Cytological examination of smears from the cervix allows one to assess the condition of the mucous membrane, the presence or absence of signs of pathological processes (reactive, precancerous, tumors). When other laboratory methods identify an infectious agent (human papillomavirus, bacterial and parasitic infections), the cytological method allows one to evaluate the body’s response to the infectious agent, the presence or absence of signs of damage, proliferation, metaplasia or transformation of the epithelium. It is also possible, by examining a smear, to determine the cause of changes in the epithelium (the presence of inflammation with an approximate or confident determination of pathogenic microbiota (microflora), pathological processes associated with hormonal, medicinal, mechanical, radiation effects on the woman’s body and the cervix, conditions fraught with the risk of dysplasia and cervical cancer, and when they develop, establish the correct diagnosis.In this regard, cytological examination is used both for screening (smears from a visually normal cervix) and in the presence of changes in the mucous membrane visible during a gynecological examination.
Receiving material
Cervical cancer most often develops in the transformation zone, it is preceded by background processes and intraepithelial lesions (epithelial dysplasia), which can be located in small areas, so it is important that material is obtained from the entire surface of the cervix, especially from the junction of squamous and columnar epithelium . The number of altered cells in a smear varies, and if there are few of them, then the likelihood increases that pathological changes may be missed when viewing the specimen. For effective cytological examination it is necessary to consider:
- During preventive examinations, cytological smears should be taken from women, regardless of complaints, the presence or absence of changes in the mucous membrane. Cytological examination should be repeated at least once every three years;
- it is advisable to obtain smears no earlier than on the 5th day of the menstrual cycle and no later than 5 days before the expected start of menstruation;
- you cannot take material within 48 hours after sexual intercourse, use of lubricants, vinegar or Lugol’s solution, tampons or spermicides, douching, insertion of medications, suppositories, creams into the vagina, including creams for performing ultrasound examinations;
- pregnancy is not the best time for screening, as incorrect results are possible, but if you are not sure that the woman will come for examination after childbirth, it is better to take smears;
- for symptoms of acute infection, it is advisable to obtain smears for the purpose of examining and identifying pathological changes in the epithelium, the etiological agent; Cytological control is also necessary after treatment, but not earlier than 2 months. after completing the course.
Material from the cervix should be taken by a gynecologist or (during screening, preventive examination) by a well-trained nurse (midwife).
It is important that the smear contains material from the transformation zone, since about 90% of tumors come from the junction of the squamous and columnar epithelium and the transformation zone, and only 10% from the columnar epithelium of the cervical canal.
For diagnostic purposes, material is obtained separately from the ectocervix (vaginal portion of the cervix) and endocervix (cervical canal) using a spatula and a special brush (such as Cytobrush). When conducting a preventive examination, Cervex-Brush, various modifications of the Eyre spatula and other devices are used to obtain material simultaneously from the vaginal part of the cervix, the junction (transformation) zone and the cervical canal.
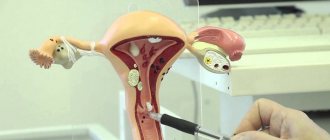
Before obtaining the material, the cervix is exposed in “mirrors”; no additional manipulations are performed (the cervix is not lubricated, mucus is not removed; if there is a lot of mucus, it is carefully removed with a cotton swab without pressing on the cervix). A brush (Eyre spatula) is inserted into the external os of the cervix, carefully guiding the central part of the device along the axis of the cervical canal. Next, its tip is rotated 360° (clockwise), thereby obtaining a sufficient number of cells from the ectocervix and from the transformation zone. The instrument is inserted very carefully, trying not to damage the cervix. Then the brush (spatula) is removed from the canal.
What does a smear for cytology mean?
Cytology is a painless and completely safe test that takes little time. Material for research is collected in a gynecological office.
Cytological examination of scrapings of the cervix, cervical canal, vagina with Papanicolaou staining in Moscow from 2 days
from 250 ₽
Sign up
Cytological examination of material obtained during separate diagnostic curettage in Moscow from the 2nd day.
from 600 ₽
Sign up
Cytological examination of smears from the surface of the cervix and cervical canal in Moscow from 3 days of age.
from 1850 ₽
Sign up
The patient sits down on a chair, after which the doctor, using a mirror and a special brush, collects epithelial cells from the cervix. The material is transferred to glass, stained and examined under a microscope.
