Broad-spectrum antibiotics: TOP 20 best
The list of broad-spectrum antibiotics includes drugs from different groups. Drugs from a number of fluoroquinolones, nitroimidazoles, glycopeptides and from the phosphoric acid group have particularly high activity.
Below you can familiarize yourself with the characteristics of the best broad-spectrum antibiotics and methods of their use. Antibiotics are prescribed by the attending physician taking into account the sensitivity of the pathogen based on laboratory data. In some cases, urgent prescription of antibacterial agents is necessary, then the choice falls on broad-spectrum antibiotics.
With long-term treatment, bacterial resistance to antibiotics may develop; in this situation, it is necessary to change drugs to others or use a complex of several drugs. The duration of antibiotic therapy is determined by the severity of the disease, the presence of complications and concomitant pathologies. Doses of modern broad-spectrum antibiotics are selected by the attending physician individually, taking into account the patient’s age and possible contraindications.
How do new generation antibiotics work?
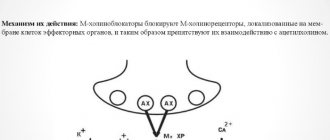
Unlike drugs from the antiseptic group, the antibiotic has a proper therapeutic effect not only after external application, but also acts systemically after oral, intravenous, and intramuscular use.
New generation antibiotics are capable of:
- Affect the synthesis of cell walls by disrupting the production of vital peptide complexes.
- Impair the functioning and integrity of the cell membrane.
- Disturb the synthesis of protein necessary for the growth and functioning of the pathogenic pathogen.
- Suppress nucleic acid synthesis.
Based on the nature of their effect on bacterial cells, antibiotics are divided into:
- Bactericidal - the pathogen will die and will then be eliminated from the body.
- Bacteriostatic - the active component does not kill bacteria, but disrupts their ability to reproduce.
It is important to determine how active the active substance of the drug is in relation to a particular pathogen of the pathological process. To do this, you need to undergo a series of laboratory tests prescribed by your doctor.
Antibiotics of the 21st century
In the first part of the article, we talked about the main myths associated with antibiotics and described in detail the penicillin group, and now let’s move on to the next generation of antibiotics.
CEPHALOSPORINS
Microbes often act, if not wisely, then quite logically.
If they are threatened by penicillin antibiotics, the microbes begin to destroy penicillins (if only we could do this - something is bothering us, but we do it! - and wipe them off the face of the earth).
The weak link of penicillins is the so-called beta-lactam ring (you will often see this term in the description of drugs, so it is better to remember it). It is this beta-lactam ring that microbes have learned to break. And the tool for breaking is special enzymes, beta-lactamases.
So, in short, antibiotics of the cephalosporin group are the same penicillins, which work in exactly the same way, but they are not afraid of beta-lactamases. This means that they can deal with microbes that the same ampicillin or even amoxicillin with clavulanic acid cannot cope with.
The “arms race” between microbes and pharmacists over time gave rise to the second, third, and then fourth generations of cephalosporin antibiotics (when pronouncing some of the names of these drugs, even doctors are scared, immediately imagining what flora these antibiotics are intended against).
WHEN DOCTORS PRESCRIBE CEPHALOSPORINS
If you are allergic to regular penicillins.
Of course, the chemical structure of penicillins and cephalosporins is similar, but the chance that the patient will not have an allergic reaction to cephalosporins is still very high; If penicillin antibiotics do not treat the infection. This often happens if a patient becomes infected with some kind of staphylococcus or streptococcus already in the hospital: the microbes there are already poisoned by anything, and therefore are especially resistant to tablets from a regular pharmacy.
Microbes, unfortunately, learn quickly and evolve quickly. This is understandable: they only need 20 minutes to change generations.
And yet, doctors always recommend starting treatment with antibiotics from the penicillin group, so as not to breed antibiotic-resistant flora.
ANTIBIOTICS OF THE MACROLIDE GROUP
Penicillin revolutionized the treatment of infections. But very soon it turned out that he was capable of killing too. Here and there, patients began to die from anaphylactic shock caused by penicillin (remember - there are allergic reactions to penicillin?).
What could the scientists do? Just develop new antibiotics.
Penicillin began to be widely used in 1943 (in the USA and USSR, and almost simultaneously). And already in 1949, Alberto Aguilar in the Philippines discovered a new (after the green mold from which penicillin was isolated) special fungus that suppresses the growth of bacteria.
In the USA, a year later, another scientist, McGuire, isolated a new antibiotic from it - erythromycin (for some reason Wikipedia persistently writes that Eli Lilly isolated it, but this is not so - McGuire simply worked for him).
Erythromycin still works: it turned out that bacteria can adapt to it much less well than to penicillin.
WHAT CAN MACROLIDES DO?
Firstly, bacteria actually adapt to macrolides much more slowly than to penicillins.
But they also adapt. That is why erythromycin has now grown into a huge family of “younger brothers” - semisynthetic macrolides and azalides, which some pharmacists classify into another separate group - the fourth and last group of antibiotics used in pediatrics.
Secondly, macrolides do not kill bacteria - they deprive them of the ability to reproduce, as a result of which bacterial cells, without causing harm to humans, very quickly die by natural causes or become victims of the immune system.
Thirdly, macrolides can penetrate inside cells and overtake the bacteria that love to settle there - chlamydia and mycoplasma.
Chlamydia and mycoplasma do not have a cell wall, due to which they are invulnerable to penicillins (penicillins kill only bacteria that have a cell wall. If there is no cell wall, the task is to strangle Kolobok - it seems that you need it by the neck, but it simply isn’t there). But for macrolides, the absence of a cell wall is not an obstacle: chlamydia and mycoplasmas die upon contact with macrolides, although not so quickly.
WHEN DO DOCTORS PRESCRIBE MACROLIDES?
- the patient is allergic to penicillins;
Rules for prescribing antibiotics
In order for antibiotics to be as effective as possible and not give serious side effects, when selecting and prescribing them, it is necessary to take into account the form of the disease and its severity, as well as determine the cause of the pathology (ideally, using culture results, find out which microbe caused the inflammation).
In addition, it is important to determine the sensitivity of bacteria to certain antibiotics that are planned to be used. Naturally, this is difficult to do in pediatric practice, and there are conditions in which a delay of several days, which is spent on performing cultures and determining sensitivity to antibiotics, can become fatal.
These include acute otitis media, tonsillitis or pneumonia, pyelonephritis and some other conditions. In these cases, antimicrobial therapy is prescribed immediately, based on clinical recommendations and treatment protocols that have been developed over years of treatment. If necessary, therapy is adjusted based on culture results already during treatment, if it is ineffective.
Antibiotics: from myth to drugs of the 21st century
There are many myths surrounding antibiotics.
To be ready for further conversation, you and I need to immediately deal with the most important things. There are only three of them.
ANTIBIOTICS CAUSE DYSBACTERIOSIS
Not really. It is extremely difficult to achieve intestinal decontamination, that is, a condition in which antibiotics kill ALL flora in the intestines and anyone can live there.
To do this, antibiotics will have to be taken for months and in fairly large dosages.
In cases where a child falls ill with an acute respiratory infection, such regimens are never prescribed by doctors.
ANTIBIOTICS SUPPRESS THE IMMUNE SYSTEM
Not really.
The immune system is formed according to the principle of clonal selection: in response to each contact with an infection, a special clone of cells is created in the immune system - “specialists” for this particular infection. Doctors call them memory cells.
When the same infection is re-infected, memory cells begin to multiply instantly and suppress this infection quickly - often faster than it can manifest itself.
By the way, this is how vaccinations work—they create clones of memory cells in response to the introduction of killed, dismembered, or even fake infections.
To create memory cells, contact with an infectious agent is sufficient; a full-blown disease is not required at all.
WITH THE FREQUENT USE OF ANTIBIOTICS, A FLORA IS FORMED THAT CANNOT BE KILLED BY ANYTHING
But this is partly true. Indeed, some infectious agents, such as pneumococcus or Staphylococcus aureus, as a result of prolonged exposure to antibiotics, become resistant to almost any antibacterial drug known to mankind.
Such pathogens can be found in surgical hospitals - especially those that have been operating for decades, and doctors, faced with an infection that is not immediately treatable, always ask the patient if he has been treated in such a hospital for a long time.
Why are they asking this question? Yes, because there is simply nowhere else to get such a microorganism that is resistant to everything. It is physically impossible to grow something like this at home, gradually poisoning some infection with pharmaceutical antibiotics. Life is not enough.
Diseases and spectrum of action of antibiotics
Back in the late 19th century, bacteriologist Hans Gram discovered that different bacteria react differently to staining. Some acquire a pronounced color, while others, on the contrary, quickly discolor. This simple experience was of great importance from a practical point of view. After all, different reactions to the dye spoke about the properties of the bacterial cell wall. This means that it suggested exactly how antibiotics should act on the microorganism.
Since then, there has been a basic division into gram-negative (non-staining) and gram-positive (staining) bacteria.
- Gram (+) is the causative agent of most infections of the respiratory tract, nasopharynx, ear, and eye. These include, in particular, staphylococci and streptococci.
- Gram (-) - Bacteria in this group can cause serious illness. These are Escherichia coli, Koch bacillus, salmonella, shigella (the causative agent of diphtheria), gonococcus, meningococcus.
The spectrum of action of antibiotics is determined by which bacteria are sensitive to a particular drug. And if narrow-spectrum antibiotics more often act on either Gram (+) or Gram (-), then a wide spectrum allows them to affect both.
Main groups of antibiotics
Antibiotics are a group of natural or semi-synthetic organic substances that can destroy microbes or suppress their reproduction. At the moment, many different types of antibiotics are known, endowed with different properties. Knowledge of these properties is the basis for proper antibiotic treatment. The individual qualities and effects of an antibiotic mainly depend on its chemical structure. In this article we will talk about the most well-known groups of antibiotics, show the mechanism of their work, their spectrum of action, and the possibility of using them to treat various infections.
Groups of antibiotics Antibiotics are substances of natural or semi-synthetic origin. Antibiotics are obtained by extracting them from colonies of fungi, bacteria, plant or animal tissues. In some cases, the original molecule is subjected to additional chemical modifications in order to improve certain properties of the antibiotic (semi-synthetic antibiotics).
At the moment, there are a huge number of different antibiotics. True, only a few of them are used in medicine; others, due to increased toxicity, cannot be used to treat infectious diseases in humans. The extreme diversity of antibiotics led to the creation of a classification and division of antibiotics into groups. At the same time, antibiotics with a similar chemical structure (derived from the same raw material molecule) and action are collected within the group.
Below we will look at the main groups of antibiotics known today: Beta-lactam antibiotics The group of beta-lactam antibiotics includes two large subgroups of the most famous antibiotics: penicillins and cephalosporins, which have a similar chemical structure.
Penicillin group . Penicillins are obtained from colonies of the mold Penicillium, hence the name of this group of antibiotics. The main effect of penicillins is associated with their ability to inhibit the formation of the cell wall of bacteria and thereby suppress their growth and reproduction. During the period of active reproduction, many types of bacteria are very sensitive to penicillin and therefore the effect of penicillins is bactericidal.
An important and useful property of penicillins is their ability to penetrate into the cells of our body. This property of penicillins makes it possible to treat infectious diseases, the causative agent of which is “hidden” inside the cells of our body (for example, gonorrhea). Antibiotics from the penicillin group have increased selectivity and therefore have virtually no effect on the body of the person receiving treatment.
The disadvantages of penicillins include their rapid elimination from the body and the development of bacterial resistance to this class of antibiotics.
Biosynthetic penicillins are obtained directly from mold colonies. The most well-known biosynthetic penicillins are benzylpenicillin and phenoxymethylpenicillin. These antibiotics are used to treat tonsillitis, scarlet fever, pneumonia, wound infections, gonorrhea, and syphilis.
Semi-synthetic penicillins are obtained on the basis of biosynthetic penicillins by adding various chemical groups. At the moment, there are a large number of semi-synthetic penicillins: amoxicillin, ampicillin, carbenicillin, azlocillin.
An important advantage of some antibiotics from the group of semisynthetic penicillins is their activity against penicillin-resistant bacteria (bacteria that destroy biosynthetic penicillins). Thanks to this, semisynthetic penicillins have a wider spectrum of action and therefore can be used in the treatment of a wide variety of bacterial infections.
