Wobenzym
It is used in complex therapy
of the following diseases:
Angiology:
thrombophlebitis, treatment of postthrombophlebitis disease and acute thrombophlebitis of superficial veins, endarteritis and obliterating atherosclerosis of the arteries of the lower extremities, prevention of recurrent phlebitis, lymphedema.
Urology:
cystitis, cystopyelitis, prostatitis, sexually transmitted infections.
Gynecology:
chronic genital infections, adnexitis, gestosis, mastopathy, to reduce the frequency and severity of side effects of hormone replacement therapy.
Cardiology:
angina pectoris subacute stage of myocardial infarction (to improve the rheological properties of blood).
Pulmonology:
sinusitis, bronchitis, pneumonia.
Gastroenterology:
pancreatitis, hepatitis.
Nephrology:
pyelonephritis, glomerulonephritis.
Endocrinology:
diabetic angiopathy, diabetic retinopathy, autoimmune thyroiditis.
Rheumatology:
rheumatoid arthritis, reactive arthritis, ankylosing spondylitis.
Dermatology:
atopic dermatitis, acne.
Surgery:
prevention and treatment of postoperative complications (inflammation, thrombosis, edema), adhesive disease, post-traumatic and lymphatic edema, plastic and reconstructive surgery.
Traumatology:
injuries, fractures, distortions, ligamentous injuries, bruises, chronic post-traumatic processes, inflammation of soft tissues, burns, injuries in sports medicine.
Neurology:
multiple sclerosis.
Pediatrics:
atopic dermatitis, infectious and inflammatory diseases of the respiratory tract (inflammation of the upper and lower respiratory tract, pneumonia), juvenile rheumatoid arthritis, prevention and treatment of postoperative complications (suppuration and poor wound healing, formation of adhesions, local edema).
Ophthalmology:
uveitis, iridocyclitis, hemophthalmos, diabetic retinopathy, ophthalmic surgery.
Prevention:
microcirculation disorders, post-stress disorders, as well as failure of adaptation mechanisms. Prevention of side effects of hormone replacement therapy, hormonal contraception. During surgical interventions to prevent infectious complications and adhesions.
WOBENZYM
recommended for the prevention of infectious complications and improvement of quality of life during chemotherapy or radiation therapy. Prevention of the development of viral infections and their complications.
We observed 53 patients with acute respiratory viral infections occurring with secondary bacterial complications at the age of 5 to 9 years (30 patients received Wobenzym in combination with antibacterial therapy, 23 - a comparison group - only antibacterial drugs). In patients with ARVI with secondary bacterial complications, Wobenzym therapy promotes faster relief of fever, intoxication syndrome, rhinitis, tonsillitis, pharyngitis, and otitis compared to the control group. During therapy with Wobenzym, a decrease in colonization of Streptococcus spp was noted. (eradication 23%), Nocardia (eradication 19%), Nocardia asteroides (eradication 29%) and an increase in the content of normal flora, which confirms the advisability of prescribing systemic enzyme therapy drugs for acute respiratory viral infections with secondary bacterial complications.
Application of polyenzimatic drug wobenzym at sickly children with acute respiratory infections
Under observation there were 53 sickly children with acute respiratory viral infection proceeding with secondary bacterial complications in the age from 5 to 9 (30 patients were taking Vobenzyme combined with antibacterial therapy, 23 - group for comparison - only antibacterial drugs). In patients with secondary respiratory acute viral infections therapy with Vobenzyme contributes to more rapid abortion of fever, intoxication syndrome, rhinitis, tonsillitis, pharyngitis, otitis appearances in comparison with the control group. Against the background therapy with Vobenzyme decrease of colonization with Streptococcus (eradication 23%), Nocardia (eradication 19%), Nocardia asteroides (eradication 29%) and increase of normal flora was noted, which confirmed expediency of drugs of system enzyme therapy prescription in sickly children with acute respiratory viral infections with secondary bacterial complications.
Currently, frequently ill children include children with a higher incidence of respiratory infections than their peers, which in domestic medicine are combined into the dispensary observation group of “frequently ill children” (FCH). These children get sick almost every month, regardless of age [1, 2].
According to the literature, in addition to etiotropic, pathogenetic and symptomatic drugs, the protocol for the treatment of acute respiratory syndrome often includes immunorehabilitation drugs, which generally leads to more rapid relief of clinical symptoms of the disease and a decrease in morbidity in the future [3, 4, 5]. However, taking into account the constantly ongoing inflammatory process in CBD, associated not only with a new infection, but also with the fact that viral and bacterial infections can persist due to a selective defect in immunological defense, the question arises of searching for new treatment methods aimed at reducing the adhesive abilities of the pathogen. Oropharyngeal Ig has such abilities in the lesion, and systemic enzyme therapy drugs have a general effect [6, 7, 8].
