Pharmacological properties of the drug Cavinton solution for infusion
Cavinton has a complex mechanism of action, exerting a beneficial effect on cerebral circulation and cerebral metabolism, as well as on the rheological properties of blood. The neuroprotective effect of Cavinton is due to the following mechanisms:
- reducing the severity of cytotoxic reactions caused by stimulating amino acids;
- inhibition of the functional activity of both cellular transmembrane sodium and calcium channels, and NMDA and AMPA receptors;
- potentiation of the neuroprotective effect of adenosine;
- stimulation of cerebral metabolism, increased absorption and assimilation of glucose and oxygen by cells;
- increasing the resistance of neurons to hypoxia: stimulating the transport of glucose, a universal source of energy for brain cells across the BBB;
- shifting glucose metabolism towards an energetically more favorable aerobic direction;
- selective inhibition of Ca2+-calmodulin-dependent cGMP phosphodiesterase, increasing the concentration of cAMP and cGMP in brain tissue, as well as the concentration of ATP and the ATP/AMP ratio;
- stimulation of cerebral metabolism of norepinephrine and serotonin, ascending noradrenergic system;
- has an antioxidant effect.
These mechanisms of action provide the cerebroprotective effect of Cavinton. Cavinton improves microcirculation in brain tissue by blocking platelet aggregation; reducing increased blood viscosity; increasing the ability of red blood cells to deform and inhibiting their absorption of adenosine; promoting interstitial oxygen transport by reducing the affinity of red blood cells for it. Cavinton selectively increases cerebral blood flow:
- increases the brain fraction of IOC;
- reduces the resistance of cerebral vessels without significantly affecting the parameters of general blood flow, has virtually no effect on blood pressure, blood flow, heart rate and peripheral vascular resistance;
- does not cause the “steal” phenomenon; on the contrary, its use primarily increases the blood supply to the ischemic but still viable area of the brain with a low level of perfusion - the “reverse steal” phenomenon.
Pharmacokinetics. When administered parenterally, the volume of distribution is 5.3 l/kg body weight. The half-life is 4.7 hours.
Cavinton solution for injection 5 mg/ml in ampoules 5 ml No. 5x2
Name
Cavinton solution d/i 5mg/ml in amp 5ml in pack No. 5x2
Description
Colorless or slightly greenish, transparent solution.
Main active ingredient
Vinpocetine
Release form
Solution
Dosage
5mg/ml in amp 10ml
Pharmacodynamic properties
Vinpocetine affects metabolism, blood circulation in the brain, as well as the rheological properties of blood. Vinpocetine exhibits neuroprotective effects: it reduces the harmful effects of cytotoxic reactions caused by stimulating amino acids. Vinpocetine inhibits voltage-gated Na+ and Ca2+ channels, as well as NMDA and AMPA receptors, and enhances the neuroprotective effect of adenosine. Vinpocetine stimulates cerebral metabolism: it increases the uptake of glucose and O2 and the consumption of these substances by brain tissue. Vinpocetine increases the brain's resistance to hypoxia; increases the transport of glucose - the exclusive source of energy for the brain - across the blood-brain barrier; shifts glucose metabolism towards the energetically more favorable aerobic pathway; selectively inhibits the Ca2+-calmodulin-dependent enzyme cGMP phosphodiesterase (PDE); increases the level of cAMP and cGMP in the brain. Vinpocetine increases ATP concentration and ATP/AMP ratio in the brain; enhances the exchange of norepinephrine and serotonin in the brain; stimulates the ascending noradrenergic system; has antioxidant activity. Vinpocetine improves microcirculation in the brain: inhibits platelet aggregation; reduces pathologically increased blood viscosity; increases the deformability of erythrocytes and inhibits the uptake of adenosine; improves O2 transport in tissues by reducing the affinity of O2 to red blood cells. Vinpocetine selectively increases blood flow in the brain: increases cerebral cardiac output fraction; reduces cerebral vascular resistance without significantly affecting systemic circulation parameters (blood pressure, cardiac output, pulse rate, total peripheral resistance); the drug does not cause a “steal effect”. Moreover, against the background of vinpocetine, blood flow to damaged (but not yet necrotic) ischemic areas with low perfusion improves (“reverse steal effect”).