There are no clear recommendations on the frequency of this procedure. Most experts say that it is advisable to take a smear every year, starting at the age of 18. Some gynecologists say that the study should be performed every 3 years for women over 25 years of age. There are also a number of gynecologists who claim that the test can be performed once every 5 years, provided that the previous tests were good.
The test should also be performed on women who have not yet had sexual intercourse. The issue is the heredity of cancer. Of course, most cases of cancer are associated with the human papillomavirus, which is often transmitted during sexual intercourse. Therefore, the risk of cervical cancer in a virgin is small, but it still exists. You should not refuse to undergo the procedure.
With the help of the study, it is possible to identify a precancerous condition at an early stage, even before the woman develops any symptoms.
Cytological examination is also used to determine other pathologies of the cervix, for example, erosion and inflammation. In addition, this analysis also shows the presence of human papillomavirus. It is the main factor in the occurrence of oncology, although the type of virus is determined only by genetic analysis.
Preparation of drugs
Transfer of the sample to a glass slide (traditional smear) should occur quickly, without drying out or losing mucus and cells adhering to the instrument. Be sure to transfer the material to the glass on both sides with a spatula or brush.
If it is intended to prepare a thin-layer preparation using the liquid-based cytology method, the brush head is disconnected from the handle and placed in a container with a stabilizing solution.
Fixation of strokes
performed depending on the intended staining method.
Papanicolaou and hematoxylin-eosin staining are the most informative in assessing changes in the cervical epithelium; any modification of the Romanovsky method is somewhat inferior to these methods, however, with experience, it allows one to correctly assess the nature of the pathological processes in the epithelium and the microflora.
The cellular composition of smears is represented by desquamated cells located on the surface of the epithelial layer. When adequate material is obtained from the surface of the mucous membrane of the cervix and from the cervical canal, the cells of the vaginal portion of the cervix (stratified squamous non-keratinizing epithelium), the junction or transformation zone (cylindrical and, in the presence of squamous metaplasia, metaplastic epithelium) and cells of the cervical canal enter the smear. columnar epithelium). Conventionally, cells of multilayered squamous non-keratinizing epithelium are usually divided into four types: superficial, intermediate, parabasal, basal. The better the epithelium’s ability to mature, the more mature cells appear in the smear. With atrophic changes, less mature cells are located on the surface of the epithelial layer.
Types of tests
Currently, two research methods are used. The main difference between them is the different efficiency.
PAP test
A PAP test is a procedure for examining biological material taken from the external uterine os and directly from the cervix. The material is stained according to the method developed by Papanicolaou. If the analysis methodology is followed, the PAP test has high reliability indicators. With its help, you can detect precancerous conditions and atypical cells in the taken material.
Often, biological material is examined that was obtained using a special cytobrush and fixed on glass with alcohol.
Such a study, in addition to precancerous conditions, determines the presence of infection.
Liquid cytology
Liquid cytology is a relatively new method for diagnosing cervical cancer. The main difference between this analysis and the traditional one is that with liquid cytology, biomaterial is collected on a liquid basis. It is not transferred to glass and is not smeared.
It has been proven many times that liquid-based cytology is much more accurate and sensitive than the Pap test. The main advantage of the liquid-based method is that it allows many more cells to be collected for further microscopic analysis. In addition, the method involves using the same material for other tests, for example, genetic testing of the human papillomavirus.
Interpretation of cytological examination results
The most common at present is the Bethesda classification (The Bethesda System), developed in the USA in 1988, to which several changes have been made. The classification was created to more effectively transfer information from the laboratory to clinical doctors and ensure standardization of treatment of diagnosed disorders, as well as follow-up of patients.
The Bethesda classification distinguishes squamous intraepithelial lesions of low grade and high grade (LSIL and HSIL) and invasive cancer. Low-grade squamous intraepithelial lesions include changes associated with human papillomavirus infection and mild dysplasia (CIN I), high-grade - moderate dysplasia (CIN II), severe dysplasia (CIN III) and intraepithelial carcinoma (cr in situ). This classification also contains indications of specific infectious agents that cause sexually transmitted diseases.
To designate cellular changes that are difficult to differentiate between reactive states and dysplasia, the term ASCUS - atypical squamous cells of undetermined significance (squamous epithelial cells with atypia of unclear significance) has been proposed. For a clinician, this term is not very informative, but it directs the doctor to the fact that this patient needs examination and/or dynamic monitoring. The Bethesda classification has now also introduced the term NILM – no intraepithelial lesion or malignancy, which combines normal, benign changes, and reactive changes.
