Composition per capsule:
Active substance:
minocycline hydrochloride dihydrate (in terms of minocycline) 50.0 mg or 100.0 mg.
Excipients:
microcrystalline cellulose (73.5 mg/147.0 mg), povidone K-17 (8.75 mg/17.5 mg), potato starch (7.0 mg/14.0 mg), magnesium stearate (1.75 mg/3.5 mg), lactose monohydrate (up to the weight of the capsule contents 175.0 mg/350.0 mg).
Hard gelatin capsules:
for 50 mg:
water (13-16%), quinoline yellow dye (0.5833-0.75%), sunset yellow dye (E110) (0.0025-0.0059%), titanium dioxide (0.9740-2.0 %), gelatin (up to 100%).
for 100 mg:
water (13-16%), titanium dioxide (2.0-2.1118%), gelatin (up to 100%).
Pharmacological properties
Pharmacodynamics
Semi-synthetic antibiotic from the tetracycline group. It has a bacteriostatic effect on cells of sensitive strains of microorganisms due to reversible inhibition of protein synthesis at the level of 30S ribosomal subunits. Has a wide spectrum of antibacterial activity.
Sensitivity of microorganisms:
Aerobic gram-positive:
Some of the microorganisms listed below have shown resistance to minocycline, so laboratory susceptibility testing is recommended before use. Antibiotics of the tetracycline group are not recommended for the treatment of streptococcal and staphylococcal infections unless sensitivity of microorganisms to minocycline is shown.
- Bacillus anthracis
- Listeria monocytogenes
- Staphylococcus aureus
- Streptococcus pneumoniae
Aerobic gram-negative:
- Bartonella bacilliformis
- Brucella species
- Calymmatobacterium granulomatis
- Campylobacter fetus
- Francisella tularensis
- Haemophilus ducreyi
- Vibrio cholerae
- Yersinia pestis
Minocycline susceptibility testing is strongly recommended for the following organisms:
- Acinetobacter species
- Enterobacter aerogenes
- Escherichia coli
- Haemophilus influenzae
- Klebsiella species
- Neisseria gonorrhoeae
- Neisseria meningitidis
- Shigella species
Additionally:
- Actinomyces species
- Borrelia recurrentis
- Chlamydia psittaci
- Chlamydia trachomatis
- Clostridium species
- Entamoeba species
- Fusobacterium nucleatum subspecies fusiforme
- Mycobacterium marinum
- Mycoplasma pneumoniae
- Propionibacterium acnes
- Rickettsiae
- Treponema pallidum subspecies pallidum
- Treponema pallidum subspecies pertenue
- Ureaplasma urealyticum
Pharmacokinetics
Food intake does not have a significant effect on the extent of absorption of minocycline. Minocycline has a high degree of lipid solubility and low affinity for Ca2+ binding. It is quickly absorbed from the digestive tract in proportion to the dose taken. The maximum concentration of minocycline in blood plasma (Cmax) after oral administration of 200 mg is 3.5 mg/l and is reached (tmax) after 2-4 hours.
Binding to blood proteins is 75%; the effect of various diseases on this parameter has not been studied.
The volume of distribution is 0.7 l/kg. Minocycline penetrates well into organs and tissues: 30–45 minutes after oral administration it is found in therapeutic concentrations in the kidneys, spleen, eye tissues, pleural and ascitic fluids, synovial exudate, exudate of the maxillary and frontal sinuses, and in the fluid of the gingival grooves. Penetrates well into the cerebrospinal fluid (20 - 25% of the level determined in plasma). Passes through the placenta and enters breast milk.
With repeated administrations, the drug may accumulate. Accumulates in the reticuloendothelial system and bone tissue. In bones and teeth it forms insoluble complexes with Ca2+. Subject to enterohepatic recirculation, 30-60% of the dose taken is excreted with intestinal contents; 30% is excreted by the kidneys within 72 hours (of which 20–30% is unchanged), in severe chronic renal failure – only 1–5%. The half-life (T1/2) of minocycline is approximately 16 hours.