Pharmacokinetic properties
Distribution: In oral dosing studies in rats, radiolabeled vinpocetine was found in highest concentrations in the liver and gastrointestinal tract. Maximum concentrations in tissues could be detected 2-4 hours after application. The concentration of the radioactive tracer in the brain did not exceed the concentration in the blood. In humans: binding to blood proteins is 66%. The absolute oral bioavailability of vinpocetine is about 7%. The volume of distribution is 246.7 ± 88.5 l, which means pronounced binding of the substance in tissues. The clearance value of vinpocetine (66.7 l/h) exceeds the values in plasma and liver (50 l/h), which indicates extrahepatic metabolism of the compound. Elimination: With repeated oral administration of the drug at a dose of 5 mg and 10 mg, vinpocetine demonstrates linear kinetics, equilibrium plasma concentrations are 1.2 ± 0.27 ng/ml and 2.1 ± 0.33 ng/ml, respectively. The half-life in humans is 4.83±1.29 hours. In studies conducted using a radioactively labeled compound, it was found that the main route of elimination is through the kidneys and intestines in a ratio of 60:40%. Larger amounts of radioactive tracer were found in the bile of rats and dogs, but no significant enterohepatic circulation was observed. Apovinamic acid is excreted through the kidneys by simple glomerular filtration; the half-life of this substance varies depending on the dose and route of administration of vinpocetine. Metabolism: The main metabolite of vinpocetine is apovincamic acid (AVA), which is formed in 25-30% of people. After oral administration, the area under the concentration-time curve of VKA is twice as high as that after intravenous administration of the drug, indicating the formation of VKA in the process of first-pass metabolism of vinpocetine. Other identified metabolites are hydroxyvinpocetine, hydroxy-AVA, dihydroxy-AVA-glycinate and their conjugates with glucuronides and/or sulfates. In any of the species studied, the amount of vinpocetine that was released unchanged was only a few percent of the drug dose taken. An important and significant property of vinpocetine is that there is no need for special selection of the dose of the drug in patients with liver or kidney diseases due to metabolism and the absence of cumulation (accumulation). Changes in pharmacokinetic properties in special circumstances (for example, at a certain age, in the presence of concomitant diseases): Since vinpocetine is indicated for the treatment of predominantly elderly patients who experience changes in drug kinetics - decreased absorption, different distribution and metabolism, decreased excretion - it was necessary to carry out studies to evaluate the kinetics of vinpocetine in this age group, especially with long-term use. The results of such studies have demonstrated that the kinetics of vinpocetine in elderly people does not differ significantly from the kinetics of vinpocetine in young people, and, in addition, there is no accumulation. In patients with impaired liver or kidney function, normal doses of the drug can be used, since vinpocetine does not accumulate in the body of such patients, which allows long-term use.
Directions for use and doses
Method of administration The drug can only be used as a slow intravenous drip infusion! (the infusion rate should not exceed a maximum of 80 drops/minute!) The drug cannot be administered intramuscularly, and the drug cannot be administered intravenously without dilution! Cavinton for injection can be diluted with any type of saline or glucose solution for infusion (for example, Salsol, Ringer, Rindeks, Reomacrodex). The medicine is used immediately after opening the ampoule. The ampoule with the drug is intended for single use only. Remains of the drug must be destroyed. From a microbiological point of view, the solution prepared for intravenous administration should be used immediately. Cavinton for injection is chemically incompatible with heparin, so these two drugs cannot be mixed in the same syringe. Cavinton for injection is also incompatible with infusion solutions containing amino acids; therefore, during infusion therapy, Cavinton should not be administered together with infusion solutions containing amino acids. Dosing. For drip infusion, the initial daily dose is usually 20 mg in 500 ml of solution for infusion. This dose can be increased to 1 mg/kg body weight per day for 2-3 days, depending on the patient's tolerance to the drug. The average duration of therapy is 10-14 days, the usual daily dose is 50 mg/day (50 mg in 500 ml solution for infusion) - based on a body weight of 70 kg. After completing the course of infusion therapy, it is recommended to continue therapy of the patient with the tablet form of the drug according to the scheme: 1 tablet of Cavinton forte or 2 tablets of Cavinton 3 times a day (3 times 10 mg each). Patients with impaired renal and liver function In patients with kidney or liver disease, no special dose adjustment is required. Pediatric population The use of Cavinton for injection in children under 18 years of age is contraindicated (see section “Contraindications”).