Since these classifications are used in the practice of a cytologist, below are parallels between the Bethesda classification and the classification common in Russia (Table 22). Cytological standardized report on material from the cervix (form No. 446/u), approved by order of the Ministry of Health of Russia dated April 24, 2003 No. 174.
The reasons for receiving defective material are different, so the cytologist lists the types of cells found in the smears and, if possible, indicates the reason why the material was considered defective.
Cytological changes in the glandular epithelium
| Bethesda Terminology developed in Bethesda (USA, 2001) | Terminology adopted in Russia |
| ASSESSMENT OF SWIM QUALITY | |
| Full material | The material is adequate (a description of the cellular composition of the smear is given) |
| The material is not complete enough | The material is not adequate (a description of the cellular composition of the smear is given) |
| Unsatisfactory for evaluation | Cellular composition is not enough to confidently judge the nature of the process |
| Satisfactory to evaluate, but limited by something (identify reason) | |
| Within normal limits Metaplasia (normal) | Cytogram without features (within normal limits) - for reproductive age Cytogram with age-related changes in the mucous membrane: - atrophic type of smear - atrophic type of smear with leukocyte reaction Estrogenic type of smear in a postmenopausal woman Atrophic type of smear in a woman of reproductive age |
| BENIGN CELL CHANGES | |
| Infections | |
| Trichomonas vaginalis | Trichomonas colpitis |
| Fungi morphologically similar to the genus Candida | Elements of Candida fungus detected |
| Cocci, gonococci | Diplococci located intracellularly were found |
| Predominance of coccobacillary flora | Flora coccobacillary, possibly bacterial vaginosis |
| Bacteria morphologically similar to Actinomyces | Flora of the Actinomycetes type |
| Other | Flora of the type Leptotrichia |
| Flora – small sticks | |
| Flora – mixed | |
| Cellular changes associated with Herpes simplex virus | Epithelium with changes associated with Herpes simplex |
| Possibly chlamydial infection | |
| Reactive Changes | |
| Inflammatory (including reparative) | The changes found correspond to inflammation with reactive changes in the epithelium: degenerative, reparative changes, inflammatory atypia, squamous metaplasia, hyperkeratosis, parakeratosis, and/or others. |
| Atrophy with inflammation (atrophic | Atrophic colpitis Atrophic type of smear, leukocyte reaction Mucosal epithelium with hyperkeratosis Mucosal epithelium with parakeratosis Mucosal epithelium with dyskeratosis Reserve cell hyperplasia Squamous metaplasia Squamous metaplasia with atypia |
| Radiation changes | Epithelium of the mucous membrane with radiation changes |
| Changes associated with the use of intrauterine contraceptives | |
| PATHOLOGICAL CHANGES IN THE FLAT EPITHELIUM | |
| Squamous epithelial cells with atypia of undetermined significance (ASC-US*) Squamous epithelial cells with atypia of undetermined significance not excluding HSIL (ASC-H) | The changes found are difficult to differentiate between reactive changes in the epithelium and dysplasia. Cells were found that were difficult to interpret (with dyskaryosis, enlarged nuclei, hyperchromic nuclei, etc.) |
| Changes in squamous epithelium (non-tumor, but worthy of dynamic observation) | |
| Low grade squamous intraepithelial lesion (LSIL): human papillomavirus infection, mild dysplasia (CIN I) | Epithelium of the mucous membrane with signs of papillomavirus infection. The changes found may correspond to mild dysplasia. |
| High-grade squamous intraepithelial lesion (HSIL): moderate, severe dysplasia and intraepithelial carcinoma (CINII, CIN III) | The changes found correspond to moderate dysplasia. The changes found correspond to severe dysplasia. The changes found are suspicious for the presence of intraepithelial cancer. |
| Invasive cancer | |
| Squamous cell carcinoma | Squamous cell carcinoma Squamous cell carcinoma with keratinization Small cell squamous cell carcinoma |
| Glandular hyperplasia The changes found correspond to endocervicosis | |
| Atypical glandular epithelial cells (possible assumptions): – Unclear significance (AGUS); – suspicious for neoplasia; – endocervical adenocarcinoma in situ (AIS); – adenocarcinoma | Glandular hyperplasia with atypia of the dysplasia type (I, II, III) Adenocarcinoma |
| Endometrial cells are cytologically benign (in a woman in menopause, etc.) | |
| Endometrial adenocarcinoma Adenocarcinoma of another location Adenocarcinoma without additional characteristics | Adenocarcinoma, possibly endometrial adenocarcinoma Adenocarcinoma NOS (not otherwise specified) |
| Other malignant tumors (if possible, determine the nosological form) | |
| Assessment of hormonal status | |
* whenever possible, ASCUS should be defined as similar to reactive, reparative or precancerous processes;
** changes associated with exposure to human papillomavirus, previously designated as koilocytosis, koilocytic atypia, condylomatous atypia, are included in the category of mild changes in squamous epithelial cells;
*** If possible, it should be noted whether the changes relate to CIN II, CIN III, whether there are signs of cr in situ;
****hormonal assessment (carried out only on vaginal smears): – the hormonal type of smear corresponds to age and clinical data; – the hormonal type of smear does not correspond to age and clinical data: (decipher); – hormonal assessment is impossible due to: (specify the reason).
