Composition and release form
Tykveol® oil for oral administration
| Oil for oral administration | 1 fl. |
| Complex of biologically active substances from pumpkin seeds (carotenoids, tocopherols, phospholipids, sterols, saturated, unsaturated and polyunsaturated fatty acids - palmitic, stearic, oleic, linoleic, linolenic) |
in dark glass bottles of 100 ml; 1 bottle in a cardboard pack.
Tykveol® capsules 450 mg
| Soft gelatin capsules | 1 caps. |
| Complex of biologically active substances from pumpkin seeds (carotenoids, tocopherols, phospholipids, sterols, saturated, unsaturated and polyunsaturated fatty acids - palmitic, stearic, oleic, linoleic, linolenic) | |
| capsule shell: medical gelatin, glycerol, sorbitol, purified water |
Capsules 450 mg, in polymer jars of 50 and 84 pcs.; in a cardboard pack 1 jar.
Tykveol® rectal suppositories
| Rectal suppositories (0.5 g based on cocoa butter) | 1 sup. |
| Complex of biologically active substances from pumpkin seeds (carotenoids, tocopherols, phospholipids, sterols, saturated, unsaturated and polyunsaturated fatty acids - palmitic, stearic, oleic, linoleic, linolenic) |
Suppositories in contour cell packaging 5 pcs.; in a cardboard pack 2 packs.
Contraindications and side effects
Taking Tykveol internally is prohibited during exacerbations of gastric ulcers, pancreatitis, as well as internal bleeding, the presence of large stones in the bile ducts and bladder. If an allergic reaction to the drug components develops, treatment should be interrupted immediately.
During therapy (when taken orally), nausea, bloating, and stool disorders may occur. If you take a large amount of oil or capsules at once, you may experience an attack of diarrhea.
Taking Tykveol orally is not recommended for children and women during pregnancy in order to avoid possible complications.
Pharmacodynamics
It has a pronounced antioxidant effect, inhibiting the processes of lipid peroxidation in biological membranes.
The hepatoprotective effect is due to membrane-stabilizing properties and manifests itself in slowing down the development of damage to hepatocyte membranes and accelerating their recovery. Normalizes metabolism, reduces inflammation, slows down the development of connective tissue and accelerates the regeneration of damaged liver parenchyma. It has a choleretic effect, normalizes the impaired functional state of the gallbladder and the chemical composition of bile, and reduces the risk of developing cholelithiasis.
Essential phospholipids are a structural element of cell membranes and hepatocyte membranes, regulate membrane permeability and the processes of oxidative phosphorylation, and contribute to the restoration of its structure and function.
It has a direct effect on the structure of epithelial tissues, ensuring differentiation and physiological function of the epithelium, reduces swelling and improves microcirculation in trophic disorders and at the stage of epithelialization, has a protective effect on the granulation process, and stimulates metabolic processes in tissues. Inhibits the proliferation of prostate cells during benign hyperplasia, reduces the severity of inflammatory processes. Eliminates dysuric disorders in prostatic hypertrophy, pain syndrome in patients with prostatitis, and has a bacteriostatic effect. With long-term use, it reduces cholesterol levels in the blood (has a hypolipidemic effect).
How does Tykveol work?
Pumpkin oil has a pronounced anti-inflammatory, choleretic, antiproliferative, antioxidant effect, accelerates tissue regeneration, and protects the liver from toxic damage. Phospholipids, fatty acids, vitamins and minerals regulate metabolic processes, stimulate the restoration of damaged mucous membranes and skin, cleanse the bile ducts, reduce the lithogenicity of bile, prevent increased cholesterol levels and the deposition of fatty plaques in the lumen of blood vessels.
Tykveol has a protective effect on prostate tissue in men. Thanks to its high zinc content, the oil supports normal testosterone production, slowing down the development of benign prostate tumors.
The high content of antioxidants ensures the general protective properties of the drug when exposed to free radicals and toxic substances. Long-term ingestion of the oil helps stabilize the level of low-density lipids, triglycerides and prevent the progression of atherosclerosis.
Directions for use and doses
Tykveol® oil for oral administration
Inside
, 1 teaspoon 30 minutes before meals 3-4 times a day. The course of treatment is at least 3–4 weeks. If necessary, the course is repeated 3-4 times a year. Shake before use.
