is highly effective in Tourette syndrome [62].
Safety of risperidone
The use of risperidone is associated with a dose-dependent increase in the incidence of extrapyramidal disorders. However, the risk of this complication is minimal when doses are selected correctly and therapeutic concentrations of the drug in the blood are maintained in the range from 25 to 150 mcg/l. Moreover, even when used in doses up to 16 mg/day, it causes less change in baseline Extrapyramidal Symptom Rating Scale (ESRS) scores than haloperidol [63].
Risperidone may cause orthostatic hypotension and reflex tachycardia. To prevent orthostatic hypotension, treatment should begin with low doses, for example, in elderly patients the initial dose should be 0.5 mg. Other common side effects of risperidone include sedation, decreased sexual desire, erectile dysfunction, and weight gain [23, 41]. However, according to the results of a systematic review of 80 clinical trials of various atypical antipsychotics, the latter effect is weaker for risperidone than for most other drugs, with the exception of ziprasidone [64]. Another systematic review found that weight gain during risperidone treatment decreased with age [65]. This side effect is most common in children under adolescence, and in people over 65 years of age it practically does not appear. In general, weight gain due to risperidone is more pronounced in adolescence than in adulthood.
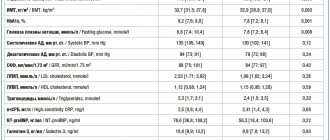
Another problem with the safety of therapy with atypical antipsychotic drugs, which has attracted special attention recently, is the increased risk of developing diabetes mellitus and other metabolic disorders under their influence. In this regard, risperidone compares favorably with clozapine and olanzapine (Table 1) [66–68]. Moreover, switching to risperidone treatment contributed to the restoration of glycemic control in patients with hyperglycemia that developed during the use of other atypical antipsychotics [2]. The cause-and-effect relationship between the use of these drugs and diabetes has not been definitively established. Perhaps the slight increase in the incidence of its development in this case is due to the increased risk of diabetes in patients with schizophrenia. However, in 2004, the manufacturer of the original drug risperidone, Janssen, added a corresponding warning to the instructions for its use.
Risperidone, like other drugs that block dopamine neurotransmission, can cause an increase in prolactin secretion [69]. Clinical consequences of elevated blood prolactin levels include gynecomastia, galactorrhea, amenorrhea, sexual dysfunction and predisposition to osteoporosis. The results of a retrospective analysis suggest that a transient increase in prolactin levels does not have a negative effect on the growth and puberty of children of both sexes under 10 years of age [70].
Unlike clozapine, risperidone does not increase the risk of agranulocytosis. It affects the QTc interval to a lesser extent than ziprasidone and quetiapine and is therefore associated with a lower risk of developing ventricular arrhythmias.
During treatment with risperidone, cases of the development of neuroleptic malignant syndrome have been described [71], however, they, like extrapyramidal disorders and many other side effects, can be prevented by proper dose selection and monitoring of drug concentrations in the blood. The optimal dose of the drug for the treatment of recurrent schizophrenia is 4 mg/day [72]. Doses above 6 mg/day do not lead to an increase in the therapeutic effect, and at daily doses of 10 mg and above there is even a tendency to reduce the effectiveness of risperidone while significantly increasing the risk of side effects. It is recommended to start treatment with a low dose and gradually titrate it until a therapeutic (but not toxic) effect is obtained [41]. Usually the initial dose is 1 mg 2 times a day, in elderly patients - 0.5 mg/day. Thereafter, it is recommended to increase the dose by 1 mg every 5–7 days.
Reducing the cost of risperidone treatment
One of the main factors limiting the use of atypical antipsychotics is their high price and cost of treatment (Table 2). The most effective way to reduce the cost of treatment is to replace original drugs with high-quality generics.
Currently, the antipsychotic drug Risdonal (risperidone), jointly produced in Russia by the pharmaceutical companies Alkaloid (Macedonia) and Makiz-Pharma (Russia), has appeared on the domestic pharmaceutical market. The results of its 8-week, randomized, blinded, comparative study with haloperidol in 40 patients with chronic schizophrenia suggest that it is an effective and well-tolerated formulation of risperidone [17].
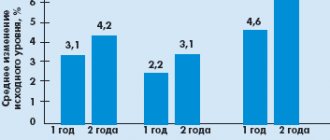
Risdonal was superior to haloperidol in terms of effectiveness in improving the positive (by PANSS subscale by 64.1 vs. 52.91%; p < 0.01) and especially negative (by PANSS subscale by 49.63 vs. 32.46%; p < 0. 01) symptoms of schizophrenia. In addition, it improved the clinical condition of patients to a greater extent than the comparator drug as assessed by the Clinical Global Impression Scale (CGI) and its subscales - global improvement and therapeutic effectiveness (Fig. 1). Risdonal was more effective than haloperidol in terms of improving quality of life. It should be noted that in most patients the therapeutic effect was achieved when taking Risdonal at a daily dose of 4–6 mg, which corresponds to the recommended doses of the original drug risperidone.
Accordingly, Risdonal was better tolerated than haloperidol. Severe extrapyramidal syndrome was recorded in only three (15.79%) patients in the Risdonal group compared to 11 (55%) in the control group. Eight (42%) patients receiving Risdonal required antiparkinsonian medications compared with 17 (80%) receiving the comparator drug (p < 0.01). These data are consistent with the results of clinical studies of the original drug risperidone. The dynamics of the combined indicator of parkinsonism, dyskinesia and dystonia, assessed according to the ESRS, are presented in Fig. 2. Similar results were obtained when separately assessing the symptoms of parkinsonism, dyskinesia and dystonia according to the ESRS subscales.
Thus, data on the effectiveness and tolerability of Risdonal allow us to consider it a worthy alternative to the original drug risperidone, which may reduce the cost of treatment with this effective antipsychotic.