Ceraxon tablets: composition and action
Ceraxon is sold in several forms:
- injections;
- pills;
- solution for systemic use.
The tablets contain the main active ingredient – citicoline sodium. In addition to this, the composition includes:
- talc;
- castor oil;
- magnesium stearate.
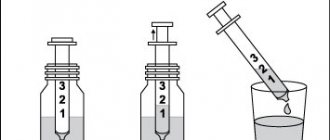
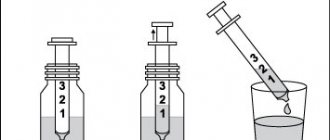
Tablets are packed in 5 pieces in a blister, each box contains 4 blisters. The solution intended for drinking contains the same basic substance - citicoline, and along with it glycerin, sorbidol, sodium citrate, sodium saccharinate, strawberry flavoring. This composition is packaged in unpainted glass bottles with plastic caps. The volume of one bottle is 30 ml. It is convenient to dose using the syringe included in each package.
The drug for administration into a vein is packaged in glass ampoules, with a break line marked. One cardboard package can contain 3 or 5 pieces. Ceraxon mainly acts on brain cells, strengthening membranes, improving the functions of ion exchange pumps of the nervous system, which is due to the production of phospholipids.
In case of severe swelling, the action of the drug relieves pressure at the site of the lesion, which returns the cognitive functions of the brain. In addition, the ability to concentrate is restored and memory improves. If Cerakson is applied promptly at the first symptoms of a stroke, it will be possible to minimize the area of spread of necrotic tissue.
Citicoline is one of the constant elements of the human body, which does not make it possible to evaluate its pharmacokinetics. The substance entering through the gastrointestinal tract is absorbed almost completely. As a rule, 1-2% of the total ingested volume remains unabsorbed. Citicoline is excreted through the kidneys in the urine.
As a result of hepatic metabolism processes, two substances are formed: cytidine and choline. Citicoline is first concentrated in the structures and tissues of the brain. At the same time, fractions of the resulting choline pass into phospholipids, and cytidine derivatives into the composition of nucleic acids.
Mechanisms of action of drugs
Citicoline is the active ingredient of Recognan and Ceraxon. In the body, it is involved in the synthesis of cell membrane components called phospholipids: this means that the administration of citicoline helps neurons - the cells of the nervous tissue - recover faster. Additionally, the drug inhibits the work of phospholipases responsible for the destruction of membrane components, prevents the formation of free radicals and interferes with the process of programmed cell death - apoptosis.
The conduction of nerve impulses also improves: citicoline helps synthesize acetylcholine, which is a transmitter and transmits excitation from one neuron to another. The complex effect reduces the area affected by a stroke, helps already damaged areas recover, and helps the patient recover.
The substance has a beneficial effect on memory, level of consciousness, attention, and helps in the treatment of movement disorders.
Citicoline is well absorbed and enters the brain both when administered orally and in the form of injections.
Cereton works due to choline alfoscerate. Breaking down into choline and glycerophosphate, the substance becomes materials for the synthesis of acetylcholine and phosphotidylcholine in cell membranes, and thanks to metabolic protection, choline is released in the brain. Neurons recover faster, are protected from damage, receptors work better, and blood flow and metabolism become more efficient.
When is Cerakson prescribed?
Treatment with Ceraxon is indicated in the following cases:
- first 4 hours during primary stroke;
- for rehabilitation after a stroke;
- emergency assistance and to restore the condition impaired as a result of severe head injury;
- cerebrovascular diseases that reduce cognitive function and provoke behavioral changes.
If the patient was administered Ceraxon in the first hours after a traumatic brain injury, the duration of post-traumatic coma will be less than without the help of the drug. In addition, there is a high probability of reducing the severity of neurological abnormalities typical of this type of injury.
If chronic hypoxia has become the reason for the predominance of lack of initiative in a person’s behavior, complete inability in matters of self-care, the drug effectively affects certain areas of the brain, restoring cognitive functions.
Medical Internet conferences
Chronic cerebral ischemia is a multifactorial disease, occurs mainly in older age groups, is characterized by clinical and morphological heterogeneity, and is characterized by a progressive course. Modern angioneurology has achieved great success in the treatment of patients with chronic cerebrovascular insufficiency. The use of drugs that help optimize metabolism, stabilize the cell membrane, and influence the processes of neuroplasticity is justified from a pathogenetic point of view.