The main adverse reactions associated with the use of penicillins are allergic in nature and are sometimes the reason for refusing to use these drugs.
Group of cephalosporins . Cephalosporins also belong to the group of beta-lactam antibiotics and have a structure similar to that of penicillins. For this reason, some of the side effects of the two groups of antibiotics are the same (allergy).
Cephalosporins are highly active against a wide range of different microbes and are therefore used in the treatment of many infectious diseases. An important advantage of antibiotics from the cephalosporin group is their activity against microbes resistant to penicillin (penicillin-resistant bacteria).
There are several generations of cephalosporins: I generation cephalosporins (Cefalothin, Cephalexin, Cefazolin) are active against a large number of bacteria and are used to treat various infections of the respiratory tract, urinary system, and to prevent postoperative complications. Antibiotics of this group are usually well tolerated and do not cause serious adverse reactions.
II generation cephalosporins (Cefomandol, Cefuroxime) are highly active against bacteria inhabiting the gastrointestinal tract, and therefore can be used to treat various intestinal infections. These antibiotics are also used to treat respiratory and biliary tract infections. The main adverse reactions are associated with allergies and disorders of the gastrointestinal tract.
III generation cephalosporins (Cefoperazone, Cefotaxime, Ceftriaxone) are new drugs with high activity against a wide range of bacteria. The advantage of these drugs is their activity against bacteria insensitive to the action of other cephalosporins or penicillins and the ability to remain in the body for a long time. These antibiotics are used to treat severe infections that cannot be treated with other antibiotics. Side effects of this group of antibiotics are associated with disruption of the intestinal microflora or the occurrence of allergic reactions.
Antibiotics from the macrolide group Macrolides are a group of antibiotics with a complex cyclic structure. The most well-known representatives of antibiotics from the macrolide group are Erythromycin, Azithromycin, Roxithromycin.
The effect of macrolide antibiotics on bacteria is bacteriostatic - antibiotics block bacterial structures that synthesize proteins, as a result of which microbes lose the ability to reproduce and grow.
Macrolides are active against many bacteria, but the most remarkable property of macrolides, perhaps, is their ability to penetrate into the cells of our body and destroy microbes that do not have a cell wall. These microbes include chlamydia and rickettsia - the causative agents of atypical pneumonia, urogenital chlamydia and other diseases that cannot be treated with other antibiotics.
Another important feature of macrolides is their relative safety and the possibility of long-term treatment, although modern treatment programs using macrolides provide ultra-short courses lasting three days.
The main areas of use of macrolides are the treatment of infections caused by intracellular parasites, the treatment of patients with allergies to penicillins and cephalosporins, the treatment of young children, pregnant women and nursing mothers.
Antibiotics from the tetracycline group The most well-known antibiotics from the tetracycline group are Tetracycline, Doxycycline, Oxytetracycline, Metacycline. The action of antibiotics from the tetracycline group is bacteriostatic. Just like macrolides, tetracyclines are capable of blocking protein synthesis in bacterial cells, however, unlike macrolides, tetracyclines have less selectivity and therefore, in large doses or with long-term treatment, can inhibit protein synthesis in the cells of the human body. At the same time, tetracyclines remain indispensable “helpers” in the treatment of many infections. The main areas of use of antibiotics from the tetracycline group are the treatment of respiratory and urinary tract infections, the treatment of severe infections such as anthrax, tularemia, brucellosis, etc.
Despite their relative safety, long-term use of tetracyclines can cause severe side effects: hepatitis, damage to the skeleton and teeth (tetracyclines are contraindicated in children under 14 years of age), developmental defects (contraindicated for use during pregnancy), allergies.
Ointments containing tetracycline are widely used. Used for local treatment of bacterial infections of the skin and mucous membranes.
Antibiotics from the aminoglycoside group Aminoglycosides are a group of antibiotics, which includes drugs such as Gentamicin, Monomycin, Streptomycin, Neomycin. The spectrum of action of aminoglycosides is extremely wide and even includes tuberculosis pathogens (Streptomycin).
Aminoglycosides are used to treat severe infectious processes associated with massive spread of infection: sepsis (blood poisoning), peritonitis. Aminoglycosides are also used for local treatment of wounds and burns.
The main disadvantage of aminoglycosides is their high toxicity. Antibiotics from this group have nephrotoxicity (kidney damage), hepatotoxicity (liver damage), ototoxicity (can cause deafness). For this reason, aminoglycosides should be used only for life-saving indications, when they are the only treatment option and cannot be replaced by other drugs.
Levomycetin Levomycetin (Chloramphenicol) inhibits the synthesis of bacterial proteins, and in large doses causes a bactericidal effect. Levomycetin has a wide spectrum of action, but its use is limited due to the risk of serious complications. The greatest danger associated with the use of the antibiotic Chloramphenicol is damage to the bone marrow, which produces blood cells.
Antifungal antibiotics Antifungal antibiotics are a group of chemicals that can destroy the cell membrane of microscopic fungi, causing their death.
The most famous representatives of this group are the antibiotics Nystatin, Natamycin, Levorin. The use of these drugs in our time is noticeably limited due to low efficiency and a high incidence of side effects. Antifungal antibiotics are gradually being replaced by highly effective synthetic antifungal drugs.
Bibliography:
- I.M. Abdullin Antibiotics in clinical practice, Salamat, 1997
- Katsunga B.G. Basic and clinical pharmacology, Binom; St. Petersburg: Nev. Dialect, 2000.
Source
Broad-spectrum antibiotics: list of drugs
Broad-spectrum antibiotics are universal bactericidal drugs that will help get rid of many diseases. Most often they are prescribed for the treatment of various infections, the causative agent of which remains unknown. They are also prescribed if a person has become infected with a fast-growing and dangerous virus.
The list of modern antibiotics is presented in the table below:
| Group | A drug | Mechanism of action |
| Tetracyclines | Doxycycline, Tetracycline | Kills bacteria and has an antiviral effect |
| Levomycetin | Moxifloxacin, Levofloxacin | Antimicrobial, antifungal and antibactericidal |
| Semi-synthetic penicillins | Carbenicillin, Ticarcillin | Inhibits the synthesis of the pathogen cell wall |
| Cephalosporins | Ceftriaxone | Changes the activity of a virus that has entered the RNA |
| Rifampicins | Streptomycin, Amphenicol | Interferes with protein production |
| Carbapenems | Meropenem, Meropenem, Cyronem, Imipenem | Antibacterial and anti-inflammatory, prolonged action |
Also, such drugs may be indicated as prophylaxis after major surgical interventions. Remember that not all cheap drugs are so bad.
Cephalosporins
Active against staphylococcal infections, as well as Proteus, Klebsiella, E. coli, pathogens of sore throat and pneumonia, urinary tract diseases, osteomyelitis, meningitis.
Antibiotics in this group include:
Parenteral 3rd generation:
| Representatives | Trade name and method of application |
| Cefotaxime | Klaforan : powder for injection solution: 0.5-2.0 g. x 1 time per day intramuscularly or intravenously slowly. Cefosin : powder for injection solution: 1.0 g each. every 8-12 hours intramuscularly, intravenously slowly/drip. |
| Cefoperazone | Cephobid : powder for injection solution: 2.0-4.0 g. per day for 2 intramuscular injections. Cefpar : powder for injection solution: 2.0-4.0 g each. every 12 hours intravenously/intramuscularly. |
| Ceftriaxone | Ceftriaxone : powder for injection solution: 1.0-2.0 g. x 1 time per day intramuscularly/intravenously. Azaran : powder for injection solution: 1.0 g. dissolve in 3.5 ml of 1% solution of lidocaine hydrochloride, intramuscular injections 1 time per day. |
| Ceftazidime | Fortum : powder for injection solution: 1.0-6.0 g each. x 1 time per day for 2-3 intravenous/intramuscular infusions. Ceftidine : powder for injection solution: 1.0-6.0 g. x 1 time per day intravenously/intramuscularly. |
Parenteral 3rd generation:
| Representatives | Trade name and method of application |
| Cefditoren | Spectracef : tablets: 0.2-0.4 g. x 2 times a day. |
| Ceftibuten | Tsedex : capsules: 0.4 g each. once a day. |
| Cefixime | Suprax Solutab: effervescent tablets: 0.4 g each. x 1 time per day or 0.2 g. x 2 times a day, dissolve in advance in a glass of water. Suprax: capsules: 0.4 g each. x 1 time per day. Pancef : tablets: 0.4 g each. once a day or 0.2 g. twice a day. |
5th generation (parenteral):
| Representatives | Trade name and method of application |
| Ceftaroline | Zinforo : powder for injection solution: 0.6 g. every 12 hours intravenously for an hour. |
| Ceftobiprole | Zeftera : lyophilisate for injection solution: not used in the Russian Federation. |
Macrolides
Macrolides tend to accumulate in tissues, and not in blood serum, like other groups. They are used in the treatment of bronchitis and community-acquired pneumonia as a monomedicine (in case of intolerance to penicillins), pathologies of the ENT organs (pharyngitis, sinusitis, otitis, laryngitis and others) and sexually transmitted diseases (syphilis, gonorrhea, benorrhea).