Externally.
Lubricate the affected areas of the skin 2-3 times a day.
Microclysters:
5 ml of the drug is injected into the anus every other day (after the procedure, the patient should lie down for 10–15 minutes).
Intravaginally.
In gynecology - vaginal tampons 2 times a day.
Tykveol® capsules 450 mg
Inside
, 4 caps. during or after meals 3–4 times a day for 1–3 months. For prostatic hyperplasia - 1-2 caps. 3 times a day for 3–4 months; for chronic prostatitis - 1-2 caps. 3 times a day for 10 days to 3 months; for hyperlipidemia - 1-2 caps. 3 times a day for 3–4 months; for the prevention of atherosclerosis - 1-2 caps. per day for a long time; for diffuse liver damage, chronic cholecystitis, biliary dyskinesia - 3-4 caps. 3 times a day after meals, duration of treatment is at least 3-4 weeks.
Tykveol® rectal suppositories
Rectally.
For hemorrhoids and prostatitis (along with internal use) - 1 supp. 1–2 times a day. The course of treatment for benign prostatic hyperplasia is from 1 to 3 months or short courses of 10–15 days for 6 months. When treating hemorrhoids - 1 supp. 1–2 times a day from 10 days to 1 month.
When to use Tykveol: instructions
Capsules of the drug are recommended:
- for cholecystitis, gallbladder dyskinesia, hepatitis, fatty liver;
- for gastritis, non-infectious form of colitis;
- with atherosclerosis;
- with lesions of the prostate gland.
Tykveol oil is prescribed:
- in the complex treatment of diseases of the gastrointestinal tract and liver;
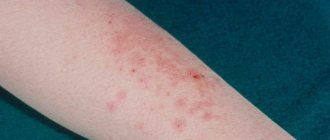
- for dermatological pathologies: dermatitis, herpetic skin lesions, psoriasis, eczema;
- for moderate burns;
- with inflammatory processes of the cervix, endocirvicitis;
- for stomatitis, periodontitis;
- for anal fissures, external hemorrhoids.
Rectal suppositories are prescribed for proctological and andrological pathologies, such as: proctitis, internal hemorrhoids, prostatitis, prostate adenoma, insufficient sexual function.
Various forms of Tykveol are recommended for recovery after surgical interventions: abdominal operations on the intestines. It is allowed to use medicines based on pumpkin oil from the age of 12.
Antifibrotic effect of the drug Tykveol in patients with chronic alcoholic steatohepatitis
In recent years, the number of patients with liver cirrhosis (LC) and hepatocellular carcinoma (HCC) has increased (1, 5). The progression of chronic hepatitis (CH) to cirrhosis and HCC depends on two circumstances: the activity of the inflammatory (dystrophic) process and the intensity of fibrosis formation (4). In alcoholic liver disease, the main treatment method is persistent abstinence, but even in this case, the progression of hCG can continue due to the activity of fibrosis formation (1). Recently, data have emerged on the reversibility of the process of fibrosis formation, which has served as a powerful incentive for the development of various methods of antifibrotic treatment (2, 3).
Table 1. Duration of alcohol abuse (according to medical history)
Table 2. Duration of illness
Table 3. Dynamics of indicators of cytolysis and cholestasis syndromes in patients
Table 4. Dynamics of lipid profile parameters in patients treated with Tykveol
Table 5. Dynamics of the liver fibrosis index during Tykveol therapy
As part of the research, both new drugs (Safironil, Etapersent) and drugs that have proven themselves as hepatoprotectors (Silymarin, Heptral, essential phospholipids) and etiotropic drugs (standard and prolonged interferons, lamivudine, glucocorticoids) are being studied (5).