Use of minocycline for mycoplasma infection
Compared to other mycoplasmas Mycoplasma genitalium
most often causes urethritis, infections of the accessory gonads in men and other infectious and inflammatory processes [11].
The standard approach to the treatment of such diseases is the administration of macrolides. However, the emergence of strains resistant to standard antibiotic therapy has been described; in these cases, minocycline was successfully used at a dose of 100 mg twice a day for 2 weeks. [12]. An extended course of minocycline works well even with mutations that make M. genitalium
resistant to fluoroquinolones and macrolides [13].
Mycoplasma hominis
It is rarely an independent causative agent of infectious and inflammatory diseases, but is characterized by significant antibiotic resistance.
Y. Zhou et al. indicated the high sensitivity of M. hominis
to minocycline;
it was higher than the sensitivity of this microorganism to most macrolides [14]. In a study by S. Zhu et al. this type of mycoplasma was sensitive only to minocycline, doxycycline and josamycin [15]. QY Wang et al. Using real-time PCR, they confirmed that the activity of tetracycline antimicrobial compounds against M. hominis
is 100%, and against isolates coinfected with
M. hominis
and
Ureaplasma urealyticum
- 84.3% [16].
According to M. He et al., resistance to minocycline in co-infection with M. hominis
and
U. urealyticum
is minimal and amounts to 6.5%;
for comparison: in the same work, resistance to doxycycline and josamycin reaches 7.2% and 13.5% [17]. In the work of H.Y. Zeng et al. this figure is also less than 10% [18]. In the Greek population, minocycline was also one of the most effective antibiotics against M. hominis
[19].
The susceptibility of microbes to antibacterial drugs is a parameter that is highly dependent on geographic and population factors. Nevertheless, it makes sense to take into account the data of S. Zhu et al., according to which U. urealyticum
remains highly sensitive only to doxycycline, minocycline and clarithromycin [15].
Different biovars and serovars of U. urealyticum
are characterized by different resistance to antibacterial drugs, but the presence of any serovar with resistance to minocycline has not yet been reported [20]. Domestic authors also report high sensitivity of ureaplasmas and mycoplasmas to minocycline [21–23].
An important aspect is the stability of population indicators of resistance to antibiotic therapy. One study demonstrated that the rate of mycoplasma resistance to minocycline remained consistently low in one population for at least 6 years [24].
Indications for use
Minocycline hydrochloride is used to treat the following diseases, subject to the sensitivity of pathogenic microorganisms:
- Acne
- Skin infections
- Spotted fever, typhoid fever, typhoid fever, Q fever (coxiellosis), vesicular rickettsiosis and tick fever
- Respiratory tract infections
- Lymphogranuloma venereum
- Psittacosis
- Trachoma (infectious keratoconjunctivitis)
- Conjunctivitis with inclusions (paratrachoma)
- Nongonococcal urethritis, cervical and anal infections in adults
- Cyclic fever
- Chancroid
- Plague
- Tularemia
- Cholera
- Brucellosis
- Bartonellosis
- Granuloma inguinale
- Syphilis
- Gonorrhea
- Yaws (tropical granuloma, non-venereal syphilis)
- Listeriosis
- anthrax
- Angina Vincent
- Actinomycosis
In the case of acute intestinal amebiasis, the use of minocycline as an addition to amoebicidal drugs is allowed.
For severe acne, minocycline may be used as adjunctive therapy.
The use of minocycline is indicated for asymptomatic carriage of Neisseria meningitidis for the eradication of meningococci from the nasopharynx.
To prevent the emergence of resistance, the use of minocycline is recommended in accordance with the results of laboratory tests, including serotyping and determination of pathogen sensitivity. For the same reason, the use of minocycline for prophylactic purposes is not recommended in cases of high risk of meningococcal meningitis.