Our attention was drawn to the systemic enzyme therapy drug Wobenzym, which has anti-inflammatory, immunomodulatory, antiaggregation, fibrino-, thrombolytic and potentiating effects [9].
Materials and methods
Under observation were 53 children with acute respiratory viral infections occurring with secondary bacterial complications at the age of 5 to 9 years. The study group consisted of 30 patients who received Wobenzym in combination with antibacterial therapy. 23 patients formed a comparison group who received only antibacterial drugs as etiotropic therapy.
The development of a bacterial infection was evidenced by the presence of serous-purulent discharge from the nose with an unpleasant odor (100%), the development of otitis media (68%), the phenomena of granulosa pharyngitis with overlays of mucopurulent secretion on the posterior wall of the pharynx (64%), swelling and hypertrophy of the palatine tonsils (100%), loosening of the mucous membrane and “gaping” of the lacunae of the tonsils, as well as bright diffuse hyperemia of the mucous membranes of the oropharynx (100%).
All observed children were classified as frequently and long-term sufferers of ARVI (up to 8 times a year). 36 (68%) patients had grade II hypertrophy of the tonsils, 21 (40%) had chronic tonsillitis, 22 (41.5%) had grade II adenoids, 2 (3.8%) had adenectomy, 34 ( 64%) - phenomena of granulomatous pharyngitis, 19 (36%) - chronic pharyngitis.
The severity of the disease in patients with ARVI with bacterial complications was determined subjectively by a set of clinical indicators and objectively by nuclear and leukocyte intoxication indices (NII, LII). All patients had moderate severity of the disease. However, 70.5% were children with severe and 29.5% with moderate signs of intoxication.
Wobenzym was prescribed to children in a daily dose of 1 tablet per 6 kg of weight for 10 days.
All children had their sputum and blood examined using gas chromatography-mass spectrometry before treatment was prescribed. The method is based on highly accurate determination of the presence of molecular characteristics of microorganisms (markers) from among their cellular lipids - higher fatty acids, aldehydes, alcohols and sterols in the analyzed sample. The determination is made using a highly sensitive and selective method of gas chromatography - mass spectrometry (GC-MS), which allows the simultaneous measurement of more than a hundred microbial markers directly in the analyzed material, blood, urine, biopsies, punctates, sputum, and other biological fluids and tissues without prior culture for nutrients environment [10].
Results and discussion
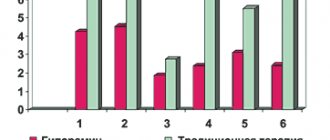
As a result of the examination before the start of treatment, the presence of 27 microorganisms with high colonization was detected in the sputum, among which the largest proportion was Streptococcus (17%); Nocardia (8.7%); Clostridium ramosum (14%); Staphylococcus intermedius (1.9%); Lactobacillus (9.1%); Candida (4.1%); E. moniliforme (9.7%); Propionibacterium spp (5.7%); Ruminicoccus (1.6%); Peptostreptococcus anaerobius (8.4%); Streptomyces (3.4%) [Fig. 1].
Figure 1. Results of studying the composition of microbial markers in the saliva of children using gas chromatography-mass spectrometry (data in microbial cells/ml of sample multiplied by x10*5) (n=18)
21 microorganisms with high colonization were identified in the blood, among which the largest proportion was Streptococcus (3.7%); Nocardia (8.6%); Clostridium ramosum (12.2%); Staphylococcus intermedius (6.3%); Lactobacillus (23.1%); Candida (4.1%); E. moniliforme (9.1%); Propionibacterium spp. (6.1%); Ruminicoccus (3.9%); Streptomyces (1.5%) [Fig. 2].
Figure 2. Results of studying the composition of microbial markers in the blood using gas chromatography - mass spectrometry (n=18)
A comparative analysis found that the microorganisms of saliva and blood are identical, however, the colonization of Streptococcus in saliva is 13.3% higher than in the blood, and the colonization of Staphylococcus intermedius is 4.4% and Lactobacillus is 14% higher in the blood, which is associated with the course of acute respiratory infection.
As a result of the treatment in the microbiocenosis of the nasopharynx, a decrease in colonization of Streptococcus by 15%, Nocardia by 7%, and Peptostreptococcus anaerobius by 4.2% was noted; moderate increase in opportunistic flora Clostridium ramosum by 4.3%; Propionibacterium spp by 7.4%. In the blood there was a decrease in Streptococcus by 1.3%, Nocardia by 3% and a moderate increase in Clostridium ramosum by 2.7%. In a comparative analysis, the microflora of saliva and blood is also homogeneous. At the same time, there was no predominance of Streptococcus colonization in saliva; in the blood, the predominance of Staphylococcus intermedius colonization decreased by 2% and the predominance of Lactobacillus increased by 2.1%.