Antibiotics in this group include:
14-member:
| Representatives | Trade name and method of application |
| Erythromycin | Erythromycin:
|
| Oleandomycin | Oleandomycin phosphate : powder substance. Practically not used at present. |
| Roxithromycin | RoxyHEXAL: tablets: 0.15 g each. twice a day or 0.3 g. at one time, the course is taken for 10 days. Esparoxy: tablets: 0.15 g each. twice a day 15 minutes before meals or 0.3 g. once, the course of administration is 10 days. Rulid : tablets: 0.15 g each. twice a day, course of treatment is 10 days. |
| Clarithromycin | Klacid: tablets: 0.5 g. twice a day, taken for 2 weeks. Fromilid: tablets: 0.5 g twice a day, taken for 2 weeks. Clarithrosin : tablets: 0.25 g. twice a day, taken for 2 weeks. |
15-member:
| Representatives | Trade name and method of application |
| Azithromycin | Sumamed: capsules: 0.5 g each. x 1 time per day before or 2 hours after meals. Azitrox: capsules: 0.25-0.5 g each. x 1 time per day. Azitral : capsules: 0.25-0.5 g. x 1 time per day before or after meals. |
16-member:
| Representatives | Trade name and method of application |
| Spiramycin | Spiramycin-vero : tablets: 2-3 tablets (3 million IU) in 2-3 doses per day. Rovamycin : tablets: 2-3 tablets (3 million IU) or 5-6 tablets (6-9 million IU) for 2-3 doses per day. |
| Josamycin | Vilprafen : tablets: 0.5 g. twice a day, without chewing, with water. Vilprafen solutab : tablets: 0.5 g each. x twice a day, without chewing or dissolved in 20 ml of water. |
| Midecamycin | Macropen : tablets: 0.4 g each. three times a day, course of administration for 2 weeks. |
Systemic antibiotics in the treatment of bacterial infections of the skin and soft tissues: focus on macrolides
Bacterial infections of the skin, causing purulent inflammation, were identified as a group of infectious dermatoses by the French scientist H. Leloir in 1891 under the name pyodermatitis (pyon - pus, derma - skin). Abroad, pyoderma is usually classified as a broad group of skin and soft tissue infections (SSTI), which includes, in addition to infections of the skin and its adnexal structures, infections of the subcutaneous fatty tissue and underlying tissues. In economically developed countries, SSTIs account for 1/3 of all infectious diseases. According to domestic studies, pustular skin infections account for 30–40% of all dermatological pathology in people of working age; in military personnel this figure reaches 60%. In pediatric dermatological practice, this pathology is one of the most common and accounts for 30 to 50% of all cases of visits to the doctor [1–3]. Etiology The main source of SSTIs are microorganisms that contaminate and colonize the surface of the skin. Gram-positive cocci S. aureus and S. Pyogenes, capable of penetrating into the thickness of the epidermis in the presence of its damage, undoubtedly play a leading role in the etiology of pustular skin infections. Moreover, S. aureus is the most common pathogen; infections caused by S. pyogenes, as well as a mixed infection involving both microorganisms, are somewhat less common. According to the results of foreign multicenter studies, in addition to S. aureus, S. pyogenes, Corynebacterium diphtheriae, P. aeruginosa, Enterobacteriaceae, Streptococcus spp. may be involved in the development of SSTI. The type of infection is of great importance in determining the etiological role of the suspected pathogen (Table 1). Unlike primary pyodermas, secondary ones, like most necrotizing SSTI infections, have a polymicrobial etiology. The virulence of the microorganism and the degree of bacterial contamination play an important role in the development of infection. It has been shown that the probability of developing an infection is directly proportional to the degree of bacterial contamination and virulence of the microorganism and inversely proportional to the strength of the body's protective reaction. The likelihood of colonization increases in the presence of skin diseases of allergic origin. Thus, in patients with atopic dermatitis, colonization of the affected areas with S. aureus is detected in 90% of cases [3]. Pathogenesis In the occurrence of one or another form of pyoderma, an important role is played by: the type of pathogen, its virulence, the state of the macroorganism, as well as various endogenous and exogenous predisposing factors that reduce the barrier and protective functions of the skin. The virulence of staphylococci and streptococci is determined by a number of pathogenic toxins and enzymes they secrete (coagulase, leukocidin, streptokinase, hyaluronidase streptolysin, hemolysins, etc.), which facilitate the penetration of pathogens into the skin, lead to damage and stratification of all layers of the epidermis, cause hemolysis and necrotization of the dermis and underlying tissues, disrupting their normal metabolism [4,5]. In the occurrence and development of SSTIs, the reactivity of the body and its mechanisms of resistance to microbial aggression are of great importance. The insufficiency of the immunocompetent system in this case is, as a rule, of a secondary (acquired) nature. It can form in the premorbid period as a result of previous or concomitant severe diseases. Diseases of the endocrine system (obesity, diabetes, insufficient activity of the pituitary-adrenal system, thyroid, gonads) contribute to a decrease in the body's anti-infective defense mechanisms. More than half of patients (52%) with chronic pyoderma abuse carbohydrates (usually easily digestible), which creates a constant overload of the insular apparatus of the pancreas and can contribute to carbohydrate metabolism disorders of varying degrees, the accumulation of carbohydrates in tissues, which are a favorable breeding ground for pyococci. A significant role is also assigned to the seborrheic skin condition. Due to an increase in the amount of sebum and changes in its chemical composition, a decrease in the sterilization properties of the skin and activation of pyogenic cocci occurs [6]. Of no small importance in the development of pustular skin diseases are chronic infectious diseases of various organs and tissues: periodontal disease, caries, gingivitis, tonsillitis, pharyngitis, infections of the urogenital tract, dysbacteriosis, intestinal intoxications, which reduce the general and local antibacterial resistance of the body and contribute to the development of subsequent specific sensitization in patients , which aggravates the course of the infectious process. A significant role in the development of chronic pyoderma is played by diseases of the central and autonomic nervous system, mental or physical overstrain, “debilitating diseases” - alcoholism, fasting, malnutrition (lack of proteins, vitamins, mineral salts, hypovitaminosis, especially A and C. Vitamin A is involved in In the process of keratin formation, vitamin C regulates the permeability of the vascular wall and is a synergist of corticosteroids). A major role in the development of pyoderma is played by various immunodeficiency conditions that arise as a result of congenital or acquired immunodeficiency (HIV infection, use of glucocorticosteroids, cytostatics and immunosuppressants). Defects in cellular antibacterial defense in the form of inhibition of the phagocytic activity of neutrophils, impaired chemotaxis, as well as a decrease in opsonic factors of blood serum and immunoglobulins contribute to chronic infection and frequent relapses [7]. Violations of the T-cell immune system are of major importance in the pathogenesis of SSTIs. The basis for disorders of specific mechanisms of immunological reactivity is a decrease in the number of T-lymphocytes in the peripheral blood, a decrease in the number of CD3 and CD4 cells and a change in their relationship with monocytes, which leads to a weakening of the T-cell immune response. Insufficiency of the patient’s immune system (immunological imbalance) and antigenic mimicry of the pathogen often lead to chronic infection and the formation of bacterial carriage, and irrational use of antibiotics leads to pathogen resistance [8]. Unfavorable environmental influences that violate the integrity of the skin and create an “entry gate” for infection are of significant importance in the development of bacterial skin infections. These primarily include the influence of high or low temperature, high humidity, leading to maceration of the skin, increased pollution and microtraumatization by occupational factors (oils, cement, coal dust). The entry point for infection occurs due to household microtraumas (cuts, injections), scratching and itchy dermatoses. Violation of the skin barrier in the form of dryness and thinning of the stratum corneum contributes to the penetration of microorganisms into the deep layers of the skin and underlying tissues, which leads to the development of the pyodermic process. Clinical types of SSTIs SSTIs are a fairly numerous and clinically heterogeneous group of diseases that lead to lesions of varying depth, prevalence and severity. A common symptom characteristic of all is the presence of local purulent inflammation, which in severe cases is accompanied by the development of a systemic inflammatory reaction. Clinical forms depend on the type of etiological factor, anatomical localization, association with skin appendages, depth and area of the lesion, and duration of the process. In domestic dermatology, the classification of primary pyoderma was adopted, proposed by J. Jadasson back in 1934 and built on an etiological principle. It includes: staphyloderma, mainly affecting the skin around the appendages (sebaceous follicles, sweat glands); streptoderma, affecting smooth skin mainly around natural openings and mixed strepto-staphylococcal infections. In each of the three groups, depending on the depth of the lesion, superficial and deep forms are distinguished. In addition, pustular skin diseases are divided into primary, occurring on unchanged skin, and secondary, developing as complications against the background of an existing dermatosis, usually itchy (scabies, eczema, atopic dermatitis). According to the duration of the course, acute and chronic pyoderma are distinguished. Staphylococcal pyoderma is usually associated with skin appendages (hair follicles, apocrine glands). They are characterized by the formation of a deep pustule, in the center of which a cavity is formed, filled with purulent exudate. Along the periphery there is a zone of erythematous-edematous inflammatory skin. The suppurative process ends with the formation of a scar (Fig. 1). Streptococcal pyoderma most often develops on smooth skin, around natural openings (oral cavity, nose) and begins with the formation of phlyctena - a superficially located bubble with a flabby folded tire, inside which contains serous-purulent contents. The thin walls of the phlyctena quickly open, and the contents pour out onto the surface of the skin, drying out into honey-yellow layered crusts. The process tends to spread along the periphery as a result of autoinuculation (Fig. 2). Staphyloderma more often affects men, streptoderma – women and children [3,4]. In foreign literature, from a practical point of view, all SSTIs are divided into three main groups: primary pyoderma, overwhelmingly caused by S. aureus and pyogenic b-hemolytic streptococci (mainly group A), and developing on unchanged skin (folliculitis, impetigo, erysipelas) ; secondary pyoderma developing against the background of skin damage or concomitant somatic pathology (for example, bedsores, diabetic foot ulcers, infections after animal bites, postoperative wound and post-traumatic infections), as well as against the background of dermatoses accompanied by itching and scratching (allergic dermatitis, psoriasis, scabies and etc.); necrotizing infections, representing the most severe form of SSTI (cellulitis of polymicrobial etiology - synergistic cellulitis, necrotizing fasciitis, myonecrosis - gas gangrene) (Fig. 3). With this pathology, determining the depth and extent of the lesion is the priority of the surgeon, because Only with surgical treatment can the true extent of the infection be most accurately determined. The initial management of these patients is the same. It consists of early surgical intervention and the appointment of adequate antimicrobial therapy [9]. Treatment of SSTIs Treatment of patients with bacterial skin infections should be comprehensive (etiotropic and pathogenetic) and carried out after a thorough anamnestic, clinical and laboratory examination of the patient. It is necessary to identify and treat concomitant diseases, examine for foci of focal infection, and in the case of a long-term persistent process, study the immunostatus. The main and only method of etiotropic treatment of patients with SSTIs are antibiotics. In acute superficial non-common processes (impetigo, folliculitis, paronychia), therapy may be limited to the local use of antibiotics and antiseptics. In all other cases, systemic antibiotic therapy is required. Indications for systemic antibiotic therapy are deep forms of pyoderma: boils (especially localized on the face and neck), carbuncle, hidradenitis, erysipelas, cellulite. The listed forms of bacterial skin infections have a long, often chronic, recurrent course, a high prevalence of the process and are often accompanied by symptoms of general intoxication in the form of fever, headache, weakness, as well as the development of regional complications (lymphadenitis, lymphangitis). Antibiotics are used as an etiotropic agent in the treatment of bacterial dermatosis – Lyme disease. They are the drugs of choice for the treatment of acne vulgaris. In dermatovenerological practice, antibiotics are widely used both for the treatment of infectious dermatoses and diseases caused by sexually transmitted infections (STIs) [4]. Before prescribing an antibacterial drug, it is advisable to culture the pus to determine the sensitivity of the isolated microorganism to various antibiotics and, based on the results of the study, prescribe the appropriate drug. However, this is not always feasible, especially if complications of infection threaten or develop. As an analysis of modern literature and our own clinical experience shows, today the following groups of antibiotics are most often used in the treatment of bacterial skin infections: 1. β-lactams: a) natural penicillin, its durant forms and semi-synthetic penicillins; b) cephalosporins (1st–4th generation). 2. Macrolides. 3. Tetracyclines. 