Clinical experience shows the effectiveness of minocycline in the treatment of Mycobacterium marinum infections, however, these data are currently not confirmed by the results of controlled clinical trials.
Effect of minocycline on male fertility
One of the most important aspects of the treatment of infections of the accessory male reproductive glands is the independent effect of antibacterial drugs on fertility. The benefits of treating “asymptomatic” infectious and inflammatory processes accompanied by leukocytospermia should outweigh the risks associated with possible disruption of spermatogenesis and damage to sperm under the influence of antibiotics. Unfortunately, studies examining the reproductive safety of antibacterial drugs in vivo are limited.
not so much.
Z. Merati et al. studied the effect of adding minocycline to the medium during sperm cryopreservation in veterinary medicine [25]. In samples to which minocycline was added, the viability and motility of sperm after thawing were significantly higher compared to samples where media with a different composition were used. An anti-apoptotic effect and a decrease in the concentration of reactive oxygen species have been demonstrated. Since the study was conducted in vitro
, we can conclude that minocycline had an independent protective and antioxidant effect on sperm, not associated with the effect on infection of the gonads and the relief of leukocytospermia. In an experimental study using mouse testicular tissue, S. Matsuki et al. demonstrated the protective effect of minocycline against damage to spermatogonial cells during overheating with an increase in temperature to 42 °C [26]. In the presence of minocycline, signs of spermatogonial apoptosis associated with heat stress were significantly attenuated, and the DNA fragmentation index, determined by the TUNEL method, decreased among mature sperm. At the same time, minocycline is currently considered as an inhibitor of one of the internal cascades of apoptosis, and the lipophilicity of the molecule provides it with the ability to penetrate histohematic barriers, including the blood-testis barrier [27]. M. Orazizadeh et al. demonstrated the additive antiapoptotic effect of minocycline in combination with dexamethasone (which, being a glucocorticoid, has a known protective effect) on testicular tissue of mice, confirmed by histological examination with the calculation of the Johnsen index [28].
In a clinical study by F. Lombardo et al. the use of minocycline in different regimens for the treatment of chlamydial infection was studied with further study of spermogram parameters [29]. The authors concluded that the most justified use of minocycline is at a dose of 100 mg twice a day for 10 days. At the same time, control examination after 3 and 6 months. after a course of antibiotic therapy showed the restoration of normozoospermia in the patients included in the study. YA Malallah et al. showed that minocycline therapy led to the elimination of leukocytospermia, which was accompanied by an increase in the number and motility of sperm [30]. The effectiveness of minocycline therapy 200 mg/day did not depend on the duration (1 or 2 weeks).
Directions for use and dosage:
Inside, after eating. It is recommended to drink enough liquid (milk) to reduce the risk of irritation and ulceration in the esophagus.
The initial dose of Minolexin® is 200 mg (2 capsules of 100 mg or 4 capsules of 50 mg), then take 100 mg (1 capsule of 100 mg or 2 capsules of 50 mg) every 12 hours (twice a day) .
The maximum daily dose should not exceed 400 mg.
Infections of the genitourinary system and anogenital area caused by chlamydia and ureaplasma:
100 mg (1 capsule of 100 mg or 2 capsules of 50 mg) every 12 hours for 7-10 days.
Inflammatory diseases of the pelvic organs in women in the acute stage:
100 mg (1 capsule of 100 mg or 2 capsules of 50 mg) every 12 hours, sometimes in combination with cephalosporins.
Primary syphilis in patients with hypersensitivity to penicillins:
100 mg (1 capsule of 100 mg or 2 capsules of 50 mg) twice a day for 10 to 15 days.
Gonorrhea:
100 mg (1 capsule of 100 mg or 2 capsules of 50 mg) twice a day for 4-5 days, or once 300 mg.
Uncomplicated gonococcal infections (excluding urethritis and anorectal infections) in men
: initial dose - 200 mg (2 capsules of 100 mg or 4 capsules of 50 mg), maintenance dose - 100 mg (1 capsule of 100 mg or 2 capsules of 50 mg) every 12 hours for at least 4 days followed by microbiological assessment of recovery 2-3 days after stopping the drug.