Purpose of the study. Studying the effectiveness of the drug "Ceraxon" in patients with chronic cerebral ischemia of stage II of mixed origin.
Materials and methods. A comprehensive clinical and instrumental examination was carried out on 86 patients (47 women and 39 men) who were treated in the neurological department of the Municipal Clinical Hospital No. 9, Saratov, with chronic cerebral ischemia (CHI) stage 2 of mixed origin with focal neurological symptoms and moderate cognitive impairment . The average age of the patients was 68+1.2.
The patients were divided into 2 groups. The main group (MG) included patients (55 people) in whose treatment, in addition to basic therapy, the drug “Ceraxon” was used at a daily dose of 1000 mg intravenously in 150 ml of 0.9% saline solution for 10 days. The comparison group (CG) consisted of patients (31 people) who received standard therapy (nootropics, antioxidants, antiplatelet agents, physiotherapeutic treatment, therapeutic exercises). The groups were comparable by gender, age, and nature of vascular pathology.
The diagnosis was established based on the results of a neurological and neuropsychological examination, laboratory and instrumental data (ultrasound of the head and neck, nuclear magnetic resonance imaging) research methods.
Before and after the end of the course of treatment, complaints and neurological status of patients were analyzed, and neuropsychological indicators were assessed. Screening assessment of cognitive functions was carried out using the MMSE scale. Memory was assessed using the method of memorizing 10 words and an associative memory test, attention was studied using the Kraepelin counting method, spatial and dynamic praxis were studied, the level of anxiety was determined using the Spielberger-Hanin scale.
Results and discussion. Analysis of the structure of complaints revealed the presence of general cerebral symptoms, such as headache, dizziness, general weakness, as well as sleep disturbance and emotional lability. The neurological status was represented by pyramidal, cerebellar, extrapyramidal, and pseudobulbar syndromes. Before starting treatment, a neuropsychological study revealed a moderate cognitive deficit in all patients in the form of a disorder of mnestic function, attention deficit, decreased cognitive activity, and rapid exhaustion. The majority of patients had anxiety of varying degrees of severity.
After the course of treatment, the subjective well-being of patients both in the MG and GS significantly improved. Positive dynamics in the form of a decrease in headaches, dizziness, improved sleep, and normalization of mood prevailed in the MG. As a result of the therapy, the severity of focal neurological symptoms decreased, especially in relation to cerebellar and pseudobulbar syndromes. In the OG, the cerebellar syndrome regressed in 69.1% of patients, in the GS – in 48.4% of cases.
After the course of treatment, activation of the functions of perception and memory of information was noted, and the number of words during reproduction increased. Activation of the mnestic function was observed in the OG before treatment - 7.2 + 0.2, after - 9.0 + 0.1*, in the GS before - 7.1 + 0.2 after - 8.5 + 0.2; stability and concentration of attention increased in the MG before treatment - 65.5+1.8, after - 52.6+1.8*, in the GS before - 64.9+2.3, after - 58.1+2.2 ; dynamic praxis indicators improved in the MG before treatment - 3.8+0.3, after - 4.6+0.2*, in the GS before - 3.8+0.1, after - 4.4+0.2* , spatial praxis in the OG before treatment - 10.2+0.2, after -12.8+0.2*, in the GS before - 9.2+0.2, after - 10.7+0.2* ( * - reliability criterion in comparison with indicators before treatment < 0.05). Positive dynamics were recorded during a repeated study of the level of anxiety: OG before treatment – 60.2+6.9, after – 51.6+8.1*, in GS before – 60.6+9.9, after – 54.9+ 10.1 (* - reliability criterion in comparison with indicators before treatment < 0.05).
Conclusions. This study confirms that the use of the drug "Ceraxon" in patients with chronic cerebral ischemia stage II leads to regression of neurological syndromes and improvement of the cognitive sphere. The use of this drug allows you to achieve the best therapeutic result and contributes to effective medical rehabilitation.