Who is the study for?
Cervical cytology is necessary if:
- A woman is planning to get pregnant.
- Childbirth occurred several times in a row (for example, 3 times in 4–5 years).
- The first time a woman gave birth was at an early age.
- Frequent change of sexual partners.
- It is planned to install a spiral.
- For a long time, no cytological examination was carried out or it never happened at all.
- The results of the latest study are poor.
- Diseases associated with the human immunodeficiency virus (HIV) have been discovered.
- One of the relatives suffered from cancer, that is, the family history is burdened.
In general, it is advisable for every woman to undergo a screening cytological examination every year. If there are significant deviations from the norm, then the procedure must be completed every 6 months.
There are also contraindications to the study:
- Active inflammatory process in the vagina and directly on the cervix.
- Menstruation.
Although this procedure is completely harmless and painless, it is not recommended if these factors are present. During inflammation or infection, the number of white blood cells increases, which can greatly affect the accuracy of the results.
Integration of various laboratory methods
In the diagnosis of cervical diseases, clinical data and microflora test results (classical microbiological (culture), ANC methods (PCR, RT-PCR, Hybrid Capture, NASBA, etc.) are important).
If it is necessary to clarify the pathological process (ASC-US, ASC-H), cytological examination is, if possible, supplemented with molecular biological ones (p16, oncogenes, methylated DNA, etc.).
HPV detection tests have low prognostic significance, especially in young women (under 30 years of age), due to the fact that in most patients in this age group, HPV infection is transient. However, despite the low specificity of the test for intraepithelial tumors and cancer, it can be used as a screening test in women under 30 years of age, followed by cytological examination. Sensitivity and specificity increase significantly with the combined use of the cytological method and research to detect HPV, especially in patients with questionable cytological data. This test is important in the management of patients with ASC-US, during follow-up to determine the risk of relapse or progression of the disease (CIN II, CIN III, carcinoma in situ, invasive cancer).
Possibilities for early diagnosis of cervical cancer
The article identifies problems in organizing the prevention of cervical cancer and discusses the diagnostic algorithm for cervical diseases: standardized sampling of cellular material from the cervix; application of liquid-based cytology; immunocytochemical study of tumor marker p16. An algorithm for the actions of a gynecologist is proposed.
1. Relevance of the problem
Cervical cancer is a malignant tumor, the etiological factor of which is human papillomaviruses (HPV) serotypes No. 16, 18, 33, 35, etc. According to the Global Cancer Incidence Database - GLOBOCAN (GLOBOCAN 2002 Database Jun. 2006), the frequency of newly diagnosed cases of cervical cancer in the Russian Federation are 16.2 per 100 thousand population per year, while mortality from this tumor is 10.1 per 100 thousand population per year. The organization of cytological screening has significantly reduced the incidence and mortality from cervical cancer. However, a number have been preserved:
1. Lack of standardized sampling of material - inadequately taken material from the cervix leads to false-negative results;
2. The problem of false-positive cytological conclusions (when a smear shows dysplasia where it actually does not exist) does not discredit the idea of cytological screening in general, because in most cases, it is resolved with relatively minimally invasive interventions (cervical biopsy, repeat cytology after trial treatment). However, in each specific case, this problem creates a number of inconveniences for the doctor and the patient:
-need to perform a biopsy (cost, invasion, time)
-the need for repeated visits to the doctor
-negative psychological impact on the patient associated with information about the possibility of developing cancer
3. Even if the diagnosis of dysplasia is verified histologically, the probability of dysplasia turning into cancer is less than 50% (Hanson K.P., Imenitov E.N. Practical Oncology 2002). However, both at the clinical and microscopic level, there are currently no reliable prognostic criteria for the further biological behavior of dysplasia (Nasser SM, Cibas ES, Crum C, et al. Cancer 2003).