Uncomplicated gonococcal urethritis in men:
100 mg (1 capsule of 100 mg or 2 capsules of 50 mg) every 12 hours for 5 days.
Acne:
50 mg (1 capsule of 50 mg) per day, for a long course of 6–12 weeks.
While taking the drug, due to the anti-anabolic effect inherent in drugs of the tetracycline group, an increase in the level of urea in the blood plasma may be observed. In patients with normal renal function, this does not require discontinuation of the drug. In patients with severe renal impairment, azotemia, hyperphosphatemia and acidosis may develop. In this situation, it is necessary to control the level of urea and creatinine in the blood plasma, and the maximum daily dose of minocycline should not exceed 200 mg.
The pharmacokinetics of minocycline in patients with renal failure (creatinine clearance less than 80 ml/min) has not yet been studied sufficiently to conclude that dose adjustment is necessary.
In case of liver dysfunction, use the drug with caution.
For children over 8 years of age for infections caused by pathogens sensitive to minocycline: initial dose – 4 mg/kg, then 2 mg/kg every 12 hours.
| Initial dose | Maintenance dose | |
| Children weighing more than 25 kg | 100 mg (1 capsule of 100 mg or 2 capsules of 50 mg) | 50 mg (1 capsule of 50 mg) every 12 hours |
Relevance
Acne vulgaris (acne vulgaris) is a chronic inflammatory disease manifested by open or closed comedones and inflammatory skin lesions in the form of papules, pustules, and nodules.
Approximately 95% of the global population will experience acne during their lifetime [1]. In 2010, acne was the eighth most common disease in the world [2], while acne is the most common dermatological disease (13.2% of all dermatological consultations) [3]. Acne can occur in any age group, but most often it develops during adolescence. This is a multifactorial dermatosis, in the pathogenesis of which genetically determined hyperandrogenism and a genetically determined type of secretion of the sebaceous glands play an important role. There are 4 main parts of the pathogenesis of acne: increased sebum production, excessive follicular hyperkeratosis, proliferation of Cutibacterium acnes and inflammation. Inflammation in acne is primary, present in all forms and precedes follicular hyperkeratosis, and C. acnes takes an active part in the formation of microcomedones. The pathogenesis of acne involves many different inflammatory mediators, some of which are activated by the proliferation of bacteria C. acnes (strains involved in the pathogenesis of acne are different from strains of healthy skin) in the blocked sebaceous gland duct [4–6].
According to the modern classification, there are four degrees of severity: comedonal acne, papulopustular acne of moderate and severe severity (with the latter, there may be single nodes in the clinical picture) and very severe acne (nodular, conglobate, cystic).
Patient management tactics depend on the severity, effectiveness of previous treatment (if courses of therapy have already been carried out) and quality of life (for example, for excoriated acne, even with minimal manifestations of acne, a systemic retinoid may be recommended). Current recommendations have revised the use of systemic and topical antibiotics (AB), which is associated with problems of antibiotic resistance. The highest incidence of antibiotic resistance is observed in patients with moderate to severe acne [7]. Thus, according to a number of authors, the sensitivity of C. acnes strains to doxycycline was 28.8%, to tetracycline – 31.1%, to erythromycin – 53.3%, to clindamycin – 51.1% [8]. In this regard, it is not recommended to use ABs in the form of monotherapy; the duration of courses should be limited in time to 8 weeks; the appointment of ABs for maintenance therapy and the simultaneous use of systemic and topical ABs, especially those with different chemical structures, should be avoided [9].
In recent years, there has been a trend towards the use of anti-inflammatory doses of AB in the treatment of patients with acne, while at the same time the need to use benzoyl peroxide (BPO) or a fixed combination of adapalene + BPO in the treatment of acne, which have shown effectiveness even in the presence of antibiotic-resistant strains of BPO, has been indicated. Kruglova, N.V. Gryazeva [7, 9].