Use during pregnancy and lactation
During pregnancy and breastfeeding, the use of vinpocetine is contraindicated. Pregnancy: Vinpocetine crosses the placenta, but is found in lower concentrations in both the placenta and fetal blood than in maternal blood. Neither teratogenic nor embryotoxic effects were noted. In animal studies, administration of large doses of vinpocetine was associated in some cases with placental hemorrhage and miscarriage, primarily as a result of increased placental blood flow. Breastfeeding period: Vinpocetine is excreted in breast milk. In studies using labeled vinpocetine, the radioactivity of breast milk was ten times higher than that in the mother's blood. The amount excreted in milk within 1 hour is 0.25 percent of the administered dose of the drug. Since vinpocetine is excreted in mother's milk, and there is no data on the effect on the newborn, the use of vinpocetine during breastfeeding is contraindicated.
Precautionary measures
If the patient has increased intracranial pressure, arrhythmia or long QT syndrome, as well as while using antiarrhythmic drugs, a course of drug therapy can be started only after a thorough analysis of the benefits and risks associated with its use. ECG monitoring is recommended in the presence of long QT syndrome or while taking a drug that prolongs the QT interval. Excipients Due to the fact that the drug contains a small amount of sorbitol (160 mg/2 ml), it is necessary to monitor blood glucose levels in patients with diabetes mellitus during the course of therapy. If the patient has fructose intolerance, treatment with the drug should not be carried out. Benzyl alcohol can cause toxic and anaphylactoid reactions. Sodium metabisulfite can in rare cases cause severe hypersensitivity reactions and bronchospasm.
Interaction with other drugs
During clinical studies, when vinpocetine was used concomitantly with beta-blockers such as cloranolol and pindolol, as well as when used concomitantly with clopamide, glibenclamide, digoxin, acenocoumarol or hydrochlorothiazide, no interaction between these drugs was identified. In rare cases, some additive effect has been observed with the concomitant use of alpha-methyldopa and vinpocetine, so regular monitoring of blood pressure is necessary while using this combination. Although data from clinical studies have not confirmed an interaction, caution is recommended in the case of simultaneous use of vinpocetine with drugs that affect the central nervous system, as well as in the case of concomitant antiarrhythmic and anticoagulant therapy. Cavinton for injection is chemically incompatible with heparin, so these two drugs cannot be mixed in the same syringe. However, the concomitant use of anticoagulants is allowed (without mixing in one syringe or solution). Cavinton for injection is also incompatible with infusion solutions containing amino acids; therefore, during infusion therapy, Cavinton should not be administered together with infusion solutions containing amino acids.
Contraindications
Acute phase of hemorrhagic cerebral stroke, severe ischemic heart disease, severe forms of arrhythmia. Pregnancy, lactation. Hypersensitivity to the active substance or to any of the excipients listed in the section “List of excipients”. The use of the drug in children under 18 years of age is contraindicated (due to the lack of data from relevant clinical studies).
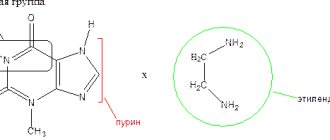
Compound
1 ml of injection solution contains 5 mg of vinpocetine. Excipients with a known effect: 1 ml of injection solution contains 80 mg of sorbitol, 10 mg of benzyl alcohol and 1 mg of sodium metabisulfite. A complete list of excipients is presented in the “List of excipients” section.
Overdose
There were no cases of overdose. Based on the literature data, administration of the drug at a dose of 1 mg/kg body weight can be considered safe. Since there is no data on the use of the drug in doses exceeding this dose, administration of the drug in higher doses is not allowed.
Side effect
Adverse reactions are listed below by organ system class and frequency of occurrence according to MedDRA terminology: Organ system class (MedDRA) Uncommon (? 1/1000 -
Storage conditions
Store at temperatures from +15 °C to +25 °C, protected from light. Keep out of the reach of children.
Indications for use of the drug Cavinton solution for infusion
In neurology: cerebrovascular insufficiency, accompanied by neurological or mental disorders: transient ischemic attack, ischemic stroke, condition after a stroke, vascular dementia, cerebral atherosclerosis, post-traumatic and hypertensive encephalopathy, vertebrobasilar insufficiency. In ophthalmology: for the treatment of chronic vascular diseases of the choroid and retina (including vasospasm and thrombosis of the central artery or retinal vein). In otiatrics: for the treatment of hearing loss of vascular, toxic (including medicinal) or other origins (idiopathic, caused by noise overload), Meniere's disease, idiopathic tinnitus.