Currently, the “gold standard” in determining fibrosis is a liver puncture biopsy (LBP), followed by a morphological assessment of the biopsy and determination of the stage of fibrosis according to METAVIR. However, PBP is an invasive method, subject to hit errors, and cannot be reproduced frequently enough. Therefore, non-invasive methods for assessing liver fibrosis have been actively developed recently: instrumental (short-term elastography using the Fibro Scan device, calculating the arterio-portal ratio with Doppler ultrasound angiography, antipyrine and galactose breath tests), determination of serum markers (type IV collagen, aminoterminal propeptide III procollagen, tissue inhibitors of metalloproteases, matrix metalloproteases, hyaluronic acid), diagnostic scales (Bonacini discriminatory scoring scale, Fibrotest and APRI systems, Foris score) (4, 5). Among the latter, we use a discriminant scoring scale (DSS), which allows us to evaluate the fibrosis index (IF) and through it determine the stage of liver fibrosis according to METAVIR, using 3 parameters: international normalized ratio, platelet count and ALT / AST activity ratio. We compared the data obtained from liver puncture biopsy with the results of studying the fibrosis index (using a discriminatory scoring scale) and obtained a very high result of agreement. DSS showed a sensitivity of 98% and a specificity of 46% (1, 4).
Target
This work is to study the effect of the drug
Tykveol
on fibrosis formation in the liver according to the fibrosis index in the treatment of chronic alcoholic steatohepatitis (CASH). The selection of CASH patients was carried out taking into account the criteria included in the clinical trial protocol.
Inclusion criteria
patients in the study:
- men and women of any ethnic origin aged 20 to 75 years with a body mass index between 19 and 29; hepatomegaly and signs of fatty liver (according to ultrasound);
- ALT levels should be at least 1.5 to 3 times the upper limit of normal.
Exclusion criteria:
- patients with chronic hepatitis of other etiology;
- patients with chronic hepatitis in the stage of liver cirrhosis;
- patients with severe concomitant diseases - heart pathology with congestive heart failure, endocrine diseases (diffuse toxic goiter with thyrotoxicosis, myxedema, Cushing's syndrome, stage III-IV obesity, acromegaly), pulmonary obstructive diseases in the "pulmonary heart" stage, severe purulent processes , malignant tumors, long-term use of hepatotoxic drugs;
- pregnancy and lactation;
- drug addicts and persons with any drug dependence.
To assess the effectiveness of the drug Tykveol
A group of 30 patients was recruited, whose age ranged from 23 to 67 years, with an average age of 50 ± 4.16 years. The patients were distributed by gender as follows: 24 were men (80%) and 6 were women (20%). All patients had a history of alcohol abuse; the duration of exposure to the etiological factor (Table 1) averaged 14.5 years (from 5 to 40 years).
The patients had CASH of varying degrees of activity: 14 patients (46%) had minimal activity and 8 patients each (27%) had mild and moderate activity. The duration of the disease averaged 5 years (from first diagnosis to 10 years). The results of the study are presented in Table 2.
Concomitant diseases were recorded in 25 patients (83% of cases): chronic alcoholic pancreatitis in remission - in 8 patients (27%), duodenal ulcer in remission - in 3 patients (10%), gastric ulcer in remission - in 1 patient (3%), gastroesophageal reflux disease – in 2 patients (7%), postcholecystectomy syndrome – in 1 patient (3%), cholelithiasis – in 2 patients (7%), chronic acalculous cholecystitis – in 4 patients ( 13%), chronic bronchitis in remission - in 2 patients (7%), ischemic heart disease - in 7 patients (23%), hypertension - in 19 patients (63%), peripheral polyneuropathy of the lower extremities of alcoholic origin - in 2 patients ( 7%), type 2 diabetes mellitus – in 2 patients (7%) and urolithiasis – in 3 patients (10%). The study group consisted of 30 patients. The study was open controlled.
Treatment method:
patients received
Tykveol
in a dose of 3-4 capsules 3 times a day after meals for 3 months, with the exception of 2 patients who developed side effects, in these cases the dose was reduced to 2 capsules 3 times a day.
The effectiveness of treatment was assessed according to:
- Ultrasound of the liver and spleen
- changes in the size and structure of organs initially and after 3 months of treatment; - blood biochemistry
- initially and after treatment, changes in indicators of cytolysis syndrome (ALT, AST), cholestasis syndrome (GGT, alkaline phosphatase, total bilirubin), creatinine and glucose were assessed; - lipid profiles
– initially and after treatment, changes in cholesterol, triglycerides, alpha-cholesterol (HDL), beta-cholesterol (LDL), atherogenic index; - blood test
- initially and after treatment, changes in hemoglobin, red blood cells, leukocytes, platelets and ESR; - blood coagulograms
– initially and after INR treatment; - fibrosis index
was calculated using the Bonacini discriminatory scoring scale (based on platelet count, INR and ALT/AST ratio) initially and after treatment; - clinically
- according to the timing of relief of pain and dyspeptic syndromes (pain in the right hypochondrium, heartburn, belching, dysphagia, nausea, vomiting, heaviness after eating and a feeling of rapid satiety).