In terms of systemic antibacterial therapy for acne, minocycline (7-dimethylamino-6-dimethyl-6-deoxytetracycline), the most commonly prescribed AB for acne, which is a semi-synthetic analogue of second-generation tetracycline, can be considered promising [10]. Minocycline has a wide spectrum of antibacterial action, including both gram-positive and gram-negative flora, incl. strains resistant to penicillins and cephalosporins (staphylococci, streptococci, etc.). The drug has a better pharmacokinetic profile than first-generation tetracyclines; it is completely absorbed when taken orally and has high bioavailability due to its lipophilicity [11]. It should be noted that food intake does not affect the bioavailability of minocycline. The drug has a high safety profile and is quite well tolerated. The most common side effects include nausea and dizziness, which are completely reversible when the drug is stopped [12]. Minocycline has anti-inflammatory, anti-apoptotic and immunomodulatory effects and has also been shown to have neuroprotective activity [13–15]. The CD40/40L pathway regulates a number of inflammatory processes. Minocycline reduces CD40L levels on T cells, thereby exerting anti-inflammatory effects. These properties are independent of its antimicrobial activity. The described non-antimicrobial properties are also explained by the ability of minocycline to inhibit the activity of key enzymes such as caspase-1 and -3, and enhance BcL-2 [16, 17]. Many of the pharmacological advantages of minocycline over first-generation tetracyclines (oxytetracycline and tetracycline) are explained by its increased lipid solubility. A greater percentage of the drug is absorbed from the gastrointestinal tract, and the serum half-life is increased by several hours. Sustained blood levels are thought to promote higher concentrations in the skin and increased sebum penetration, although this view is controversial [18].
As a result of the high bioavailability of minocycline, lower doses can be used, which minimizes the risk of developing colonial resistance disorders [14].
According to the clinical recommendations of the Russian Society of Dermatovenerologists and Cosmetologists for the treatment of acne vulgaris, minocycline is recommended for the treatment of nodular acne of moderate and severe severity; acne conglobata of moderate to severe forms at a dose of 50–200 mg/day with a total duration of therapy of no more than 8 weeks (level of strength of recommendations – C, level of certainty of evidence – 2) [19]. However, there are no studies on the use of low doses of minocycline in combination with topical therapy in the available literature, which determined the purpose of this study.
Purpose of the study: to increase the effectiveness and safety of therapy for papulopustular acne by including the systemic antibiotic minocycline in the treatment regimen at a minimum daily dosage of 50 mg in combination with a topical fixed combination of adapalene + BPO in accordance with clinical recommendations.
Methods
The work assessed the effectiveness and safety of the use of minocycline in a daily dosage of 50 mg in combination with topical therapy with a fixed combination of adapalene + BPO in patients with severe papulopustular acne.
In the Russian Federation, since 2012, the only minocycline preparation has been registered and successfully used - Minolexin capsules of 50 and 100 mg No. 20 per package (Russia). 20 patients aged from 14 to 39 years were observed. Among them are 16 women and 4 men. Depending on age, patients were divided into 2 groups: 1st (n=11) – acne vulgaris (patients 14–24 years old), 2nd group (n=9) – adult acne (patients over 25 years old). All patients received a course of minocycline at a dose of 50 mg per day orally for 6–8 weeks, a topical drug (adapalene + BPO) and specialized dermatocosmetics (cleansing, moisturizing).
The effectiveness assessment was carried out taking into account the dynamics of the IGA (Investigator's Global Assessment) scale indicators [20]. According to this scale, effectiveness was defined as IGA 0 (complete clearance) or IGA 1 (almost complete clearance) or an improvement of 2 points compared to baseline. In addition, the impact of acne on quality of life was assessed. For this purpose, the HRQOL (Health-related quality of life) index was used. An assessment was made on a 5-point scale in four aspects: self-perception, emotional sphere, social sphere, acne symptoms [21].