4. If a consensus in treatment tactics in relation to severe dysplasia and Ca in situ has already been reached, then with moderate and mild dysplasia much depends on the opinion of the attending physician. He must compare the cytological conclusion, the clinical and colposcopic picture, the patient’s age, etc. and make a decision (ranging from observation without treatment to cone excision of the cervix). At the same time, the doctor often faces a difficult choice, because aggressive tactics are not always justified (especially in young patients), and an unreasonably chosen wait-and-see strategy threatens the development of cancer. The situation is aggravated by the fact that on this issue there is neither unity of opinion among doctors nor certain generally accepted standards (Ashrafyan L.A., Kharchenko N.V., Ogryzkova V.L., Antonova I.B. Practical Oncology 2002).
5. The problem of false-negative cytological conclusions in an established cytological screening system is leveled by the annual repetition of the cytological smear. However, with a one-time cytological examination (when the patient has not previously been screened), in some cases additional guarantees of the absence of a neoplastic process are required.
6. The problem of false-positive and false-negative cytological conclusions is particularly acute in relation to glandular epithelial cells. While cervical adenocarcinoma may not appear at all on a cytological smear, the cause of pronounced changes in glandular epithelial cells (even with a cytological picture of adenocarcinoma) may be reactive processes or endometriosis (Krane JF, Granter SR Cancer 2001).
7. There is no consensus among experts regarding tactics for patients who are infected with HPV strains of high oncogenic risk, but there is no dysplasia in the cytological smear. The opinion that it is necessary to treat all carriers and contact persons has already been discredited today, because most carriers will never get cervical cancer.
To resolve these problems, the following diagnostic algorithm for cervical diseases is proposed:
1. Standardized collection of cellular material from the cervix;
2. Application of liquid cytology;
3. Immunocytochemical study of the p16ink4α tumor marker.
Each of these points is discussed on subsequent pages of the article.
2. Material collection
In the absence of visible pathology, a scraping must be taken from the cervix for cytological examination.
Given the absence of visible pathology, material is taken from the entire surface of the cervix and from the cervical canal. Due to the fact that cancer most often develops at the junction of the squamous epithelium of the cervix with the columnar epithelium of the cervical canal, along the perimeter of the uterine pharynx (the so-called transformation zone), a scraping for collecting material during screening must necessarily include this zone and the epithelium of the cervical canal.
Of fundamental importance in this case is the use of a special tool that guarantees the collection of material from all specified areas and ensures that the preparation is informative. The use of adapted tools and means is unacceptable, as this leads to a decrease in the effectiveness of screening, up to zero results.
3. Liquid cytology
More than fifty years ago, Dr. Papanicolaou, after whom the method (Pap test) was later named, developed a method for cytological examination of cervical canal cells using a smear on glass. The Pap test is widely used in screening studies to diagnose cervical cancer, precancerous and background conditions. In northern European countries, where screening studies are well organized, treatment of precancerous conditions and early forms of cancer has reduced mortality from invasive cervical carcinoma by 50 - 70%. According to published data, in Finland in 1992 the incidence was reduced to 2.8 cases per 100,000 women. In Russia in 1998, 11,937 new cases of the disease were identified, which was 15.3 per 100,000 women, of which 6,078 died (Syrjänen S., Shabalova I., Petrovichev N. et al. J Lower Genital Tract Disease 2002). In England, according to data for 1987/88. the incidence was 9 per 100,000 women, mortality in 1997 was 3.7 per 100,000. At the same time, about 4 million women per year underwent cytological screening examination of cervical smears (Payne N, Chilcott J, McGoogan E. University of Sheffield 2000.) . Currently, identifying a risk group through cytological examination of cervical smears during preventive examinations and further observation makes it possible to catch the process of malignancy at the earliest stages.
A necessary prerequisite for accurate assessment of cell morphology in a smear is a properly made and well-stained smear. The effectiveness of cytological diagnostics is largely determined by the quality of preparation of preparations. Currently, a new technology for the preparation of cytopreparations, the so-called liquid cytology, is becoming increasingly widespread. An important technological feature of the liquid cytology (LC) method, which improves the quality of the study, is that the material being studied is taken into a special stabilizing solution, which ensures its preservation without destruction or loss of cells. In this case, all cellular material retains its morphological and immunocytochemical properties without changes. Several systems are known for the life cycle method: Cytospin, AutoCytePrep (CytoRich), LABONORD Easy Prep, Thin Prep.