4. Fluoroquinolones. In recent years, penicillin and its durant drugs have rarely been used in the treatment of SSTIs, since the overwhelming number of pyococcal strains have acquired the ability to produce the enzyme b-lactamase (penicillinase), which suppresses the antibacterial activity of penicillin. In addition, β-lactams are drugs that have a high incidence of allergic reactions. Tetracyclines and aminoglycosides are currently used much less frequently. This is due to the large number of strains of microorganisms resistant to these antibiotics (which implies their low therapeutic activity), as well as the presence of severe side effects. It should be remembered that tetracyclines are contraindicated in pregnancy, children and patients with liver failure. Fluoroquinolones are prescribed mainly for the treatment of sexually transmitted diseases, due to the high sensitivity of urogenital infections to them, and for pyoderma they are used only when other groups of antibiotics are ineffective. However, in diseases of the central nervous system, in pregnant women, as well as in pediatrics, the range of their use is limited - they are prescribed mainly for health reasons. It is also necessary not to forget about the photosensitizing effect of fluoroquinolones and the associated precautions, especially in spring and summer [10]. Modern medical practice imposes certain requirements on the choice of antibiotic. First of all, the drug must have a wide spectrum of antimicrobial action and minimally expressed antibiotic resistance to microbial agents, have no severe side effects, have a minimal risk of allergic reactions, be convenient to use for the patient (availability of an oral form, a convenient dosage regimen) and affordable. In addition, it is very important that the antibiotic does not have clinically significant interactions with other drugs. Today, antibiotics – macrolides – fully meet these requirements. Classification and mechanisms of pharmacotherapeutic action of macrolides Macrolides have been widely used in clinical practice for more than 50 years. The first natural antibiotic of this group, erythromycin (a metabolite of Streptomyces erythreus), was obtained back in 1952. Macrolides can be classified by chemical structure and origin. The basis of the chemical structure of this class of antibiotics is the macrocyclic lactone ring. Depending on the number of carbon atoms in the ring, macrolides are divided into 14-, 15- and 16-membered (Table 2). Among macrolides, there are 3 generations: a) first generation: erythromycin, oleandomycin; b) second generation: spiramycin, roxithromycin, josamycin, clarithromycin, etc.; c) third generation: azithromycin (Azitral). The antibacterial effect of macrolides is based on disruption of the synthesis of ribosomal proteins of the microbial cell and thereby inhibiting the process of pathogen reproduction. They mainly have a bacteriostatic effect, which makes it advisable to prescribe them in the acute phase of inflammation. Macrolides belong to “tissue antibiotics”, i.e. when distributed in the body, they accumulate predominantly not in the bloodstream, but in those organs and tissues where there is inflammation, thereby creating high concentrations of the drug. Well distributed in the body, macrolides are able to overcome histohematological barriers (with the exception of the blood-brain barrier), significantly superior to β-lactam antibiotics. However, widespread (and often unjustified) use quickly led to the emergence of a high percentage of erythromycin-resistant strains of pathogens, especially staphylococci. This, in turn, has significantly reduced the use of erythromycin in clinical practice [11]. Interest in macrolides arose again in the early 80s of the 20th century, after the emergence of new generations of antibiotics of this group - azalides (in particular, azithromycin). Azithromycin was synthesized in 1983 from erythromycin. The drug in its pharmacokinetic properties surpassed all the indicators of its predecessor and became the first representative of the new group of antibiotics - azalids. The uniqueness of azithromycin is based on its exceptional pharmacokinetics. Azithromycin is stable in an acidic environment, due to which it is well absorbed after oral administration. Simultaneous intake with food reduces the absorption by 50%, so the drug is taken 1 hour before or 2 hours after eating. The lipophilicity of the azithromycin molecule provides, in addition to a high level of absorption in the intestines, also an excellent penetration of the drug into the tissue. The rapid penetration of azithromycin from the blood in the tissue is also ensured by a low level of binding of azithromycin with blood proteins, which makes it possible to achieve a rapid therapeutic effect in infections that affect the cells and tissue. A high concentration of the drug in the area of lesion, 10-100 times higher than the concentration in the bloodstream, allows you to actively affect the pathogenic focus, thereby providing a quick clinical effect and an early recovery. Современные макролиды (в частности, азитромицин) проявляют наибольщую эффективность в отношении таких возбудителей, как S. pyogenus, S. aureus, S. pneumoniae, некоторых грамотрицательных микроорганизмов (гонококи), а также внутриклеточных возбудителей (в частности, Chlamidia trachomatis и Ureaplasma urealyticum) What causes their high demand in dermatovenerological practice [12]. The second -generation macrolides are important for antibacterial activity of the second -generation macrolides. Due to their ability to penetrate neutrophils and create high concentrations in them, many macrolides positively modify the functions of these cells, influencing, in particular, chemotaxis, the activity of phagocytosis and killing. Along with the antimicrobial effect, these antibiotics have moderate anti -inflammatory activity. Activating the cells of the macrophage row, they are able to penetrate them and during the migration of phagocytic cells into the focus of inflammation to go there with them. The uniqueness of these drugs also lies in the fact that they have a pronounced plain effect, that is, they retain high concentrations in the focus of inflammation for 5-7 days after the abolition. This sanogenetic effect made it possible to develop short treatment courses not exceeding 3-5 days, and a convenient dosage regimen (1 time per day). This, in turn, ensures the compliance of treatment and improves the quality of life of the patient. The most pronounced postbiotic effect in azithromycin is, which allows you to create an antibiotic concentration in the foci of infection, which is many times higher than the IPC in relation to active pathogens in the treatment of both acute and chronic infections. Recently, evidence of the immunomodulating action of azithromycin in an experiment on healthy volunteers has been obtained. The first phase of the immunomodulating effect is to degenerate neutrophils and oxidant explosion, which contributed to the activation of protective mechanisms. Upon reaching the eradication of pathogens, it was noted to reduce IL -8 products and the stimulation of neutrophil apoptosis, which minimized the severity of the inflammatory reaction [13]. Macrolides, both natural and semi -synthetic, compared to other antibiotics have a minimal effect on the normal microflora of the human body and do not cause dysbiosis. Therefore, azithromycin is considered not only as a highly effective, but also the safest antibiotic with a minimum number of contraindications to the appointment. Unwanted reactions when taking it as a whole are extremely rare and do not exceed 5%. The most common side effects are symptoms from the gastrointestinal tract (nausea, severity in the epigastric region), which are usually expressed moderately, do not require the cancellation of the drug and quickly pass when taking drugs after eating [11]. The clinical efficiency of azithromycin as comparative studies indicate, with IKMT among antibiotics used in outpatient practice, the most effective macrolides of the new generation, primarily 15- and 16 -member (azithromycin, josamycin, roxyromycin). The 20 -year positive experience in the use of azithromycin in domestic dermatovenerological practice has already been accumulated. In dermatology, it is the basic therapy of staphylococcal and streptococcus lesions of the skin and soft tissues (boil, impetigo, cellulite), and in venereological practice - in the treatment of SPPPs. Unlike most macrolides, azithromycin does not have clinically significant interactions with other drugs. It is not associated with the enzymes of the Cytochrome R450 complex, as a result of which it does not show a reaction of drug interaction with drugs metabolizing along this path. This property is important, since in real clinical practice, most patients who occur IKMT have background or related diseases, about which they receive appropriate treatment. It must also be emphasized that, along with good tolerance and lack of pronounced adverse reactions of macrolides (azithromycin), have another unconditional advantage compared to other groups of antibiotics - this is that it can be prescribed for pregnant women and children [14]. Currently, one of the most commonly used drugs in clinical practice is the Azitral (azithromycin) drug, produced by pharmaceutical. Azitral (azithromycin) is similar to the original azithromycin - the first representative of the Azalids subgroup from the group of macrolide antibiotics used in the treatment of IKMT and urogenital infections. Studies have shown that the clinical effectiveness of the drug prescribed in a single dose of 500 mg for 3 days is comparable to the effectiveness of most widely used antibacterial agents. This allows you to reduce the usual course of antibiotic therapy by 2-3 times, and the unique pharmacokinetic profile of Azitral provides one -time daily intake and high compliance of therapy [15]. Due to the features of pharmacokinetics and a kind of antimicrobial spectrum covering the main pathogens of the genitourinary tract infections, azithromycin is the first choice in the therapy of combined IPPPs, including chronic complicated urogenital chlamydia and in non -understanding women, and an alternative tool for the treatment of this disease during the period of pregnancy. With a single use of 1 g of azithromycin (azitral), its concentration in the tissue of the prostate and uterus exceeds the IPC for C. trachomatis (0.125 μg/ml) by 42.5 times, and in the cervical canal - 12 times, which is the therapeutic concentration for the treatment of this infection. Moreover, even after 2 weeks, the therapeutic concentration of azithromycin in the prostate tissue exceeds the MPC for C. trachomatis by 13.6 times. The authors proved that it was with such a technique in tissues where C. trachomatis is vegetated that a high therapeutic concentration of the drug is supported during 6–8 development cycles. The data obtained indicate the high efficiency of pulse - therapy with Azitral (1 g 1 time per week, a course dose of 3 g). In the complex treatment of chronic chlamydial urethropostatitis and mycouraplasmic and wardenelle infection associated with it. It is important to note that the drug Azitral is well tolerated by patients, is available in price and therefore can be widely used in therapy of complicated urogenital chlamydia and VZ [16,17]. The study of the effectiveness, safety and tolerance of azithromycin in 30 children from 6 months to 3 years with staphylococcal infections of various localization of ENT organs and skin showed that asytromycin (Azitral) is not inferior in effectiveness with anti -staphylococcal penicillins. Along with high efficiency, characterized by quick and persistent reverse dynamics of the main clinical symptoms and local inflammatory changes in 100% of cases, good tolerance of the drug and the lack of side effects in all children were noted. A wide range of antimicrobial activity, features of pharmacokinetics, a low percentage of unwanted phenomena and a number of advantages over other macrolides determine the priority of using the drug for various skin infectious processes (impetigo, furunculosis, folliculitis, cellulite, paronichia) in children. The effectiveness of azithromycin in pediatric practice, proved by clinical trials, allows you to recommend it as an alternative to b -lactam antibiotics, and in children with burdened allergoannesis - as a drug of choice [18,19]. One of the most important pharmacoeconomic indicators that determine the choice of antibiotic is the ratio of cost/efficiency. It is determined how the ratio of the cost of drug treatment (for oral drugs is equal to the cost of a course dose) to the share of successfully treated patients. It should be noted that Azitral among the existing drugs of azithromycin shows the optimal price/quality ratio [20]. It is known that the inefficiency of antibiotic therapy is largely determined by a decrease in sensitivity to the drug used. Currently, there is no clinically significant resistance to azithromycin. According to antibiotic resistance monitoring, resistance to azithromycin and other macrolides of the latest generation among the pathogens of IKMT does not exceed 2-10%. The sensitivity of S. Pyogenes is allocated in Russia to the antibiotic of azithromycin is 92%. As shown in a number of studies, the clinical effectiveness of azithromycin is higher than that of tetracyclines and B - lactam antibiotics. Comparative clinical and microbiological study of effectiveness in deep staphyloderma of the 5 -day course of azithromycin and 10 -day taking cephalexin showed higher therapeutic activity of macrolide. The eradication of the pathogen when using azithromycin was noted in 94%, with cephalexin in 90% of cases, clinical cure - respectively, in 56 and 53% of cases. At the same time, the frequency of adverse reactions, as a rule, does not require the abolition of the drug, does not exceed 5%, which is much lower than erythromycin (up to 14%) or oral forms of B - lactams [21,22]. Thus, azithromycin has a wide spectrum of antimicrobial action, high bacteriostatic activity in relation to sensitive infections for it, high bioavailability with selective effects in the focus of inflammation, it has low toxic, has a minimum of side effects and a convenient regime of administration. Consequently, the drug meets the modern requirements of rational antibiotic therapy and can be recommended for effective use in dermatovenerological practice.
References 1. Jones ME, Karlowsky JA, Draghi DC, Thornsberry C., Sahm DF, Nathwani D. Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial treatment. Int J Antimicrob Agent 2003; 22:406–19. 2. N.N. Murashkin, M.N. Gluzmina, L.S. Galustyan. Pustular skin lesions in the practice of a pediatric dermatologist: a fresh look at an old problem. RZHKVB: Scientific and practical journal, 2008, No. 4, p. 67–71. 3. Belkova Yu.A. Pyoderma in outpatient practice. Diseases and pathogens. Clinical microbiology and antimicrobial chemotherapy: No. 3, volume 7, p. 255–270, 2005. 4. T.A. Belousova, M.V. Goryachkina. Bacterial skin infections: the problem of choosing the optimal antibiotic. RMJ 2005, volume 13, no. 16, p. 1086–1089. 5. Takha T.V., Nazhmutdinova D.K. Rational choice of antibiotic therapy for pyoderma. RMJ 2008, volume 16, no. 8, p. 552–555. 6. Novoselov V.S., Plieva L.R. Pyoderma. RMJ 2004, volume 12, no. 5, p. 327–335. 7. Masyukova S.A., Gladko V.V., Ustinov M.V., Vladimirova E.V., Tarasenko G.N., Sorokina E.V. Bacterial skin infections and their significance in the clinical practice of a dermatologist. Consilium medicum 2004, volume 6, no. 3, p. 180–185. 8. T. File. Diagnosis and antimicrobial therapy of skin and soft tissue infections. Ohio, USA. Clinical microbiology and antimicrobial chemotherapy: No. 2, volume 5, p. 119–125, 2003 9. Shlyapnikov S.A., Fedorova V.V. The use of macrolides for surgical infections of the skin and soft tissues. GRM, 2004.–t.12, no. 4, pp.204–207 10. Guchev I.A., Sidorenko S.V., Frantsuzov V.N. Rational antimicrobial chemotherapy for skin and soft tissue infections. Antibiotics and chemotherapy. 2003, v. 48, 10, pp. 25–31 11. Parsad D., Pandhi R., Dogras S. A guide to selection and appropriate use of macrolides in skin infection Am J Clin Dermatol 2003; 4:389–97 12. Yakovlev S.V., Ukhtin S.A. Azithromycin: basic properties, optimization of application regimens based on pharmacokinetic and parameters. Antibiotics and chemotherapy. 2003 vol. 48, no. 2. - With. 22–27 13. Turovsky A.B., Kolbanova I.G. Macrolides in the treatment of respiratory tract infections from the position of an ENT doctor: pros and cons Consilium medicum, 2010, No. 4, vol. 12, p. 11 -14. 14. Prokhorovich E.A. Azithromycin. From clinical pharmacology to clinical practice. RMJ 2006, volume 14, no. 7, p. 567–572 15. Berdnikova N.G. Current aspects of the use of azithromycin (Azitral) in the treatment of community-acquired pneumonia in adults. RMJ 2006, volume 14, no. 22, p. 1625–1628. 16. Khryanin A.A., Reshetnikov O.V. Macrolides in the treatment of chlamydial infection in pregnant women (efficacy, safety, cost-effectiveness). RMJ 2008, volume 16, no. 1, p. 23–27. 17. Serov V.N., Dubnitskaya L.V., Tyutyunnik V.L. Inflammatory diseases of the pelvic organs: diagnostic criteria and principles of treatment. RMJ 2011, volume 19, no. 1, p. 46–50. 18. Talashova S.V. Some aspects of the use of antibacterial drugs in pediatrics using the example of macrolides. RMJ 2009, volume 17, no. 7, p. 464–466 19. Mazankova L.N., Ilyina N.O. The place of azalides in pediatric practice. RMJ 2008, volume 16, no. 3, p. 121–125. 20. Solovyov A.M., Pozdnyakov O.L., Tereshchenko A.V. Why is azithromycin considered the drug of choice for the treatment of urogenital chlamydial infection. RMJ 2006, volume 14, no. 15, p. 1160–1164. 21. Gurov A.V., Izotova G.N., Yushkina M.A. Possibilities of using the drug Azitral in the treatment of purulent-inflammatory diseases of the ENT organs. RMJ 2011, volume 19, no. 6, p. 405. 22. Klani R. Double-blind, double-dummy comparison of azithromycin and cephalexin in the treatmen of skin and skin structure infection. Eur.J. Clin. Microbiol. Infect.Dis. 1999, Oct. 10 (10) – p.880–84
Aminoglycosides
The first generation is used in the treatment of plague and tuberculosis only in combination with tetracycline. Third and fourth for tuberculosis, sepsis, severe hospital infections such as pneumonia.