Safety monitoring included general and biochemical blood tests (before therapy, after 1, 2 months from the start of therapy). A biochemical blood test determined aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), γ-glutamyltransferase (GGTP), antinuclear antibodies (ANA), adverse events such as headache, nausea, vomiting, dyspeptic symptoms, pigmentation, hepatotoxicity, lupus-erythematous -like syndrome.
results
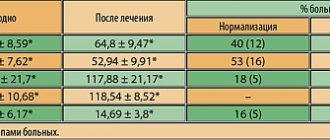
In accordance with the dynamics of the IGA index, the results of group 1 were as follows: 7 (64%) patients had complete cleansing of the skin - IGA=0, 4 (36%) had almost clear skin - IGA<1. In group 2, 5 (56%) patients had complete skin cleansing – IGA=0, 4 (44%) had almost clear skin – IGA<1 (Fig. 1).
According to the HRQOL questionnaire, in patients of group 1, indicators of self-perception improved by 42.7%, emotional sphere - by 37%, social sphere - by 38.7%, acne symptoms - by 44% (Fig. 2).
In patients of group 2, indicators of self-perception improved by 53%, emotional sphere - by 41.7%, social sphere - by 47.4%, acne symptoms - by 51.6% (Fig. 3).
In total, according to the HRQOL scale, there was an improvement in patients of group 1 by 40.5%, in patients of group 2 by 48.6% (Fig. 4).
No changes in the blood count were noted in any patient. Indicators of ASAT, ALAT, GGTP in some (15%) patients after a month tended to increase, but did not exceed 10% of the initial values.
This negative dynamics did not require discontinuation of the drug and the prescription of hepatoprotective therapy. Liver enzyme levels remained within normal limits throughout the observation period. ANA values in patients in the study groups did not change during the observation period. Side effects included headache (5%), nausea (10%), which were temporary and did not require special correction.
Discussion
Systemic antibacterial agents are included by the American Academy of Dermatology (AAD), the European Academy of Dermatology and Venereology (EADV), and the Russian Society of Dermatovenerologists and Cosmetologists (RODVK). Minocycline is approved by the FDA and included in all foreign guidelines in Europe, America, and China since 2012 [1, 7, 19]. Minocycline has a wide spectrum of antibacterial action, including both gram-positive and gram-negative flora, incl. strains resistant to penicillins and cephalosporins (staphylococci, streptococci, etc.), which, in addition to C. acnes, may play a role in maintaining inflammation in acne.
The main problem of antibiotic therapy is the growing resistance of microorganisms to all antibiotics, which also develops in C. acnes. However, resistance to minocycline is now rare. According to modern studies, the resistance of C. acnes to erythromycin is 98%, to clindamycin - 98.4%, to azithromycin - 100%, to tetracycline - 30.8%, to doxycycline - 44.2%, to levofloxacin - 9, 6%, to minocycline – 1.9% [22].
Minocycline is the most lipophilic antibiotic of the tetracycline group, so it easily overcomes the lipid layer of the bacterial cell membrane - one of the main mechanisms of bacterial antibiotic resistance. It should be taken into account that minocycline was not widely used in Russia, therefore, the vast majority of pathogenic microorganisms did not have time to develop resistance. In addition, minocycline does not have cross-resistance with other tetracycline drugs and, therefore, pathogenic microorganisms resistant to other tetracycline drugs remain sensitive to minocycline [23–25].
The effect of minocycline in the treatment of acne is not dose-dependent; therefore, a dosage of 50 mg/day is recommended [26], while a number of authors have shown that when using 1 mg/kg/day (compared to doses of 2 and 3 mg/kg /day) the incidence of side effects is comparable to placebo [27].