In the dynamics of the disease in patients treated with Wobenzym, dysbiotic changes in the microflora of the oropharynx tended to normalize both qualitative and quantitative composition, which significantly affected the duration of the main clinical symptoms and the duration of the disease [Table. 1].
Table 1.
Clinical symptoms of ARVI with bacterial complications in children treated with Wobenzym
| Main group (with Wobenzym) ( n =30) | Control group (n= 23 ) | ||
| Duration of fever | 4,8±0,15 | 5,0±0,19 | |
| Symptoms of intoxication | Headache | 5,4±0,23 | 5,6±0,16 |
| Adynamia | 5,3±0,27 | 5,5±0,2 | |
| Anxiety | 6,1±0,27 | 6,3±0,26 | |
| Decreased appetite | 5,3±0,29* | 6,7±0,21 | |
| Pallor | 5,6±0,26 | 5,5±0,26 | |
| Catarrhal symptoms | Nasal congestion | 6,8±0,18* | 8,0±0,10 |
| Rhinitis | 7,0±0,20 | 7,2±0,10 | |
| Rhinitis mucoserous | 6,5±0,29 | 6,7±0,28 | |
| Rhinitis mucopurulent | 4,9±0,31 | 5,3±0,33 | |
| A sore throat | 5,2±0,25 | 5,5±0,19 | |
| Throat hyperemia | 6,9±0,22 | 7,0±0,11 | |
| Granularity of the posterior pharyngeal wall | 7,0±0,21 | 7,4±0,18 | |
| Swelling of the tonsils | 6,9±0,26 | 7,4±0,15 | |
| Conjunctivitis | 2,4±0,80 | 2,5±0,63 | |
| Tearing | 3,3±0,41 | 3,2±0,47 | |
| Cough | Dry | 3,8±0,42 | 4,2±0,6 |
| Wet | 5,2±0,33* | 6,7±0,24 | |
| Total duration of cough | 7,2±0,23* | 8,9±0,14 | |
| Auscultatory changes in the lungs | 5,3±0,27* | 6,4±0,15 | |
Note: *—difference from the control group is significant, p<0.05
As can be seen from the presented data, in patients treated with Wobenzym, changes in such indicators as “decreased appetite”, “nasal congestion”, “wet cough”, “total duration of cough”, “auscultatory changes in the lungs” turned out to be significant. The duration of other clinical symptoms tended to decrease. The maximum clinical effect was observed in 38% of children on the 6th day of taking the drug. At the same time, 42% of children showed a change in sputum density in favor of its dilution, while in the comparison group these changes were recorded only in 38% of children.
According to expert scoring, in patients treated with Wobenzym, the clinical effect was excellent in 53.3%, and good in 46.7%. In the control group, 48 and 52%, respectively (Table 2).
Table 2.
Expert assessment of the clinical effectiveness of the drug Wobenzym in patients with ARVI with bacterial complications and the results of their treatment
| Treatment | Clinical effect on the 10th day of treatment | Total | ||
| Great | Good | Weakly expressed or absent | ||
| Main group Wobenzym (n=30) | 16 (53,3%) | 14 (46,7%) | 0 | 30 (100%) |
| Control group(n=23) | 11 (48%) | 12 (52%) | 0 | 23 (100%) |
We considered treatment effective in cases where clinical recovery or marked improvement with normalization of temperature, relief of clinical manifestations of infection and disappearance of symptoms of intoxication occurred before the 6th day from the start of treatment. The clinical effect was assessed as “excellent” in cases where a significant change in the patient’s condition occurred already on the 3rd day of treatment.
As a result of the treatment, 41.2% of children were discharged with recovery, in 58.8% of observation cases there was a good effect with an improvement in condition.
At the same time, the level of eradication of pathogens in saliva was: Streptococcus - 95%; Nocardia - 92%; Peptostreptococcus anaerobius - 78%; Streptomyces - 48%; Clostridium ramosum - 43%; Candida - 42% [Fig. 3].
Figure 3. Results of studying the composition of microbial markers in the saliva of children using gas chromatography - mass spectrometry before and after treatment with Wobenzym. Data in microbial cells/ml sample multiplied by x10*5(n=18)
In the blood, the level of pathogen eradication was: Streptococcus - 18%; Nocardia - 17% [Fig. 4].
Figure 4. Results of studying the composition of microbial markers in the blood of children using gas chromatography - mass spectrometry before and after treatment with Wobenzym (n=18)
The level of eradication of pathogens corresponded to a 2-fold decrease in the severity of the disease according to LII and 1.5 times according to NII [Fig. 5].