Antibiotics in this group include:
1st generation:
| Representatives | Trade name and method of application |
| Neomycin | Neomycin : external aerosol: on the affected areas of the skin, shake well and place the balloon at a distance of 15-20 cm, apply for 3 seconds; Repeat application 1-3 times a day. |
| Streptomycin | Streptomycin : powder for injection solution: 0.5–1.0 g. x 2 times a day intramuscularly. To prepare the solution, use sterile water/saline solution/0.25% novocaine. Calculation: per 1.0 g. medications - 4 ml of solvent. Streptomycin sulfate . Substance-powder: for intramuscular administration - 0.5-1.0 g. per day. For intratracheal/aerosol administration – 0.5-1.0 g. x 2-3 times every 7 days. |
| Kanamycin | Kanamycin : powder for injection solution: 1.0-1.5 g. for 2-3 injections intravenously (a single dose (0.5 g) is dissolved in 200 ml of a 5% dextrose solution). Kanamycin sulfate : for intramuscular administration 0.5 g./1.0 g. dissolve in 2/4 ml of sterile water or 0.25% novocaine. For intravenous administration 0.5 g. dissolve in 200 ml of saline or 5% glucose solution. |
2nd generation:
| Representatives | Trade name and method of application |
| Tobramycin | Tobrex: eye drops: 1-2 drops, pulling back the lower eyelid, every 4 hours; for severe eye infections - 2 drops every hour. Tobriss: eye drops: 1 drop, pulling back the lower eyelid, 2 times a day (morning and evening); for severe eye infections - 1 drop x 4 times a day. Bramitob : solution for inhalation: 1 ampoule of medication (0.3 g) every 12 hours, administered by inhalation using a nebulizer, course 28 days. |
| Gentamicin | Gentamicin : solution for injection: 0.003-0.005 g. per 1 kg of weight for 2-4 injections, administered intravenously/intramuscularly. Eye drops: 1-2 drops every 1-4 hours, retracting the lower eyelid. Ointment: 3-4 applications per day to affected areas of the skin. Gentamicin sulfate : powder up to 1.2 mg per 1 kg of body weight per day for 2-3 injections (urinary infections); 2.4-3.3 mg per 1 kg of body weight per day for 2-3 injections (severe infections, sepsis). Administer the medicine intramuscularly/intravenously. |
3rd generation:
| Representatives | Trade name and method of application |
| Framycetin | Isofra : nasal spray: 1 injection into each nasal passage x 4-6 times a day, course no more than 10 days. |
| Spectinomycin | Kirin : powder for making a suspension: 2.0 g each. (5 ml)/4.0 g. (10 ml) deep intramuscularly into the upper outer part of the buttock. To prepare the suspension, you need to add 3.2 ml of sterile water to the vial. Suspension for single use, storage is prohibited. |
| Amikacin | Amikacin: solution for infusion: 0.01-0.015 g. per 1 kg of body weight per day for 2-3 injections, administered intramuscularly/intravenously (stream, drip). Amikacin sulfate: substance-powder: 0.005 g each. per 1 kg of weight every 8 hours or 0.0075 g. per 1 kg of body weight every 12 hours, administered intramuscularly/intravenously. |
| Netilmicin | Nettacin : eye drops: 1-2 drops, pulling back the lower eyelid, 3 times a day. Vero-Netilmicin : solution for injection: 4-6 mg per 1 kg of body weight per day intravenously/intramuscularly; for severe infections, the daily dose can be increased to 7.5 mg per 1 kg. |
ANTIBIOTICS OF DIFFERENT GROUPS
MODERN ANTIMICROBIAL CHEMOTHERAPY
MODERN ANTIMICROBIAL CHEMOTHERAPY
L.S. Strachunsky, S.N. Kozlov. Guide for doctors
| Content | ANTIBIOTIC.ru |
Antibacterial drugs
- Fusidic acid
- Chloramphenicol
- Rifampicin
- Fosfomycin trometamol
- Spectinomycin
- Mupirocin
- Dioxidine
FUSIDIIC ACID
Fuzidin, Fucidin
Fusidic acid and its salts have a bacteriostatic effect and have a narrow spectrum of activity. Their main clinical significance lies in their effect on staphylococci.
Activity spectrum
| Gram(+) cocci: | staphylococci, including PRSA and many MRSA. |
| Anaerobes: | C. difficile. |
Does not affect streptococci, including pneumococci (!), and enterococci. Resistance to fusidic acid quickly develops.
Pharmacokinetics
Well absorbed from the gastrointestinal tract, bioavailability when taken on an empty stomach is 90%. Accumulates in bones and joints. Does not penetrate the BBB. It is excreted mainly through the gastrointestinal tract. T1/2 - 9-14 hours.
Adverse reactions
- Dyspeptic and dyspeptic phenomena.
- Hepatotoxicity (increased transaminase activity, jaundice). Risk factors:
long-term use (especially intravenously), underlying liver pathology. - Phlebitis (with intravenous administration).
Indications
- Staphylococcal infections with allergy or resistance to β-lactams (usually in combination with rifampicin, erythromycin, lincosamides).
- C. difficile
- associated diarrhea, pseudomembranous colitis (as a reserve drug).
Dosage
Adults
Orally - 0.5-1.0 g every 8 hours 1 hour before meals; intravenous drip (over 2 hours) 0.5 g every 8 hours.
Children
Orally - 30-60 mg/kg/day in 3 divided doses; intravenous drip 20 mg/kg/day in 3 injections.
Release forms
Tablets of 0.125 g and 0.25 g; granules for preparing a suspension; bottles of 0.25 g and 0.5 g of powder for preparing a solution for infusion (in the form of diethanolamine salt); ointment, 2%; cream, 2%; eye drops, 1% (“Fucitalmic”).
Included in the combination preparations "Fucidin-N cream" (1 g - 20 mg of fusidic acid, 10 ml of hydrocortisone acetate), "Fucicort cream" (1 g - 20 mg of fusidic acid, 1 mg of betamethasone valerate).
CHLORAMPHENICOL
Levomycetin
Has a wide spectrum of activity. It has a bactericidal effect on pneumococcus, meningococcus and hemophilus influenzae, and a bacteriostatic effect on other sensitive microflora.
Its use is limited due to severe adverse reactions and secondary resistance of many pathogens.
Activity spectrum
| Gram(+) cocci: | streptococci, including S. pneumoniae (penicillin-resistant pneumococci are usually resistant); staphylococci (however, many strains are resistant); enterococci. |
| Gram(-) cocci: | meningococci, gonococci, M.catarrhalis . |
| Gram(-) sticks: | H. influenzae (including ampicillin-resistant strains), E. coli, Salmonella, Shigella, Brucella, Yersinia. It should be taken into account that in Russia 50-90% of Shigella and more than 10% of Salmonella are resistant. |
| Anaerobes: | clostridia, anaerobic cocci, bacteroides (including multidrug-resistant B.fragilis ). |
| Rickettsia. |
Pharmacokinetics
It is well absorbed from the gastrointestinal tract, bioavailability is 70-80% and does not depend on food. It is well distributed in the body: it penetrates the BBB, GOB, creates high concentrations in brain tissue, bronchial secretions, and bile. Metabolized in the liver. It is excreted through the kidneys mainly in an inactive state, so in case of renal failure the dose does not need to be reduced. T1/2 in adults - 1.5-3.5 hours, in children - 3-6.5 hours.
Chloramphenicol is used orally in the form of a base, parenterally - in the form of inactive succinate, the activation of which occurs after the succinic acid residue is eliminated, but before this a significant part of the drug can be excreted from the body. Therefore, higher doses are administered intravenously than orally, and intramuscular administration is irrational due to the unpredictability of pharmacokinetics.
Adverse reactions
- Hematotoxicity:
a) reversible
- reticulocytopenia, thrombocytopenia, anemia, depending on the daily and course dose;
b) irreversible
- aplastic anemia with an almost 100% fatal outcome, which is rare (1 case in 10-40 thousand patients), can develop 6-8 weeks after discontinuation of the drug, after a single and even local administration.
Indications
Given the high frequency and danger of adverse reactions, for all the indications below, chloramphenicol is considered as a reserve antibiotic.
- Bacterial meningitis.
- Brain abscess.
- Intra-abdominal and pelvic infections.
- Generalized forms of salmonellosis.
- Typhoid fever.
- Rickettsial diseases.
- Gas gangrene.
Warning
For early detection of hematotoxicity, it is necessary to conduct a clinical blood test every 3 days to determine reticulocytes and platelets.
Dosage
Adults
Orally and parenterally - 50-75 mg/kg/day in 4 divided doses (administrations), regardless of food.
Children
The first week of life - 25 mg/kg/day in 1 injection, 2-4 weeks - 25-50 mg/kg/day in 1-2 injections, over 4 weeks - 25-50 mg/kg/day in 2-3 injections . Children over 1 year old - 50-75 mg/kg/day in 4 doses.
Release forms
Tablets of 0.25 g and 0.5 g; capsules of 0.1 g, 0.25 g and 0.5 g; 0.25% eye drops; bottles of 0.5 g and 1.0 g of powder for preparing a solution for infusion.
RIFAMPICIN*
Rimactan
Semi-synthetic bactericidal antibiotic with a wide spectrum of activity.
Activity spectrum
| Mycobacteria: | M.tuberculoisis, M.leprae , “atypical” mycobacteria ( M.avium, M.kansasii, M.marinum , etc.). |
| Gram(+) cocci: | streptococci, including many penicillin-resistant pneumococci; staphylococci, including PRSA and many MRSA. Enterococci are resistant. |
| Gram(-) cocci: | gonococci, meningococci. |
| Gram(-) sticks: | H. influenzae (including strains resistant to ampicillin and chloramphenicol), Legionella, F. tularensis . Bacteria of the intestinal group ( E.coli , salmonella, etc.) are insensitive. |
Anaerobes are resistant.
Pharmacokinetics
Well absorbed from the gastrointestinal tract, bioavailability when taken on an empty stomach is 95%, but decreases in the presence of food. Penetrates into various fluids and tissues, passes through the BBB. Metabolized in the liver. It is excreted in bile and urine, partly with saliva and tear fluid. T1/2 - 1-4 hours, does not change with renal failure.
Adverse reactions
Small:
- dyspeptic and dyspeptic phenomena;
- discoloration of urine, saliva and tear fluid in an orange-red color.
Large:
- hepatotoxicity (up to the development of hepatitis);
- hematotoxicity: thrombocytopenia, hemolytic anemia;
- influenza-like syndrome (fever, arthralgia, myalgia). It often develops when taking the drug again after a break in treatment.