The undoubted advantage of minocycline is the minimal increase in skin photosensitivity compared to other tetracyclines, so the drug can be prescribed in the treatment of patients with acne throughout the year [28].
conclusions
- The systemic antibacterial drug minocycline is highly effective when used in combination with a fixed combination of adapalene + BPO in patients with severe papulopustular acne, which is confirmed by the results of a clinical study.
- As a result of the therapy, in total, 12 (60%) patients with severe papulopustular acne achieved complete skin cleansing - IGA = 0 and 8 (40%) patients achieved almost clear skin - IGA <1.
- The quality of life of patients with severe papulopustular acne significantly improved as a result of therapy with the inclusion of minocycline, as evidenced by the HRQOL scale index (improvement in patients of the 1st group by 40.5%, and in patients of the 2nd group by 48. 6%.
- Therapy with the antibacterial drug minocycline in combination with a fixed combination of adapalene + BPO is safe, as evidenced by the results of general and biochemical blood tests before and after treatment, as well as the absence of adverse events in patients, incl. dyspeptic symptoms from the gastrointestinal tract.
Source of financing
The article was published with the support of JSC AVVA RUS, Russia.
Side effect:
The spectrum of adverse events associated with minocycline does not differ from other tetracyclines.
From the digestive system:
anorexia, nausea, vomiting, diarrhea, dyspepsia, stomatitis, glossitis, dysphagia, hypoplasia of dental enamel, enterocolitis, pseudomembranous colitis, pancreatitis, inflammatory lesions (including fungal) in the oral cavity and anogenital area, hyperbilirubinemia, cholestasis, increased content “liver” enzymes, liver failure, including terminal, hepatitis, including autoimmune.
From the genitourinary system:
vulvovaginal candidiasis, interstitial nephritis, dose-dependent increase in urea content in blood plasma.
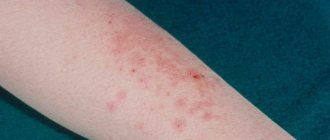
From the skin:
alopecia, erythema nodosum, nail pigmentation, pruritus, toxic epidermal necrosis, vasculitis, maculopapular and erythematous rash, Stevens-Johnson syndrome, exfoliative dermatitis, balanitis.
From the respiratory tract:
shortness of breath, bronchospasm, exacerbation of asthma, pneumonia.
From the musculoskeletal system:
arthralgia, arthritis, limited mobility and swelling of joints, discoloration of bone tissue, muscle pain (myalgia).
Allergic reactions:
urticaria, angioedema, polyarthralgia, anaphylactic reactions (including shock), anaphylactoid purpura (Henoch-Schönlein purpura), pericarditis, exacerbations of systemic lupus, pulmonary infiltration accompanied by eosinophilia.
From the hematopoietic organs:
agranulocytosis, hemolytic anemia, thrombocytopenia, leukopenia, neutrocytopenia, pancytopenia, eosinopenia, eosinophilia.
From the central nervous system:
convulsions, dizziness, numbness (including limbs), lethargy, vertigo, increased intracranial pressure in adults, headaches.
From the senses:
tinnitus and hearing impairment.
From the side of metabolism:
thyroid gland: an isolated case of a malignant neoplasm, discoloration (according to the results of pathomorphological studies), dysfunction
.
Others:
Changes in the color of the oral cavity (tongue, gums, palate), changes in the color of tooth enamel, fever, color of secretions (for example, sweat).
Interaction with other drugs:
Tetracycline group drugs reduce prothrombin activity of blood plasma, which may require reducing doses of anticoagulants in patients,
on anticoagulant therapy.
Due to the fact that bacteriostatic drugs affect the bactericidal effect of penicillins, simultaneous administration of drugs from the penicillin and tetracycline groups should be avoided.
The absorption of tetracyclines is impaired when taken simultaneously with antacids containing aluminum, calcium, magnesium or iron-containing drugs, which may lead to a decrease in the effectiveness of antibiotic therapy.
Cases of terminal renal toxicity have been reported while taking methoxyflurane and tetracycline drugs.