Treatment results
According to liver ultrasound data,
Initially, hepatomegaly was found in 28 patients (93%), normal liver sizes were found in 2 patients (7%), all patients had signs of fatty liver degeneration. The dimensions of the spleen and the vessels of the portal vein system were within normal limits.
After treatment, in 17 patients (57%), the size and structure of the liver did not change; in 4 patients (13%), the size of the liver decreased by an average of 1.05 cm in the thickness of both lobes; in the rest, fluctuations in liver size were within the statistical range.
Biochemical blood test indicators
were assessed over time; The results obtained are presented in Table 3.
As can be seen from the table, initially there was an increase in ALT by 2.5 times, AST by 2.4 times, GGT by 3.5 times and total bilirubin by 1.1 times, while the level of alkaline phosphatase was within normal limits. After treatment, a significant decrease in ALT and AST levels was recorded to figures exceeding the norm by 1.5 times. At the same time, the ALT level normalized in 12 patients (40%), decreased to figures exceeding the norm by 1.9 times in 14 patients (47%), and in 4 patients (13%) the ALT level increased 2.7 times.
The AST level returned to normal in 16 patients (53%), decreased to 1.6 times the normal value in 11 patients (37%) and increased in 3 patients (10%) to 4 times the normal value.
The GGT level was normalized in 7 patients (22%), decreased in 14 patients (50%) and increased in 8 patients (28%).
The level of total bilirubin was normalized in 17 patients (63%), decreased to a figure 1.5 times higher than the norm in 5 patients (20%) and increased to a figure 3.2 times higher than the norm in 8 patients (27%) .
The alkaline phosphatase level decreased to a figure 1.5 times higher than normal in 4 out of 7 patients who initially had elevated values; and increased on average from 98.9 units to 153.1 units in 10 patients (33%). Glucose and creatinine values did not change significantly.
Results of studying the lipid spectrum
in patients in the dynamics of treatment are presented in Table 4.
As follows from the table, initially the average values of cholesterol, triglycerides, HDL, LDL and atherogenic index were within normal limits in 11 patients (37%), cholesterol was elevated in 7 patients (23%), and they also had elevated LDL and atherogenic index .
After treatment, there was a trend towards a decrease in cholesterol, triglycerides, LDL and atherogenic index, but the data obtained were unreliable. Among 11 patients with initial hypercholesterolemia, normalization of cholesterol was recorded in 5 people (46%), a decrease by an average of 0.27 mmol/l in 4 people (37%) and an increase by an average of 0.45 mmol/l in 3 people (18 %); in the remaining 19 patients with normal cholesterol levels, no dynamics were recorded. Among 7 patients with elevated LDL levels and an increased atherogenic index, the indicators were normalized in 4 people (57%) and remained at the original position in 3 (43%); in 23 patients, LDL and atherogenic index values did not change.
According to a general blood test,
indicators of erythrocytes, leukocytes, ESR, hemoglobin did not change significantly.
Dynamics of fibrosis index
liver in patients is presented in Table 5.
As follows from Table 5, initially an FI of 0-3 points (weak fibrosis according to METAVIR) was recorded in 20 patients (67%), an FI of 4-6 points (moderate fibrosis according to METAVIR) - in 8 patients (27%) and an FI of 7 points and more pronounced fibrosis (in the absence of physical signs of liver cirrhosis) – in 2 patients (7%).
After treatment, IF did not change in 10 patients (33%), decreased from 4 to 1.6 points in 15 patients (50%) and increased from 2 to 3.2 points in 5 patients (17%). At the same time, in patients with IF 0-3 points (weak fibrosis according to METAVIR), IF did not change in 7 people (35%), decreased from 2.6 to 0.88 points in 9 people (45%) and increased from 1. 5 to 2.5 points – for 4 people (20%).