Over the past five years, many studies have been conducted in different countries that compared the effectiveness of traditional techniques and life cycle, using histological examination as the “gold standard” and evaluation of cytospecimens according to the TBS (The Bethesda System) classification to confirm diagnoses. The study involved both outpatient and inpatient departments, examining thousands of patients (Grace A et al. Cytopathology 2002.). According to generalized data, the sensitivity of the traditional method fluctuates in a wide range of values: 34.5 - 89%; and for the life cycle method it has more stable values: 71.4 - 95%. Accordingly, the specificity of the methods was: 36 - 76.9% and 58 - 76.2%. The authors of the studies summarized that the LC method is a more reliable laboratory test, reduces the number of false negative results, reduces the number of drugs unsatisfactory for analysis and the time required for a cytologist to evaluate the drug.
A study of Pap smears obtained by the traditional method of collecting material shows that not all, but only from 6.5 to 18% of the cells taken are applied to the smear. In addition, many of these cells are difficult or impossible to analyze as a result of poor application. There is no doubt that the traditional method of cytological smear analysis has high diagnostic efficiency. However, there is an opinion that the method has from 6 to 55% false negative results. According to Fahey's review, the sensitivity of the traditional method is 55-65%, and the specificity is 65-70% (Fahey MT, Irwig L, Macaskill P. American Journal of Epidemiology 1995).
Figure 1. Smears obtained G6 - in the traditional way, P20 - using liquid cytology.
The cause of the majority (about 2/3) of false results, which reduces the quality of laboratory work, is an error in taking or transferring material for preparing smears. Material for cytological examination is obtained from the surface of the mucous membrane. The mucus present in the taken material prevents the cells from being transferred to the smear, and the material cannot be mixed evenly. The material may be unevenly distributed on the glass. A smear that is too thick results in incomplete material. When transferring material onto glass in the traditional way, cells from an entire region of the cervix may not be included in the preparation. Drying and loss of cells adhering to the instrument significantly reduces the diagnostic information value of microslides (Fig. 2). The life cycle method allows you to exclude these negative factors (Amelyushkina V.A. Laboratory Medicine 2002).
Figure 2. Smears obtained traditionally (a) and using liquid-based cytology (b).
However, routine smear staining gives us information about the current state of the cervix, but it is not able to assess the oncogenic potential of changes in cases where cancer is not detected. Thus, the use of cytology alone is not sufficient for screening for cervical cancer.
The advantage of liquid cytology is that it can produce up to 5-6 “serial” (that is, identical in cellular composition) smears from one sample of material. This makes it possible to use additional research methods. For a long time, this additional research method was used to determine the DNA of the human papillomavirus of high oncogenic risk. However, it is now known that screening for HPV infection in age-appropriate women can identify patients at low risk of developing cervical cancer if the test is negative, but HPV PCR is not useful in identifying patients at high risk of progression. To achieve this goal, we need a test that allows us to detect the manifestation of the oncogenic potential of the virus - thus, our attention must be transferred from the agent to the host. (Miller AB. Lancet 2001).
4. Immunocytochemical study of tumor marker p16 The normal cell cycle consists of G1, S, G2 and M phases. The cervical epithelium is a dynamic tissue with constant cellular renewal. The kinase that ensures the cell passes from G1 to the S phase of the cell cycle is E2F. Normally, it is inactive, being bound to the suppressor protein Rb (product of the retinoblastoma gene) (Fig. 3).
Figure 3. Normal cell cycle.
The p16(INK4a) protein controls the uncoupling of the E2F-Rb complex, preventing uncontrolled cell proliferation. However, the synthesis of p16(INK4a) is normally inhibited by a feedback mechanism, thus the concentration of this protein in a normal cell is extremely low, which is manifested by a negative immunocytochemical reaction (Fig. 4).
Figure 4. Negative reaction to p16ink4α in normal squamous epithelium of the cervix
The E7 protein of the human papillomavirus HPV of high oncogenic risk, when interacting with the retinoblastoma gene product, leads to the uncoupling of the E2F-Rb complex.