Simultaneous use of tetracycline antibiotics and oral contraceptives may lead to a decrease in the effectiveness of contraception.
Avoid taking isotretinoin immediately before, at the same time, or immediately after taking minocycline, as both drugs can cause a benign increase in intracranial pressure.
Concomitant use of drugs from the tetracycline group with ergot alkaloids and their derivatives increases the risk of developing ergotism.
Minocycline and prostate infections
There is virtually no new data on the pharmacokinetics of minocycline and its ability to create adequate concentrations in the prostate gland. However, available publications suggest that all antibacterial drugs of the tetracycline group penetrate well into the prostate [31]. N. Kawashima et al. showed that the ratio between the concentration of minocycline in the prostate and in the blood serum is 0.76 ± 0.33 [32]. In a study by J. Kamei et al. Minocycline has been successfully used before transrectal prostate biopsy as part of antibiotic prophylaxis based on the results of bacteriological examination of the rectal smear [33].
Special instructions:
With long-term use of minocycline, the cellular composition of peripheral blood should be regularly monitored, liver function tests should be performed, and the concentration of nitrogen and urea in the serum should be determined.
When using contraceptives with estrogens, additional contraceptives or a combination of contraceptives should be used during minocycline therapy.
A false increase in the level of catecholamines in urine is possible when they are determined by the fluorescent method.
When examining a biopsy of the thyroid gland in patients who have been receiving tetracyclines for a long time, one should take into account the possibility of dark brown staining of the tissue in micropreparations.
While taking the drug and 2-3 weeks after stopping treatment, diarrhea caused by Clostridium difficile (pseudomembranous colitis) may develop. In mild cases, it is sufficient to discontinue treatment and use ion exchange resins (colestyramine, colestipol); in severe cases, replacement of loss of fluid, electrolytes and protein, and the appointment of vancomycin, bacitracin or metronidazole are indicated. Do not use medications that inhibit intestinal motility.
To avoid the development of resistance, minocycline should be used only in accordance with the results of a sensitivity study of pathogenic microorganisms. If susceptibility testing of microorganisms is not possible, the epidemiology and susceptibility profile of microorganisms in a particular region should be taken into account.
In the case of sexually transmitted diseases, if concomitant syphilis is suspected, before starting treatment it is necessary to conduct dark-field microscopy studies. Serological diagnostics of blood serum is recommended to be carried out at least once every four months.
Periodic laboratory diagnostics of body functions, including hematopoietic and renal functions, as well as the condition of the liver, are necessary.
Use of minocycline in implant surgery
Minocycline is an interesting antibacterial drug with as yet unrevealed potential. In addition to the fact that it can be used in the treatment of genital infections, pneumonia and soft tissue infections, it has established itself as an effective means for the prevention of periprosthetic infections in urology. A patented composition containing minocycline and rifampicin is known, which is used for the protective coating of penile implants [34]. The implant coated with this composition lasts for about 2 weeks. releases minocycline and rifampicin into surrounding tissues. This technology was first used in the production of vascular catheters [35]. Suppressing bacterial growth action against gram-positive flora ( Staphylococcus epidermidis, Staphylococcus aureus, Enterococcus faecalis
)
in vitro
this combination was more potent than vancomycin. Suppressive effects against gram-negative and fungal flora were comparable to ceftazidime and amphotericin B coatings [36]. In clinical studies, antibacterial coating with minocycline led to a 2-fold reduction in the incidence of periprosthetic infection after the installation of penile prostheses [37].
Algorithms for action in the event of certain side effects:
If superinfection develops, minocycline should be discontinued and adequate therapy should be prescribed.
If intracranial pressure increases, minocycline should be discontinued.
Diarrhea is a common disorder associated with antibiotic use. If diarrhea occurs during treatment with minocycline, you should immediately consult a doctor.
Antibiotics of the tetracycline group cause increased sensitivity to direct sunlight and ultraviolet radiation. If erythema occurs, you should stop taking the antibiotic.