Figure 5. Dynamics of the severity of the disease according to leukocyte LII and YII in children with ARVI with bacterial complications before (I) and after treatment with Wobenzym (II)
Thus, the microecology of the respiratory tract both during the period of acute respiratory infection and convalescence is a single environment, represented by both pathogenic and opportunistic bacterial flora. Prescribing systemic enzyme therapy drugs to children with acute respiratory viral infections with secondary bacterial complications promotes faster relief of clinical symptoms of the disease, eradication of pathogens, and a reduction in hospital stay, which generally reduces the economic costs of treating the patient and confirms the feasibility of using this therapy.
Conclusions:
1. In FBD, the severity of the inflammatory reaction and intoxication during ARVI with secondary bacterial complications subjectively (according to clinical indicators) and objectively (according to LII and NII) corresponds to a moderate form of severity.
2. A new direction in molecular microbial diagnostics - the determination of mixed infections, dysbiosis and inflammatory processes by specific markers (fatty acids, aldehydes and sterols) using gas chromatography-mass spectrometry in ARVI patients with secondary bacterial complications in one experiment showed the presence of heterogeneous microorganisms (aerobes, anaerobes, actinobacteria, fungi, viruses) with predominant colonization by Streptococcus, Nocardia, Streptomyces and other opportunistic flora.
3. In patients with ARVI with secondary bacterial complications, Wobenzym therapy helps relieve fever, the manifestation of intoxication syndrome (malaise, decreased appetite, weakness, decreased physical activity), symptoms of rhinitis, tonsillitis, pharyngitis, otitis after the 6th day of taking the drug.
4. In those treated with Wobenzym, complete recovery at discharge was observed in 7 (41.2%) patients, improvement in 10 (58.8%), the clinical effect was excellent in 9 (53%) patients, in 8 (47%) - good.
6. During therapy with Wobenzym, a decrease in the colonization of Streptococcus (eradication - 23%), Nocardia (eradication - 19%), Nocardia asteroides (eradication - 29%) and an increase in the content of normal flora were noted, which confirms the advisability of prescribing systemic enzyme therapy drugs for patients with acute respiratory viral infections with ARVI secondary bacterial complications.
O.V. Kladova, Yu.I. Sternin, K.Yu. Bezrukov, K.P. Yablonskaya,
L.I. Feldfiks, T.P. Legkova, L.A. Rogova, V.F. Uchaikin
Russian State Medical University, Moscow
Morozov Children's City Clinical Hospital, Moscow
Medical Academy of Postgraduate Education, St. Petersburg
Olga Viktorovna Kladova - Doctor of Medical Sciences, Professor of the Department of Infectious Diseases in Children, Faculty of Pediatrics, Russian State Medical University
Literature:
1. Results of using the drug “Aflubin” in nonspecific prevention of acute respiratory diseases in frequently ill children / V.F. Uchaikin, O.V. Kladova, A.A. Shcherbakova, Z.A. Blistinova // Epidemiology and infectious diseases. - 2001. - No. 6. - P. 56-58.
2. Prevention of respiratory infections / T.P. Markova, D.G. Chuvirov // Russian Medical Journal. - 2004. - No. 1. - P. 5-7.
3. Modern methods of immunorehabilitation of frequently ill children with acute obstructive laryngitis // O.V. Kladova, V.L. Fomina, L.I. Feldfiks, V.F. Uchaikin // Pediatrics. - 2009. - No. 2. - P.72-74.
4. The use of Arbidol and Amiksin as agents for etiotropic therapy of influenza and other acute respiratory viral infections in children / V.F. Uchaikin, O.V. Kladova, F.S. Kharlamova, S.G. Cheshik, I.I. Balabolkin, T.P. Legkova, B.L. Mednikov // Pediatrics. - 2004. - No. 5. - P. 15-19.
5. Effective treatment of croup syndrome using the immunomodulator “Gepon” / F.S. Kharlamova, A.A. Shcherbakova, T.P. Legkova, O.V. Kladova, L.I. Feldfiks, V.F. Uchaikin // Russian Medical Journal. - 2002. - No. 3. - P. 138-141.
6. Persistence of respiratory viruses / E.V. Zamakhina, O.V. Kladova // Children's infections. - 2009. - No. 3. - P. 36-38.
7. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes / CT Garnett, D. Erdman, W. Xu, LR Gooding // J. Virol. - 2002. - V. 76. - P. 10608-10616.
8. The use of systemic enzyme therapy in the treatment of influenza and acute respiratory infections / V.A. Isakov, I.V. Kabolova, G.Yu. Knorring // Russian Medical Journal. - 2006. - T. 14. - No. 22. - P. 22-25.
9. Systemic enzyme therapy in children and adolescents. Method. letter / ed. acad. A.G. Rumyantseva. M., 2007. - 38 p.
10. Quantitative in situ microbiological analysis of lipid markers in biological fluids using gas chromatography - mass spectrometry / G.A. Osipov, N.F. Fedosova, K.V. Lyadov // Healthcare - medical technologies. - 2007. - No. 5. - P. 20-23.