Drug interactions
Rifampicin is an inducer of microsomal liver enzymes, so it increases the metabolism of many drugs: cardiac glycosides, theophylline, glucocorticoids, oral contraceptives, etc., which can lead to a decrease in their therapeutic effect.
Indications
- The main one is tuberculosis, in which rifampicin is always used in combination with isoniazid and other anti-tuberculosis drugs.
- Prevention and treatment of atypical mycobacteriosis in HIV-infected patients (in combination with azithromycin, clarithromycin, etc.).
- Leprosy.
- Legionellosis (in combination with erythromycin).
- Staphylococcal infections caused by MRSA (in combination with vancomycin, co-trimoxazole, fusidic acid, fluoroquinolones).
Dosage
Adults
Orally - 0.45-0.6 g/day in 1-2 doses 1 hour before meals; for tuberculosis - 10-20 mg/day in one dose (no more than 0.6 g/day). Intravenous drip - 0.45-0.6 g/day in one injection for 3 hours. For staphylococcal (MRSA) infections, 0.45 g 2 times a day, orally or intravenously.
Children
Orally - 10-20 mg/kg/day (no more than 0.6 g/day) in 1-2 doses; intravenous drip - 10-20 mg/kg/day in one administration.
Release forms
Capsules of 0.15 g, 0.3 g and 0.45 g; tablets of 0.15 g, 0.3 g, 0.45 g and 0.6 g; bottles of 0.15 g and 0.6 g of powder for preparing a solution for infusion.
* In the US it is known by the generic name rifampin.
FOSFOMYCIN TROMETAMOL
Monural
Bactericidal antibiotic with primary activity against gram-negative flora.
Activity spectrum
| Gram(-) sticks: | E. coli , Protea and some others, including multidrug-resistant strains. |
| Gram(+) cocci: | Staphylococci are moderately sensitive. |
Resistant streptococci, enterococci, P. aeruginosa
and anaerobes.
Pharmacokinetics
Bioavailability when taken orally on an empty stomach is 60%. It is well distributed in the body, penetrating various organs and tissues. High concentrations are observed in the kidneys. It is excreted unchanged in the urine. T1/2 - 4 hours.
Adverse reactions
- Dyspeptic symptoms, diarrhea (rare).
- Headache, dizziness.
Indications
- Cystitis (acute and recurrent).
- Bacteriuria in pregnant women.
Dosage
Adults
Orally - for acute cystitis 3.0 g once; for recurrent - two doses of 3.0 g every 24 hours.
Children over 5 years old
Orally - 2.0 g once.
Release form
Granules in sachets of 3.0 g.
SPECTINOMYCIN
Trobitsin
It is structurally similar to aminoglycosides and is an aminocyclitol. It has a bacteriostatic effect and a narrow spectrum of activity. Activity against N.gonorrhoeae
. Most often used for allergies to β-lactams.
Activity spectrum
N.gonorrhoeae
, including penicillin-resistant strains.
Pharmacokinetics
Well absorbed when administered intramuscularly. Does not penetrate tissue barriers well. Not metabolized, excreted unchanged by the kidneys. T1/2 - 2-3 hours.
Adverse reactions
Overall well tolerated. Unlike aminoglycosides, it does not have nephrotoxicity or ototoxicity. Sometimes there is a rash, urticaria, fever, dizziness, nausea, and infiltrates at the injection site.
Indications
- Acute uncomplicated gonorrheal urethritis, cervicitis, proctitis.
- Disseminated gonorrhea.
Warning
Spectinomycin should not be used for gonorrheal pharyngitis as it does not produce adequate concentrations in saliva.
Dosage
Adults
Intramuscularly: for urethritis, cervicitis, proctitis - 2.0 g once; for disseminated infection - 2.0 g every 12 hours for 3 days.
Release form
Bottles of 2.0 g of powder for the preparation of a solution for injection with the addition of a solvent.
MUPIROCIN
Bactroban
A drug of natural origin that exhibits a bacteriostatic or bactericidal effect depending on the concentration. Intended for topical use. The most important distinguishing feature of mupirocin is its high activity against staphylococci, including strains resistant to penicillin, aminoglycosides, macrolides, lincosamides, including MRSA. Has virtually no effect on the normal microflora of the skin.
Activity spectrum
| Gram(+) cocci: | S. aureus (including MRSA), coagulase-negative staphylococci; streptococci of groups A, B, C, G. |
| Gram(-) sticks: | E. coli, P. multocida. |
Does not affect enterococci, gram-positive bacilli, most enterobacteria, P.aeruginosa
, anaerobes
Pharmacokinetics
Absorption through intact skin is no more than 0.25%, and increases with damaged skin. May undergo slow partial inactivation in the skin. Due to the high degree of protein binding, activity is reduced in the presence of blood serum.
Adverse reactions
- Local - itching, burning, rash (especially when applied to damaged skin and mucous membranes).
- When administered intranasally, a change in taste is sometimes observed.
Indications
- Skin infections - furunculosis, folliculitis, cellulitis, impetigo (including bullous forms), erysipelas, trophic ulcers, infected dermatoses (including infected eczema), infected wounds, abrasions, burns.
- Otitis externa (except malignant).
- Sanitation of MRSA carriers.
Warnings
- Avoid getting the ointment in your eyes. Do not use dermatological ointment intranasally.
- Use with caution in patients with renal pathology, since propylene glycol, which is included in the ointment base, can be absorbed through the skin and have a nephrotoxic effect.
- Should not be used during pregnancy due to lack of safety data.
Dosage
Adults and children
Dermatological ointment is applied to the affected areas of the skin 2-3 times a day. Nasal ointment in an amount equal to the size of a match head is injected into both halves of the nose 2-3 times a day for 5-7 days.
Release forms
Dermatological ointment, 2%; nasal ointment, 2%.
DIOXIDINE
A synthetic drug that has a bactericidal effect and has a broad antimicrobial spectrum. Due to high toxicity, it is intended mainly for topical use.
According to some Russian clinicians, for severe infections it can be used systemically as a reserve drug, since it acts on multi-resistant microorganisms. However, no controlled clinical studies have been conducted on dioxidin; There are no criteria for assessing the sensitivity of microflora.
Pharmacokinetics
When administered intravenously, it penetrates into various tissues and liquids. Not metabolized, excreted by the kidneys. Well absorbed through the wound surface when applied topically. Quantitative parameters (T1/2, etc.) have not been established.
Adverse reactions
- Acute reactions (at the time of administration) - fever, chills, headache, tremor. Prevention measures:
slow administration, premedication with antihistamines. - Dystrophy and destruction of the adrenal glands (dose-dependent) with the development of acute adrenal insufficiency.
- Embryotoxic, teratogenic and mutagenic effects.
Indications
Locally
- irrigation of infected wounds, washing of cavities, instillation into the bladder.
Intravenously
- severe infections (sepsis, peritonitis, etc.), but only for health reasons in the absence of other, less toxic antibiotics.
Contraindications
- Childhood.
- Adrenal insufficiency.
Dosage
Adults
Intravenously slowly - 10 mg/kg/day in 2-3 injections.
In the cavity - 10-50 ml of 1% solution 1-2 times a day (no more than 70 ml per day).
Release forms
Ampoules of 10 ml and 20 ml of 0.5% solution for intravenous administration; ampoules of 10 ml of 1% solution for local and intracavitary use; ointment, 5%.
| Copyright © 2000-2007 ANTIBIOTIC.ru Posted: 05/15/2004 |
The address of this page: https://www.antibiotic.ru/books/mach/mac0113.shtml
Last modified date: 05/24/2004 18:56
Carbapenems
As a rule, we encounter carbapenems extremely rarely or not at all. And this is wonderful - after all, these antibiotics are indicated for the treatment of severe hospital infections that threaten life. The spectrum of action of carabapenems includes most existing pathological strains, including resistant ones.
Antibiotics in this group include:
| Representatives | Trade name and method of application |
| Doripenem | Doriprex : powder for injection solution: 0.5 g each. intravenously every 8 hours. To prepare the solution, the powder must be dissolved in 10 ml of isotonic sodium chloride solution, the resulting mixture should be added to a bag with 100 ml of isotonic sodium chloride solution or 5% glucose solution. |
| Ertapenem | Invanz : lyophilisate for injection solution: 1.0 g each. per day, administered in 1 injection intravenously/intramuscularly. |
| Meropenem | Meronem: powder for injection solution:
The medicine is administered intravenously slowly (within 5 minutes; the solution is prepared by adding 5 ml of sterile water per 250 mg of the drug) or intravenously drip (within 15-30 minutes; the solution is prepared by adding 50-200 ml of isotonic sodium chloride). Meropenem : powder for injection solution:
|
| Imipenem + cilastatin | Tsilaspen: powder for injection solution: the method of preparing the solution and using it is similar to the above. Tiepenem: powder for injection solution: 1.0-2.0 g. per day, administered intravenously in 3-4 infusions. To prepare the solution, you need to add isotonic sodium chloride to the bottle in a ratio of 100 ml of sodium chloride per 0.5 g. drug, shake until completely homogeneous. Tsilapenem : powder for injection solution: 1.0-2.0 g. per day, administered intravenously in 3-4 infusions. To prepare the solution, add 100 ml of isotonic sodium chloride to the bottle and shake until smooth. Tienam : powder for injection solution: 2.0 g each. per day, administered in 4 injections intravenously/intramuscularly. |
Penicillins
With the discovery of an antibiotic of this particular group - Benzylpenicillin - doctors realized that microbes could be defeated. Despite its venerable age, benzylpenicillin is still used today, and in some cases it is a first-line drug. However, broad-spectrum agents include other, newer penicillin antibiotics, which can be divided into several groups.
| Representatives | Trade name and method of application |
| Ampicillin | Ampicillin:
|
| Amoxicillin + clavulanic acid | Amoxiclav: tablets: 1 tablet (250+125 mg) three times a day or 1 tablet (500+125 mg) twice a day; take with food, course of treatment for 2 weeks. Powder for suspension: use the attached tables to calculate the dose of the medicine. Augmentin: tablets: 1 tablet (250+125 mg) three times a day, course of administration for 2 weeks. Powder for suspension: add 60 ml of clean water at room temperature to the bottle with the powder, wait 5 minutes, add the volume of water to the mark on the bottle, mix. Flemoclav Solutab : tablets: 1 tablet (500+125 mg) three times a day or 1 tablet (875+125 mg) twice a day; do not chew, take at the beginning of meals, take the course for 2 weeks. |
| Amoxicillin | Flemoxin Solutab: tablets: 0.5 g. twice a day, course of administration for 2 weeks. Amoxicillin: tablets: 0.5 g. twice a day, course of administration for 2 weeks. Amosin : capsules: similar regimen and duration of administration. Powder for suspension: pour the powder from the bag into a glass with warm, clean water, stir. |
Fluoroquinolones
Probably, no doctor can imagine his medical practice without fluoroquinolone antibiotics. The first synthesized representatives of this group were distinguished by a narrow spectrum of action. With the development of pharmaceuticals, new generations of fluoroquinolone antibacterial agents were discovered and the spectrum of their activity expanded.
Antibiotics in this group include:
| Representatives | Trade name and method of application |
| Sparfloxacin | Sparflo : tablets: 0.1-0.4 g. per day (depending on the type and severity of the infection). |
| Gatifloxacin | Gatispan : tablets: 0.4 g. x 1 time per day, without chewing, course 10 days. |
| Moxifloxacin | Moflaxia : tablets: 0.4 g. x 1 time per day, without chewing, course 14 days. Avelox : tablets: 0.4 g. x 1 time per day, without chewing, course 14 days. |
| Levofloxacin | Tavanik: tablets: 0.25 gr. (2 tablets) twice a day or 0.5 g. (1 tablet) 1 time per day with water, course 14 days. Floracid : tablets: 0.5 g. twice a day, without chewing. |
Antibiotics for sore throat, bronchitis and cough
Inflammatory diseases of the upper respiratory tract are among the most common in clinical practice. Both children and adults are susceptible to diseases such as tonsillitis and bronchitis. Sometimes these pathological conditions can be caused by viruses, but often a bacterial infection is also associated. In this case, it is necessary to use antibacterial drugs (antibiotics). It should be recalled that antibiotic therapy should be carried out only after a complete examination of the patient, diagnosis and testing of the sensitivity of the flora to a particular antibiotic.