Ceraxon 1000: instructions
Intravenous administration of the drug Ceraxon 1000 should be carried out slowly and evenly, avoiding the creation of a strong stream of flow. When administering drips, the lamb should be in the middle position, which provides up to 60 drops per minute. Jet injection, including into an elastic band, is carried out by applying gentle pressure on the syringe piston. The effectiveness of the drug directly depends on the time of treatment. The sooner the patient was admitted to the care of physicians, and the earlier drug treatment was started, the more positive the result will be.
Ceraxon 1000 is a classic dosage for the treatment of stroke of various etiologies and to minimize the consequences of traumatic brain injuries, which is administered every 12 hours for 6 weeks. After which, if the patient has not lost the swallowing reflex, intravenous administration is replaced by systemic administration through tablets.
If treatment involves taking the solution orally, it must be diluted in 100 ml of drinking water. Drink the medicine with meals. Children may be prescribed the drug in cases of damage to the central nervous system. The dosage for the child is prescribed by the attending physician, depending on his weight, age and condition.
Courses of treatment with drugs containing citicoline
The solution for oral administration from a bottle of Ceraxon or Recognan is dosed with a syringe from the kit, and when choosing a product in bags, just open it and drink the already measured portion. If desired, the medicine is dissolved in half a glass of water. Drink it between meals or at the same time as them. The solution can be used even if preservative crystals appear at the bottom of the bottle: they disappear if stored correctly and do not affect the quality.
Both drugs should be used immediately after opening the ampoules. It is best to administer the medicine intravenously, slowly or using a drip. If citicoline is prescribed intramuscularly, try not to place the injections in the same place. Dosages depend on the diagnosis.
- Acute period of stroke and trauma - 1000 mg every 12 hours for 6 weeks. They start with injections, after 3-5 days you can switch to a solution.
- Recovery period, illness with impaired memory and thinking - 500-2000 mg of medication per day. The duration of treatment and forms of administration are individual.
1000 mg of medicine is contained in 10 ml of solution from a bottle or 1 sachet. The daily dose of such drugs is taken immediately or divided into two doses. Safety makes it possible not to recalculate the dosage for elderly patients, and according to the standards, Ceraxon and Recognan are identical.
Ceraxon is also available as 500 mg tablets. They are given 1-4 pieces per day. It is difficult to find medicine in this form on sale: solutions are much more popular.
Contraindications for use
If, as a result of diagnosing the patient’s condition, the following features are revealed, Cerakson is not prescribed:
- individual intolerance to one of the components of the drug;
- tone of the autonomic nervous system, in its parasympathetic part;
- hereditary fructose intolerance.
Adverse reactions during treatment with Cerakson are extremely rare, among them are:
- coordination problems;
- pain in the occipital region;
- diarrhea;
- appetite disorders;
- insomnia;
- rash.
Sometimes short-term changes in blood pressure are observed, as well as loss of sensation, mainly in the arm and leg on the paralyzed side, if the patient has suffered a stroke.
If any of these reactions occur, you should discuss changing the course of therapy with your doctor.
Differences and Benefits of Nootropics
Cereton, Recognan and Ceraxon are nootropics with the ability to accelerate the restoration of neuronal membranes and the production of the transmitter acetylcholine. Only one of them - Ceraxon - is an original drug with all the necessary studies and a high chance of being better than generics. Recognan is a cheap substitute with a different effectiveness, and Cereton reproduces the composition of the original Gliatilin. The drugs are prescribed in the form of solutions for oral administration or injection. Cereton is also available in capsules.
Indications for nootropics include acute brain damage due to stroke and trauma, as well as the recovery period and age-related brain disorders. All medications rarely cause side effects, but are not recommended for children. Cortexin injections based on polypeptides from the cerebral cortex of cattle can help in this case.
special instructions
Treatment with Cerakson is often prescribed to premature babies and newborns with brain tissue injuries received during childbirth. The treatment regimen is determined by the attending physician and depends on the Apgar assessment of the child’s condition. Storing the medicine in the refrigerator may cause crystals to form in the solution. It will take at least two months at room temperature to dissolve them. Despite the fact that this does not reduce the quality of the active substance, the drug should still be stored at room temperature not exceeding +30 C.
The empty packaging of the drug must be used within three years from the date of release.