Cavinton forte dosage
The duration of treatment and dose are determined by the doctor.
Inside, after eating.
Typically the daily dose is 15-30 mg (5-10 mg 3 times a day). The initial daily dose is 15 mg. The maximum daily dose is 30 mg.
The therapeutic effect develops approximately a week from the start of taking the drug. It takes 3 months to achieve the full therapeutic effect.
For diseases of the kidneys and liver, no adjustment of the dosage regimen is required; the absence of accumulation allows for long courses of treatment.
Application of the drug Cavinton solution for infusion
It is administered only intravenously by drip, slowly (maximum infusion rate - 80 drops per 1 min), intravenous jet or intramuscular administration is not allowed. The initial daily dose for adults is 20 mg (contents of 2 ampoules) diluted in 500 ml of infusion solution. Depending on tolerability, the dose can be increased to 1 mg/kg/day over 2–3 days. The course of treatment lasts on average 10–14 days, the usual daily dose for a patient weighing 70 kg is 50 mg (the contents of 5 ampoules are diluted in 500 ml of infusion solution). For infusion, you can use all solutions containing isotonic sodium chloride solution or glucose. Due to Cavinton's lack of hepato- and nephrotoxic effects, there is no need for dosage adjustment in patients with kidney or liver disease. After completing the course of infusion therapy, it is recommended to continue treatment with the oral form of the drug - 2 tablets (10 mg) 3 times a day.
Drug interactions
No interaction is observed when used simultaneously with beta-blockers (chloranolol, pindolol), clopamide, glibenclamide, digoxin, acenocoumarol and hydrochlorothiazide, imipramine.
The simultaneous use of Cavinton® Forte and methyldopa sometimes caused a slight increase in the hypotensive effect, therefore, when using this combination, regular blood pressure monitoring is required.
Despite the lack of data confirming the possibility of interaction, it is recommended to exercise caution when prescribing Cavinton Forte simultaneously with centrally acting drugs, antiarrhythmics and anticoagulants.
Side effects of the drug Cavinton solution for infusion
from the heart (0.9%): depression of the ST and prolongation of the P–Q , tachycardia, extrasystole. The connection of these disorders with the use of the drug is questionable due to their spontaneous occurrence; from the vascular system (2.5%): slight changes in blood pressure, mainly in the direction of its decrease; skin hyperemia, phlebitis; from the central nervous system (0.9%): sleep disturbance (insomnia, drowsiness), dizziness, headache, weakness, increased sweating may occur during use of the drug, but more often they are symptoms of the underlying disease; from the gastrointestinal tract (0.6%): dry mouth, nausea, heartburn; on the skin: in isolated cases, allergic symptoms were noted.
Use during pregnancy and children
Cavinton® Forte is contraindicated for use during pregnancy, because Vinpocetine penetrates the placental barrier. Moreover, its concentration in the placenta and in the blood of the fetus is lower than in the blood of a pregnant woman. When taken in high doses, placental bleeding and spontaneous abortion may develop, probably as a result of increased placental blood supply.
Within 1 hour, 0.25% of the administered dose of the drug is excreted in breast milk. If it is necessary to use the drug during lactation, breastfeeding should be stopped.
Cavinton's analogues
Level 4 ATX code matches:
Bravinton
Acefen
Carnicetine
Pyracesin
Nooclerin
Semax
Piracetam
Olatropil
Fezam
Vinpocetine
Cerebrocurin
Cavinton Forte
Calcium hopantenate
Glutamic acid
Cephabol
Olanzapine
Cerebrolysate
Pramistar
Sidnocarb
Vinpotropil
Similar drugs are:
- Vinpocetine
- Vicebrol
- Neurovin
- Oxopotin
The price of Cavinton's analogues is often less. For example, a medicine whose name comes from the main active ingredient is significantly cheaper.
Which is better Cavinton or Vinpocetine?
The drugs are absolutely identical. However, Cavinton is considered to be safer than Vinpocetine due to better alkaloid clearance.
Which is better Cavinton or Piracetam?
Piracetam has a slightly different purpose and is often used in combination with Cavinton to treat problems associated with circulatory and metabolic disorders.
Note!
Description of the drug Cavinton conc. d/r-ra d/inf. 5mg/ml amp. 2ml No. 10 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.