In patients with IF 4-6 points (moderate and severe fibrosis according to METAVIR), IF did not change in 3 people (38%), decreased from 5.5 to 2 points in 4 people (50%) and increased from 4 to 5 points – in 1 person (12%). In patients with an IF score of 7 or more points (in the absence of signs of liver cirrhosis), the IF decreased to 3 points (1 person) and to 5 points (1 person).
The dynamics of pain and dyspeptic syndromes are presented in Table 6.
As can be seen from Table 6, initially, pain in the right hypochondrium, heaviness in the epigastrium after eating, bitterness in the mouth, a feeling of rapid satiety and, to a lesser extent, heartburn, nausea, vomiting, and belching predominated.
After treatment, pain was relieved in 6 patients within 7 to 16 days, decreased in 5 patients (after 10-20 days) and remained the same in 6 patients. Heaviness after eating disappeared in 6 patients within 4 to 24 days, and persisted in 5 patients. The bitterness in the mouth disappeared in 7 patients within 5 to 7 days, and persisted in 2 patients. The feeling of rapid satiety bothered 4 patients and persisted during the therapy (an associated symptom associated with gastric “trouble”). Heartburn disappeared in 1 patient on day 14, and persisted in 2 patients. Nausea in 3 patients disappeared within 8 to 12 days of treatment. Belching, recorded in 2 patients, persisted.
Side effects
noted in 3 patients (10%): in 2 patients (7%) pain appeared in the epigastrium and in the right hypochondrium of low intensity on the 5th and 21st days, respectively, after reducing the dose of the drug to 2 capsules 3 times a day, the pain decreased, and one patient (3%) on the 56th day of treatment developed nausea, single vomiting and increased pain in the right hypochondrium, which may be associated with alcohol consumption.
Tolerability of Tykveol
generally good, side effects were mild and disappeared when the dose was reduced to 2 capsules 3 times a day.
Conclusion
Tykveol
consists of the following components: biologically active substances obtained from pumpkin seeds (carotenoids, tocopherols, phospholipids, sterols, phosphates, flavonoids, vitamins B1, B2, C and PP), saturated and polyunsaturated fatty acids (with a proportion of linoleic acid of at least 51, 7%), zinc, manganese, magnesium and calcium.
Pumpkin therapy
was accompanied by a decrease in liver IF in 50% of patients who had predominantly moderate and severe fibrosis (15 out of 30), IF did not change in 33% of patients who had mild fibrosis (7 out of 20), and IF increased in 17% of patients who had mainly the stage of weak fibrosis (in 4 out of 5).
Weak fibrosis according to the calculated index can characterize both weak fibrosis and its absence, so that insignificant dynamics in this group of patients can be qualified as an acceptable statistical error. Consequently, Tykveol
is a drug that effectively affects fibrosis formation in patients with chronic alcoholic steatohepatitis.
The drug has anti-inflammatory and antidystrophic effects, as evidenced by the dynamics of ALT and AST in 87-90% of patients, GGT in 68% of patients, total bilirubin in 83% of patients and ALP in 4 out of 7 patients. The recorded increase in biochemical parameters of cytolysis and cholestasis is most likely associated with the “zigzag” (ethanol load), since no other reasons that could lead to this were recorded. This explanation is suggested by the rapidity of their dynamics to the starting position and the absence of structural changes in the liver according to ultrasound data.
General antifibrotic effect of Tykveol
is expressed in a beneficial effect on the synthesis and metabolism of lipids (according to LDL and atherogenicity index, normalization of cholesterol levels in 83% of patients).
The drug was generally well tolerated; side effects occurred in 10% of patients. They were mild, did not require discontinuation of the drug and disappeared when the dose was reduced by 1.5-2 times the original dose.
Tykveol
is an effective drug in the treatment of chronic alcoholic steatohepatitis with a degree of activity ranging from minimal to moderate.
Treatment with pumpkin
led to a decrease in fibrosis (according to the IF data, calculated using a discriminant scoring scale) in 50% of patients who had mostly moderate and severe fibrosis.
Tykveol
has anti-inflammatory and anti-dystrophic effects, which is reflected in the normalization and reduction of biochemical markers of cytolysis and cholestasis. The drug effectively restores lipid metabolism (in 83% of patients the levels of cholesterol, LDL and atherogenic index are normalized).