Analogues and substitutes
Recognan and Cereton are not the only generics designed to replace expensive original drugs. Analogs with citicoline also include Neupilept, one of the domestic substitutes in the form of solutions for intravenous and intramuscular administration, as well as oral administration. The concentration of the solution in ampoules is 125 and 250 mg/ml, for oral administration - 100 mg/ml. It is used similarly to Cerakson, the price corresponds to generics.
| A drug | Active substance | Manufacturer | Price |
| Recognan solution for intravenous and intramuscular administration 500 mg/4 ml | Citicoline | GEROPHARM LLC (Russia) | 446 rub. for 5 ampoules |
| Ceraxon solution for intravenous and intramuscular administration 500 mg/4 ml | Citicoline | FERRER INTERNACIONAL SA (Spain) | 570 rub. for 5 ampoules |
| Neypilept oral solution 100 mg/ml | Citicoline | SOTEX CJSC (Russia) | 380 rub. |
| Cereton capsules 400 mg | Choline alphoscerate | SOTEX CJSC (Russia) | 410 rub. for 14 capsules |
| Cereton solution for intramuscular and intravenous administration 250 mg/ml | Choline alphoscerate | SOTEX CJSC (Russia) | 266 rub. for 3 ampoules |
| Cerepro solution for intravenous and intramuscular administration, 250 mg/ml | Choline alphoscerate | VEROPHARM JSC (Russia) | 400 rub. for 3 ampoules |
| Gliatilin solution for intramuscular and intravenous administration 1000 mg/4 ml | Choline alphoscerate | ITALFARMACO SpA (Italy) | 530 rub. for 3 ampoules |
| Cortexin lyophilisate for the preparation of solution for intramuscular administration | Livestock cerebral cortex polypeptides | GEROPHARM LLC (Russia) | 1300 rub. |
Among the substitutes for Cereton, one can highlight Cerepro: solution and capsules with choline alfoscerate. The dosage and method of use are the same for the analogues. In acute conditions, any of the drugs is prescribed intravenously, and after 10-15 days of intensive treatment, the patient can be switched to capsules. None of the complete analogues are recommended for children. To help a child with brain injuries or developmental delays, you need to choose other nootropics.
Cortexin - an option for children
Cortexin is sold as a lyophilisate and is prescribed only intramuscularly. The drug contains polypeptides from the cerebral cortex of livestock, which are able to pass through the blood-brain barrier and protect neurons. Cortexin improves memory, learning, resistance to stress, and is prescribed both for developmental delays in children and in cases of trauma, epilepsy, cerebral palsy, and impaired blood circulation in the brain.
Lidocaine as a solvent for Cortexin increases the likelihood of side effects in children and is therefore not recommended.
Cortexin is well tolerated; it is contraindicated only in cases of intolerance to the drug, during pregnancy and lactation. The usual dosage for children under 20 kg is 0.5 mg/kg, for children with greater weight and adults - 10 mg at a time. The course lasts 10 days, the injection is given once a day.
The list of side effects is limited. The injection site may become red, and the patient has a chance of insomnia, restlessness, tachycardia, and lack of coordination. Dangerous reactions include anaphylactic shock as a type of allergy to a foreign protein. In less serious cases, the allergy is expressed by a rash, itching, and redness.
Contraindications and negative reactions of the body
The main contraindications to taking the drug are hypersensitivity to the active ingredient and other components. The medicine is not prescribed before reaching the age of 18 years, as well as during pregnancy and lactation.
Contraindications include the acute stage of hemorrhagic stroke, which, as a rule, develops against the background of high blood pressure.
When taking the drug, allergic reactions may occur: rash and itching on the skin. Other adverse reactions are rare. Nausea is sometimes observed after taking the drug, which is a consequence of the effect of the drug on the peripheral nervous system. When it appears, discontinuation of the drug is not required; it is enough to reduce the dosage.
It is necessary to consult a doctor when the medicine provokes disturbances in the functioning of the central nervous system. For example, during treatment, headaches, sleep problems, and increased excitability occur. It may also be necessary to discontinue the drug if gastritis and peptic ulcers worsen during oral administration, or severe constipation or diarrhea occurs.