Figure 5. Oncogenic effect of E7 protein on the cell cycle, leading to the synthesis of p16 ink4α. E2F remains constantly active, stimulating uncontrolled cell proliferation. P16(INK4a) tries to restrain cell proliferation, which leads to its uncontrolled synthesis (Fig. 5). However, p16 is deprived of its target, which, in the absence of feedback, significantly increases its concentration in the cell. Immunocytochemically, this is manifested by a positive reaction (Fig. 6), which is a biomarker of the initiation of carcinogenesis in the cervical epithelium (Nieh S., Chen SF, Chu TY et al. Gynecol Oncol. 2003).
Figure 6. Positive reaction for p16ink4α (brown nuclear staining) in dysplastic cervical epithelium.
It has been reliably shown that p16 expression is associated with low, moderate and severe dysplasia (with intraepithelial squamous cell lesions of the cervix of both high and low Grade, LSIL and HSIL, according to the WHO classification), and p16 expression was not found in squamous epithelium without signs of dysplasia ( Bibbo M et al Acta Cytol 2002). At the same time, although there was a clear correlation between the detection of human papillomavirus DNA and dysplasias, there was also a large number of PCR-positive cases for HPV (with episomal localization of HPV), in which, upon subsequent histological examination, there was no precancer or cancer (Nieh S., Chen SF, Chu TY et al Gynecol Oncol. 2005). This phenomenon was confirmed by a negative immunocytochemical reaction for p16ink4α in dysplastic cells (Fig. 7).
Figure 7. Negative immunocytochemical reaction to p16ink4α in dysplastic cells (indicated by an arrow).
Thus, immunocytochemical determination of p16 expression on smears prepared using liquid-based cytology can allow:
1. Reliably assess the potential of dysplasia in relation to the development of cervical cancer and, accordingly, choose more conservative or more aggressive treatment tactics;
2. Clarify the cytologist’s conclusions. From our point of view, all cases of atypical cytology (except for invasive cervical cancer), indeterminate cytological findings (atypical squamous epithelial cells of unknown significance - ASCUS) and all changes in the glandular epithelium (Murphy N., Heffron B., King B. et al. Virchows Arch 2004);
3. Resolve controversial issues when a gynecologist identifies a highly abnormal colposcopic picture that is not accompanied by changes in the cytological smear;
4. In many cases it is reasonable to refuse a biopsy;
5. In the majority of elderly patients with a cytological picture of dysplasia against the background of atrophic cervicitis, it is reasonable to choose a wait-and-see approach, refusing not only treatment, but also biopsies, and increasing the intervals between clinical examinations. In a small number of p16-positive patients in this age group, on the contrary, it is reasonable to choose a more active tactic;
6. Determine individual tactics for patients infected with strains of human papillomavirus of high oncogenic potential, if they do not have cytological and colposcopic changes. In women with positive PCR for HPV, select a group of patients in whom carcinogenesis has already been initiated in the epithelium of the cervix, and direct the necessary therapeutic effects to them. Accordingly, further monitoring is necessary in the larger group of p16 negative women;
7. Improve monitoring of patients who have undergone organ-preserving treatment for “severe dysplasia” or “carcinoma in situ” by adding p16 determination to routine cytological examination. This will allow patients at greatest risk to increase the sensitivity of cytological examination and test for persistent human papillomavirus infection before the appearance of morphological changes.
5. Algorithm of actions of a gynecologist
1. collecting material using a disposable cervical brush (cervix-brush, Fig. 8): it is inserted into the vagina under eye control. The cone of the brush is carefully guided into the cervical canal. After inserting the cone into it, the brush is pressed against the surface of the neck and 5 full circular rotations are performed - three clockwise and two counterclockwise. Before carrying out these manipulations, it is necessary to thoroughly clean the cervix of mucus. The brush with the material must be lowered into a container with a liquid transport medium and rinsed thoroughly there. Liquid medium and cell medium should be stored in a refrigerator at a temperature of +2 to +8 °C. The cell suspension is stable for 10 days.
Figure 8. Cervical brush.
2. The laboratory stage takes from 3 to 5 days and includes centrifugation of the medium to obtain monolayer smears, routine Romanovsky-Giemsa staining, and immunocytochemical study for p16ink4a.
3 . Interpretation of results . The conclusion includes two subsections: liquid-based cytology and immunocytochemical study for p16ink4a. The formulation of the liquid cytology conclusion does not differ from the traditional one, however, its interpretation should be carried out depending on the identification of the p16ink4a tumor marker. This especially applies to cervical dysplasia.