The following medications may be prescribed to treat these diseases:
| Name and group of the drug | Dosage |
| Flemoxin Solutab. Penicillin group, active ingredient – Amoxicillin. |
|
| Sumamed. A group of macrolides, the active ingredient is Azithromycin. |
|
| Gatispan. A group of fluoroquinolones, the active substance is Gatifloxacin. | 1 tablet 400 mg per day. |
| Avelox. A group of fluoroquinolones, the active substance is Moxifloxacin. | 1 tablet 400 mg per day. |
| Rulid. A group of macrolides, the active ingredient is Roxithromycin. | Adults and children weighing over 40 kg – 2 tablets of 150 mg 1-2 times a day. In other cases, the dosage is calculated individually. |
| AzitRus. A group of macrolides, the active ingredient is Azithromycin. | Adults and children over 12 years old – 1 capsule or tablet of 500 mg per day. Children over 3 years old – 10 mg per 1 kg of weight per day. |
The addition of a bacterial infection may be indicated by a sharp increase in body temperature, an increase in signs of general intoxication (weakness, headache and muscle pain, dizziness), cough with the discharge of purulent sputum.
Do I need to take an antibiotic for ARVI?
Antibiotics are NOT prescribed for the treatment of ARVI, as they are ineffective against viruses. However, if colds and flu are treated incorrectly, a bacterial infection can join the virus and cause complications (sinusitis, otitis media, pneumonia, etc.). In this case, taking antibiotics will become necessary. However, the doctor will not prescribe antibiotics in advance to prevent complications, since there is a risk of the addition of other microorganisms, and there will be a need to take another antibiotic.
Principles for selecting the best antibiotics for children
In order for antibiotics to be as effective, safe and free of side effects as possible, it is important to follow certain principles and rules when prescribing them.
Then the antibiotics selected by the doctor will be the best in treating the pathology:
- antibiotics are prescribed only for a proven microbial infection or with a high chance of its development, for complicated forms of pathologies, when the risks of adverse outcomes of the disease are high
- drugs are selected according to the most likely pathogens in a given region and for a given age, based on their resistance to certain drugs
- it is important to take into account previous episodes of antibiotic treatment, if they were carried out in the previous three months, to exclude carriage of resistant strains
- When prescribing drugs in outpatient practice, only oral forms are applicable; injections are prescribed only for special indications.
Drugs that have potentially toxic effects are prohibited for home treatment - a group of aminoglycosides, chloramphenicol, fluoroquinolone drugs and biseptol. When selecting antibiotics for complex clinical situations, it is also important to take into account age restrictions - for example, for tetracyclines, which are permissible only from 12 years of age, since earlier periods of their use threaten serious health consequences.
Possible complications
Despite all the benefits that a broad spectrum of antibiotics provides, such medications cannot be considered a panacea. Their uncontrolled use can affect health.
In particular, the following complications arise:
- According to some data, children who took broad-spectrum antibiotics in the first year of life are more susceptible to developing asthma.
- Improper use of antibiotics can lead to decreased sensitivity to drugs. This is often observed in people who did not complete the full course of therapy, but stopped treatment before the period specified by the doctor. In this case, the antibiotic managed to kill only weak and sensitive bacteria. The remaining ones begin to multiply, cause a new round of disease, but can no longer be treated with the original antibiotic.
- Long-term use of some drugs leads to serious complications. Penicillins can have a toxic effect on the central nervous system, and streptomycin can damage the auditory nerve.
- Destruction of beneficial microflora and subsequent problems with the gastrointestinal tract. Broad-spectrum antibiotics destroy all bacteria, including those we need. Therefore, probiotics or prebiotics are often prescribed along with them, which help maintain normal intestinal health.
Therefore, the universal spectrum of action of antibiotics is not at all a reason to treat them yourself. Only a doctor can select the appropriate drug, prescribe doses, and prescribe the duration of the course. And, of course, it is the specialist who determines the advisability of taking antibiotics as such.
Antimicrobial agents. Classification of antimicrobial drugs
According to the spectrum of activity, antimicrobial drugs are divided into: antibacterial, antifungal and antiprotozoal. In addition, all antimicrobial agents are divided into drugs with a narrow and wide spectrum of action.
Narrow-spectrum drugs primarily targeting gram-positive microorganisms include, for example, natural penicillins, macrolides, lincomycin, fusidine, oxacillin, vancomycin, and first-generation cephalosporins. Narrow-spectrum drugs primarily targeting gram-negative bacilli include polymyxins and monobactams. Broad-spectrum drugs include tetracyclines, chloramphenicol, aminoglycosides, most semisynthetic penicillins, cephalosporins starting from the 2nd generation, carbopenems, fluoroquinolones. The antifungal drugs nystatin and levorin (only against candida) have a narrow spectrum, and clotrimazole, miconazole, amphotericin B have a wide spectrum.
According to the type of interaction with the microbial cell, antimicrobial drugs are divided into:
· bactericidal – irreversibly disrupt the functions of the microbial cell or its integrity, causing immediate death of the microorganism, used for severe infections and in weakened patients,
· bacteriostatic – reversibly block cell replication or division, used for mild infections in non-weakened patients.
According to acid resistance, antimicrobial drugs are classified into:
acid-resistant - can be used orally, for example, phenoxymethylpenicillin,
· acid-labile – intended only for parenteral use, for example, benzylpenicillin.
Currently, the following main groups of antimicrobial drugs are used for systemic use.
¨ Lactam antibiotics
Lactam antibiotics ( Table 9.2) of all antimicrobial drugs are the least toxic, since, by disrupting the synthesis of the bacterial cell wall, they have no target in the human body. Their use in cases where pathogens are sensitive to them is preferable. Carbapenems have the widest spectrum of action among lactam antibiotics; they are used as reserve drugs - only for infections resistant to penicillins and cephalosporins, as well as for hospital-acquired and polymicrobial infections.
¨ Antibiotics of other groups
Antibiotics of other groups ( Table 9.3) have different mechanisms of action. Bacteriostatic drugs disrupt the stages of protein synthesis on ribosomes, while bactericidal drugs disrupt either the integrity of the cytoplasmic membrane or the process of DNA and RNA synthesis. In any case, they have a target in the human body, therefore, compared to lactam drugs, they are more toxic, and should be used only when it is impossible to use the latter.
¨ Synthetic antibacterial drugs
Synthetic antibacterial drugs ( Table 9.4 ) also have different mechanisms of action: inhibition of DNA gyrase, disruption of the incorporation of PABA into DHPA, etc. Also recommended for use when it is impossible to use lactam antibiotics.
¨ Side effects of antimicrobial drugs,
their prevention and treatment
Antimicrobial drugs have a wide variety of side effects, some of which can lead to serious complications and even death.
Allergic reactions
Allergic reactions can occur when using any antimicrobial drug. Allergic dermatitis, bronchospasm, rhinitis, arthritis, Quincke's edema, anaphylactic shock, vasculitis, nephritis, lupus-like syndrome may develop. Most often they are observed with the use of penicillins and sulfonamides. Some patients develop cross-allergy to penicillins and cephalosporins. Allergies to vancomycin and sulfonamides are often observed. Very rarely, aminoglycosides and chloramphenicol cause allergic reactions.
Prevention is facilitated by a thorough collection of allergy history. If the patient cannot indicate which antibacterial drugs he had allergic reactions to, tests must be performed before administering antibiotics. The development of an allergy, regardless of the severity of the reaction, requires immediate discontinuation of the drug that caused it. Subsequently, the introduction of even antibiotics with a similar chemical structure (for example, cephalosporins for allergies to penicillin) is allowed only in cases of extreme necessity. Treatment of infection should be continued with drugs from other groups. In case of severe allergic reactions, intravenous administration of prednisolone and sympathomimetics and infusion therapy are required. In mild cases, antihistamines are prescribed.
Irritant effect on routes of administration
When administered orally, the irritant effect can be expressed in dyspepsia, and when administered intravenously, it can result in the development of phlebitis. Thrombophlebitis is most often caused by cephalosporins and glycopeptides.
Superinfection, including dysbacteriosis
The likelihood of dysbacteriosis depends on the breadth of the spectrum of action of the drug. The most common candidomycosis develops when using narrow-spectrum drugs after a week, when using broad-spectrum drugs - already from one tablet. However, cephalosporins cause fungal superinfection relatively rarely. Lincomycin ranks first in the frequency and severity of dysbiosis caused. Disorders of the flora during its use can take the form of pseudomembranous colitis - a severe intestinal disease caused by clostridia, accompanied by diarrhea, dehydration, electrolyte disturbances, and in some cases complicated by perforation of the colon. Glycopeptides can also cause pseudomembranous colitis. Tetracyclines, fluoroquinolones, and chloramphenicol often cause dysbacteriosis.
Dysbacteriosis requires discontinuation of the drug used and long-term treatment with eubiotics after preliminary antimicrobial therapy, which is carried out based on the sensitivity of the microorganism that caused the inflammatory process in the intestine. Antibiotics used to treat dysbiosis should not affect the normal intestinal autoflora - bifidobacteria and lactobacilli. However, the treatment of pseudomembranous colitis uses metronidazole or, alternatively, vancomycin. Correction of water and electrolyte imbalances is also necessary.
Impaired tolerance to alcohol is common to all lactam antibiotics, metronidazole, and chloramphenicol. It is manifested by the appearance of nausea, vomiting, dizziness, tremor, sweating and a drop in blood pressure when drinking alcohol simultaneously. Patients should be warned not to drink alcohol during the entire period of treatment with an antimicrobial drug.
Organ-specific side effects for various groups of drugs:
· Damage to the blood system and hematopoiesis - inherent in chloramphenicol, less commonly lincosomides, 1st generation cephalosporins, sulfonamides, nitrofuran derivatives, fluoroquinolones, glycopeptides. Manifested by aplastic anemia, leukopenia, thrombytopenia. It is necessary to discontinue the drug, in severe cases, replacement therapy. Hemorrhagic syndrome can develop with the use of 2-3 generation cephalosporins, which impair the absorption of vitamin K in the intestine, antipseudomonal penicillins, which impair platelet function, and metronidazole, which displaces coumarin anticoagulants from bonds with albumin. Vitamin K preparations are used for treatment and prevention.
· Liver damage – inherent in tetracyclines, which block the enzyme system of hepatocytes, as well as oxacillin, aztreonam, lincosamines and sulfonamides. Macrolides and ceftriaxone can cause cholestasis and cholestatic hepatitis. Clinical manifestations are an increase in liver enzymes and bilirubin in the blood serum. If it is necessary to use hepatotoxic antimicrobial agents for more than a week, laboratory monitoring of the listed indicators is necessary. In case of an increase in AST, ALT, bilirubin, alkaline phosphatase or glutamyl transpeptidase, treatment should be continued with drugs of other groups.
· Damage to bones and teeth is typical for tetracyclines, growing cartilage – for fluoroquinolones.
· Kidney damage is inherent in aminoglycosides and polymyxins that disrupt tubular function, sulfonamides that cause crystalluria, generation cephalosporins that cause albuminuria, and vancomycin. Predisposing factors are old age, kidney disease, hypovolemia and hypotension. Therefore, when treating with these drugs, preliminary correction of hypovolemia, control of diuresis, and selection of doses taking into account renal function and body mass are necessary. The course of treatment should be short.
· Myocarditis is a side effect of chloramphenicol.
· Dyspepsia, which is not a consequence of dysbacteriosis, is typical when using macrolides that have prokinetic properties.
· Various lesions of the central nervous system develop from many antimicrobial drugs. Observed:
- psychoses during treatment with chloramphenicol,
- paresis and peripheral paralysis when using aminoglycosides and polymyxins due to their curare-like action (therefore they cannot be used simultaneously with muscle relaxants),
- headache and central vomiting when using sulfonamides and nitrofurans,
- convulsions and hallucinations when using aminopenicillins and cephalosporins in high doses, resulting from the antagonism of these drugs with GABA,
- convulsions when using imipenem,
- agitation when using fluoroquinolones,
- meningism when treated with tetracyclines due to their increase in cerebrospinal fluid production,
- visual impairment during treatment with aztreonam and chloramphenicol,
- peripheral neuropathy when using isoniazid, metronidazole, chloramphenicol.