— If a positive reaction to the p16ink4a tumor marker is detected, the patient should undergo surgical treatment to the extent of conization of the cervix. We recommend that this treatment be carried out in specialized oncological institutions.
— If a negative reaction to the tumor marker p16ink4a is detected, then conservative treatment is carried out locally.
L.I. Maltseva, G.A. Raskin, L.N. Farrakhova, A.V. Akhmetzyanova, V.S. Petrov, R.Sh. Khasanov Kazan State Medical Academy
Kazan State Medical University
Clinical Oncology Dispensary of the Ministry of Health of the Republic of Tajikistan
Literature: 1. Ashrafyan L.A., Kharchenko N.V., Ogryzkova V.L., Antonova I.B. Principles of treatment of premicroinvasive cervical cancer.// Practical Oncology. - 2002. - Volume 3. - P. 173-178;17
2. Hanson K.P., Imyanitov E.N. Modern ideas about the carcinogenesis of cervical cancer.// Practical Oncology - 2002. - Volume 3. - P. 145-155; 18
3. Bibbo M., Klump WJ, DeCecco J., Kovatish AJ Procedure for immunocytochemical detection of p16INK4A antigen in thin-layer, liquid-based specimens. // Acta Cytol. - 2002. - V.46 - P. 25-29;15
4. Cripe TP, Haugen TH, Turk JP et al. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. // EMBO J. - 1987. - V. 6(12) - P.3745-3753;2
5. GLOBOCANDatabase Jun. 2006. https://www.dep.iarc.fr/globocan/globocan.htm; 3
6. Jenison SA, Yu XP, Valentine JM, Galloway DA Characterization of human antibody-reactive epitopes encoded by human papillomavirus types 16 and 18. // J Virol. 1991 - V.65(3) - P. 1208-1218;1
7. Klaes R., Froedrich T., Spitkovsky D. et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri.// Int J Cancer. 2001 - V.92(2) - P.276-84;4
8. Meyer JL, Hanlon DW, Andersen BT et al. Evaluation of p16INK4a expression in ThinPrep cervical specimens with the CINtec p16INK4a assay: correlation with biopsy follow-up results.// Cancer. - 2007. - V. 111(2). — P. 83-92;20
9. Murphy N., Heffron B., King B. et al. P16INK4A positivity in benign, premalignant and malignant cervical glandular lesions: a potential diagnostic problem.// Virchows Arch. - 2004. - V. 445 - P.610-615;12
10. Nasser SM, Cibas ES, Crum C. et al. The significance of the Papanicolaou smear diagnosis of low-grade squamous intraepithelial lesion cannot exclude high-grade squamous intraepithelial lesion.// Cancer. - 2003. - V.99. — P.272-276;16
11. Negri G., Egarter-Vigl E., Kasal A. et al. p16INK4A is a useful marker for the diagnosis of adenocarcinoma of the cervix uteri and its precursors.// Am J Surg Pathol. - 2003. - V. 27 - P.187-193;13
12. Nieh S, Chen SF, Chu TY et al. Expression of p16 INK4A in Papanicolaou smears containing atypical squamous cells of undetermined significance from the uterine cervix. // Gynecol Oncol. - 2003. - V.91(1) - P.201-8;5
13. Nieh S, Chen SF, Chu TY et al. Is p16(INK4A) expression more useful than human papillomavirus test to determine the outcome of atypical squamous cells of undetermined significance-categorized Pap smear? A comparative analysis using abnormal cervical smears with follow-up biopsies.// Gynecol Oncol. 2005 - V.99(3) - P.578-84;6
14. Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression.// Exp Cell Res. - 2001. - V.264 - P.42-55; 7
15. Ronco G., Cuzick J., Pierotti P. et al. Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening: randomized controlled trial. BMJ. - 2007. - V. 335(7609). — P. 28 — 38;19
16. Sano T., Oyama T., Kashiwabara K., Fukuda T., Nakajima T. Immunohistochemical overexpression of p16 protein associated with intact retinoblastoma protein expression in cervical cancer and cervical intraepithelial neoplasia. // Pathol Int. - 1998. - V.48 - P.580-5;8
17. Ylitalo N., Josefsson A., Melbye M. et al. A Prospective Study Showing Long-Term Infection with Human Papillomavirus 16 before the Development of Cervical Carcinoma in situ. // Cancer Research. - 2000. - V. 60 - P. 6027-6032;14