· Hearing damage and vestibular disorders are a side effect of aminoglycosides, more characteristic of the 1st generation. Since this effect is associated with the accumulation of drugs, the duration of their use should not exceed 7 days. Additional risk factors include old age, renal failure and concomitant use of loop diuretics. Vancomycin causes reversible changes in hearing. If there are complaints of hearing loss, dizziness, nausea, or unsteadiness when walking, it is necessary to replace the antibiotic with drugs from other groups.
· Skin lesions in the form of dermatitis are characteristic of chloramphenicol. Tetracyclines and fluoroquinolones cause photosensitivity. Physiotherapeutic procedures are not prescribed during treatment with these drugs, and exposure to the sun should be avoided.
· Hypofunction of the thyroid gland is caused by sulfonamides.
· Teratogenicity is inherent in tetracyclines, fluoroquinolones, and sulfonamides.
· Paralysis of the respiratory muscles is possible with rapid intravenous administration of lincomycin and cardiodepression with rapid intravenous administration of tetracyclines.
· Electrolyte disturbances are caused by antipseudomonal penicillins. The development of hypokalemia is especially dangerous in the presence of diseases of the cardiovascular system. When prescribing these drugs, monitoring of ECG and blood electrolytes is necessary. In treatment, infusion-corrective therapy and diuretics are used.
Microbiological diagnostics
The effectiveness of microbiological diagnostics, which is absolutely necessary for the rational selection of antimicrobial therapy, depends on compliance with the rules for collection, transportation and storage of the test material. Rules for collecting biological material include:
- taking material from the area as close as possible to the source of infection,
— prevention of contamination by other microflora.
Transportation of the material must, on the one hand, ensure the viability of bacteria, and on the other hand, prevent their reproduction. It is advisable that the material be stored at room temperature before the start of the study and for no more than 2 hours. Currently, special tightly closed sterile containers and transport media are used for collecting and transporting material.
To no less an extent, the effectiveness of microbiological diagnostics depends on the competent interpretation of the results. It is believed that the isolation of pathogenic microorganisms, even in small quantities, always allows them to be classified as the true causative agents of the disease. A conditionally pathogenic microorganism is considered a pathogen if it is isolated from normally sterile environments of the body or in large quantities from environments not typical for its habitat. Otherwise, it is a representative of normal autoflora or contaminates the test material during collection or research. Isolation of low-pathogenic bacteria from areas uncharacteristic of their habitat in moderate quantities indicates the translocation of microorganisms, but does not allow them to be classified as the true causative agents of the disease.
It can be much more difficult to interpret the results of a microbiological study when culturing several types of microorganisms. In such cases, they focus on the quantitative ratio of potential pathogens. More often, 1-2 of them are significant in the etiology of this disease. It should be borne in mind that the likelihood of equal etiological significance of more than 3 different types of microorganisms is negligible.
Laboratory tests for the production of ESBLs by Gram-negative microorganisms are based on the sensitivity of ESBLs to beta-lactamase inhibitors such as clavulanic acid, sulbactam and tazobactam. Moreover, if a microorganism of the Enterobacteriaceae family is resistant to 3rd generation cephalosporins, and when beta-lactamase inhibitors are added to these drugs, it demonstrates sensitivity, then this strain is identified as ESBL-producing.
Antibiotic therapy should be aimed only at the true causative agent of the infection! However, in most hospitals, microbiological laboratories cannot establish the etiology of infection and the sensitivity of pathogens to antimicrobial drugs on the day of admission of the patient, so the initial empirical prescription of antibiotics is inevitable. At the same time, the peculiarities of the etiology of infections of various localizations characteristic of a given medical institution are taken into account. In this connection, regular microbiological studies of the structure of infectious diseases and the sensitivity of their pathogens to antibacterial drugs are necessary in each hospital. Analysis of the results of such microbiological monitoring must be carried out monthly.
Table 9.2.
Lactam antibiotics.
| Group of drugs | Name | Characteristics of the drug | |||||
| Penicillins | Natural penicillins | sodium and potassium salts of benzylpenicillin | administered only parenterally, effective for 3-4 hours | highly effective in their spectrum of action, but this spectrum is narrow, in addition, the drugs are lactamase unstable | |||
| bicillin 1,3,5 | administered only par-enterally, lasts from 7 to 30 days | ||||||
| phenoxymethylpenicillin | drug for oral administration | ||||||
| Antistaphylococcal | oxacillin, methicillin, cloxacillin, dicloxacillin | have less antimicrobial activity than natural penicillins, but are resistant to staphylococcal lactamases, can be used orally | |||||
| Amino penicillins | ampicillin, amoxicillin, bacampicillin | broad-spectrum drugs that can be used orally, but not resistant to beta-lactamases | |||||
| Combined bathrooms | Ampiox - ampicillin+ +oxacillin | a broad-spectrum drug resistant to beta-lactamases, can be used orally | |||||
| Antisinopurulent | carbenicillin, ticarcillin, azlocillin, piperacillin, mezlocillin | have a wide spectrum of action, act on strains of Pseudomonas aeruginosa that do not produce beta-lactamases; during treatment, bacterial resistance to them can quickly develop | |||||
| Lactamase protected - preparations with clavulanic acid, tazobactam, sulbactam | amoxiclav, tazocin, timentin, cyazine, unasin | the drugs are a combination of broad-spectrum penicillins and beta-lactamase inhibitors, therefore they act on bacterial strains that produce beta-lactamases | |||||
| Cephalosporins | 1st generation | cefazolin | antistaphylococcal drug for parenteral approx. | you are not resistant to lactactases, they have a narrow spectrum of action | With each generation of cephalosporins, their spectrum expands and toxicity decreases; cephalosporins are well tolerated and occupy first place in frequency of use in hospitals | ||
| cephalexin and cefaclor | applied per os | ||||||
| 2 generations | cefaclor, cefuraxime | applied per os | resistant to lactams, spectrum includes both gram-positive and gram-negative bacteria | ||||
| cefamandole, cefoxitin, cefuroxime, cefotetan, cefmetazole | used only parenterally | ||||||
| 3 generations | ceftizoxime, cefotaxime, ceftriaxone, ceftazidime, cefoperazone, cefmenoxime | only for parenteral use, have anti-blue purulent activity | resistant to lactamases of gram-negative bactheniums, not effective against staphylococcal infections | ||||
| cefixime, ceftibuten, cefpodoxime, cefetamet | used per os, have anti-anaerobic activity | ||||||
| 4 generations | cefipime, cefpirone | the widest spectrum of action, used parenterally | |||||
| Cephalosporins with beta-lactamase inhibitors | sulperazone | Has the spectrum of action of cefoperazone, but also acts on lactamase-producing strains | |||||
| Carbapenems | imipenem and its combination with cilostatin, which protects against destruction in the kidneys - tienam | More active against gram-positive microorganisms | have the widest spectrum of action among lactam antibiotics, including anaerobes and Pseudomonas aeruginosa, and are resistant to all lactamases, resistance to them is practically not developed, they can be used for almost any pathogen, excluding methicillin-resistant strains of staphylococcus, and as monotherapy even for severe infections, have an aftereffect | ||||
| meropenem | More active against gram-negative microorganisms | ||||||
| ertapenem | |||||||
| Mono-bactams | Aztreons | a narrow-spectrum drug, acts only on gram-negative bacilli, but is very effective and resistant to all lactamases | |||||
Table 9.3.
Antibiotics of other groups.
| Group of drugs | Name | Characteristics of the drug | |
| Glyco-peptides | vancomycin, teicoplamin | have a narrow gram-positive spectrum, but are very effective in it, in particular they act on methicillin-resistant staphylococci and L-forms of microorganisms | |
| Polymyxins | These are the most toxic antibiotics; they are used only for topical use, in particular per os, since they are not absorbed into the gastrointestinal tract | ||
| Fuzidin | low toxic but also low effective antibiotic | ||
| Levomycetin | highly toxic, currently used mainly for meningococcal, eye and especially dangerous infections | ||
| Lincos-amines | lincomycin, clindamycin | less toxic, act on staphylococcus and anaerobic cocci, penetrate well into bones | |
| Tetra-cyclins | natural – tetracycline, semi-synthetic – metacycline, synthetic – doxycycline, minocycline | broad-spectrum antibiotics, including anaerobes and intracellular pathogens, are toxic | |
| Amino-glycosides | 1st generation: streptomycincanamycin monomycin | highly toxic, used only locally for decontamination of the gastrointestinal tract, for tuberculosis | toxic antibiotics with a fairly broad spectrum of action, have a poor effect on gram-positive and anaerobic microorganisms, but enhance the effect of lactam antibiotics on them, and their toxicity decreases in each subsequent generation |
| 2nd generation: gentamicin | widely used for surgical infections | ||
| 3 generations: amikacin, sisomycin, netilmicin, tobramycin | act on some microorganisms resistant to gentamicin; against Pseudomonas aeruginosa, tobramycin is the most effective | ||
| Macro leads | natural: erythromycin, oleandomycin | low toxic, but also low effective, narrow-spectrum antibiotics, act only on gram-positive cocci and intracellular pathogens, can be used per os | |
| semi-synthetic: rock-sithromycin, clarithromycin, flurithromycin | also act on intracellular pathogens, the spectrum is somewhat wider, in particular includes Helicobacter and Moraxella, they pass through all barriers in the body well, penetrate various tissues, and have an aftereffect of up to 7 days | ||
| azolides: azithromycin (sumamed) | have the same properties as semisynthetic macrolides | ||
| Rifampicin | used mainly for tuberculosis | ||
| Antifungal antibiotics | fluconazole, amphotericin B | amphotericin B is highly toxic and is used when pathogens are not sensitive to fluconazole | |
Table 9.4.
Synthetic antibacterial drugs.
| Group of drugs | Name | Characteristics of the drug | ||
| Sulfonamides | Resorptive action | norsulfazole, streptocide, etazol | short-acting drugs | broad-spectrum drugs; pathogens often develop cross-resistance to all drugs in this series |
| sulfadimethoxine, sulfapyridazine, sulfalene | long-acting drugs | |||
| Acting in the intestinal lumen | phthalazole, sulgin, salazopyridazine | salazopyridazine - used for Crohn's disease, ulcerative colitis | ||
| Local application | sulfacyl sodium | mainly used in ophthalmology | ||
| Nitrofuran derivatives | furagin, furazolidone, nitrofurantoin | have a wide spectrum of action, including clostridia and protozoa; unlike most antibiotics, they do not inhibit, but stimulate the immune system; they are used topically and per os | ||
| Quinoxaline derivatives | quinoxidine, dioxidine | have a wide spectrum of action, including anaerobes, dioxidin is used topically or parenterally | ||
| Quinolone derivatives | nevigramon, oxolinic and pipemidic acid | act on a group of intestinal gram-negative microorganisms, are used mainly for urological infections, resistance to them quickly develops | ||
| Fluoroquinolones | ofloxacin, ciprofloxacin, pefloxacin, lomefloxacin, sparfloxacin, levofloxacin, gatifloxacin, moxifloxacin, gemifloxacin | highly effective broad-spectrum drugs that act on Pseudomonas aeruginosa and intracellular pathogens, are well tolerated against many lactamase-producing strains, are widely used in surgery, ciprofloxacin has the greatest antipseudomonas activity, and moxifloxacin has the greatest antianaerobic activity | ||
| 8-hydroxyquinoline derivatives | nitroxoline, enteroseptol | act on many microorganisms, fungi, protozoa, are used in urology and intestinal infections | ||
| Nitroimide-ash | metronidazole, tinidazole | act on anaerobic microorganisms, protozoa | ||
| Specific antituberculosis, antisyphilitic, antiviral, antitumor drugs | used mainly in specialized institutions | |||
Bottom line
Antibiotics are powerful substances of natural, synthetic or semi-synthetic origin that help suppress the growth and activity of pathogenic microorganisms.
Broad-spectrum drugs are effective against most bacteria at the same time, and their new generation causes minimal harm to the body.
The selection of a suitable drug depends first of all on the diagnosis, then on its mechanism of action, the degree of toxicity and pharmacokinetic properties. Independent selection and use of antibacterial drugs is dangerous and unacceptable.