Pulmonary embolism (PE) - symptoms and treatment
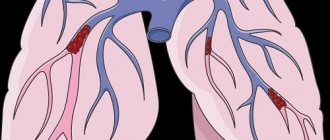
Pulmonary embolism (PE) is a blockage of the pulmonary arteries with blood clots of various natures, most often formed in the large veins of the lower extremities or pelvis.
Risk factors for pulmonary embolism include pathological conditions in which there is impaired venous blood return, endothelial damage or endothelial dysfunction, and hypercoagulability disorders.
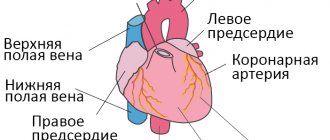
As a result of thromboembolism of the pulmonary arteries, the blood supply to the lung tissue stops, necrosis (tissue death) develops, infarction-pneumonia, and respiratory failure occur. The load on the right parts of the heart increases, right ventricular circulatory failure develops: cyanosis (blue discoloration of the skin), edema in the lower extremities, ascites (fluid accumulation in the abdominal cavity). The disease may develop acutely or gradually over several hours or days. In severe cases, the development of pulmonary embolism occurs rapidly and can lead to a sharp deterioration of the patient’s condition and death.
Every year, 0.1% of the world's population dies from pulmonary embolism. In terms of the frequency of deaths, the disease is second only to IHD (coronary heart disease) and stroke. More patients with pulmonary embolism die than patients with AIDS, breast and prostate cancer, and victims of road accidents combined. The majority of patients (90%) who died from pulmonary embolism were not correctly diagnosed in time and did not receive the necessary treatment. PE often occurs where it is not expected - in patients with non-cardiological diseases (trauma, childbirth), complicating their course. The mortality rate for pulmonary embolism reaches 30%. With timely, optimal treatment, mortality can be reduced to 2-8%.[2]
Symptoms of pulmonary embolism depend on the size of the blood clots, the suddenness or gradual onset of symptoms, and the duration of the disease. The course can be very different - from asymptomatic to rapidly progressing, up to sudden death.
PE is a ghost disease that wears the mask of other heart or lung diseases. The clinic may be infarct-like, reminiscent of bronchial asthma, acute pneumonia. Sometimes the first manifestation of the disease is right ventricular circulatory failure. The main difference is the sudden onset in the absence of other visible reasons for the increase in shortness of breath.
Etiology
PE usually develops as a result of deep vein thrombosis, which usually develops 3-5 days before the onset of the disease, especially in the absence of anticoagulant therapy.
Risk factors for pulmonary embolism
When diagnosing, the presence of risk factors for thromboembolism is taken into account. The most significant of them are: fracture of the femoral neck or limb, replacement of the hip or knee joint, major surgery, trauma or brain damage.
Dangerous (but not so strong) factors include: knee arthroscopy, central venous catheter, chemotherapy, chronic heart failure, hormone replacement therapy, malignant tumors, oral contraceptives, stroke, pregnancy, childbirth, the postpartum period, thrombophilia. In malignant neoplasms, the incidence of venous thromboembolism is 15% and is the second leading cause of death in this group of patients. Chemotherapy treatment increases the risk of venous thromboembolism by 47%. Unprovoked venous thromboembolism may be an early manifestation of malignancy, which is diagnosed within a year in 10% of patients with an episode of PE.[2]
The safest, but still risky, factors include all conditions associated with prolonged immobilization (immobility) - prolonged (more than three days) bed rest, air travel, old age, varicose veins, laparoscopic interventions.[3]
Some risk factors are common to arterial thrombosis. These are the same risk factors for complications of atherosclerosis and hypertension: smoking, obesity, sedentary lifestyle, as well as diabetes, hypercholesterolemia, psychological stress, low consumption of vegetables, fruits, fish, low level of physical activity.
The older the patient, the more likely it is to develop the disease.
Finally, today the existence of a genetic predisposition to pulmonary embolism has been proven. The heterozygous form of factor V polymorphism increases the risk of initial venous thromboembolism by three times, and the homozygous form by 15-20 times.
The most significant risk factors contributing to the development of aggressive thrombophilia include antiphospholipid syndrome with an increase in anticardiolipin antibodies and a deficiency of natural anticoagulants: protein C, protein S and antithrombin III.
Statistics on morbidity and mortality of pulmonary embolism
Statistics show that about 0.1% of the world's population dies every year from pulmonary embolism. In terms of the frequency of deaths, this pathology is second only to coronary heart disease, strokes and some cancers.
The main cause of mortality in pulmonary embolism is late diagnosis. About 90% of patients do not receive the necessary treatment in the early stages of this acute condition.
The difficulty in diagnosing thromboembolism is that this condition can masquerade as a large number of heart and lung diseases. Many patients begin receiving therapy for heart attack or asthma. As a result, valuable time is lost.
Another danger of pulmonary embolism is suddenness. It affects not only patients with cardiovascular diseases, but also women during childbirth, and practically healthy people after injuries. Most often, after a diagnosis of pulmonary embolism is made, only 70% of patients can be saved. But if the pathology was diagnosed in a timely manner and optimal treatment was started, then this figure can increase to 98% percent.
Establishment of clinical class and verification of diagnosis
Pulmonary function tests can identify obstructive or restrictive changes for the purpose of differential diagnosis of PH and clarify the severity of lung damage. Patients are characterized by a decrease in the diffusion capacity of the lungs for carbon monoxide (40-80% of normal), a slight or moderate decrease in lung volumes, a normal or slightly decreased PaO2, and usually a decreased PaCO2 due to alveolar hyperventilation.
Ventilation-perfusion lung scintigraphy is a screening method to exclude chronic thromboembolism as a cause of pulmonary hypertension. In patients after thromboembolism, perfusion defects are found in the lobar and segmental zones in the absence of ventilation disturbances. Perfusion scintigraphy has historically become one of the first methods for detecting perfusion defects in the pulmonary parenchyma in pulmonary embolism. The images obtained in acute PE and CTEPH differ significantly. Perfusion defects in acute PE are more clearly defined and contrast sharply with normally functioning tissue. In CTEPH, perfusion defects are not clearly defined and often do not correspond to the blood supply area of the large pulmonary artery.
Computed tomography and CT angiopulmonography
The CT picture of chronic thromboembolism can be represented by occlusions and stenoses of the pulmonary arteries, eccentric filling defects due to the presence of blood clots, including recanalized ones. CT angiopulmonography is performed on spiral computed tomographs during the phase of passage of the contrast agent through the pulmonary arterial bed. Among the methodological features, it should be noted that the study must be carried out using at least an 8-spiral tomograph, with a minimum step (no more than 3 mm) and a slice thickness (no more than 1 mm). A thorough scan should cover both lungs completely, from the apices to the phrenic sinuses. Contrast enhancement of the right chambers of the heart and pulmonary arteries should match or exceed the degree of contrast enhancement of the left chambers of the heart and aorta. The second, arterial phase of scanning is recommended for all patients over 40 years of age, especially if there is evidence of arterial thrombosis and coronary artery disease in the anamnesis. Modern software allows you to reconstruct images of the pulmonary arteries in any plane, construct maximum intensity projections and three-dimensional images. In most cases, to clarify the nature of the lesion, it is enough to analyze cross sections using an image viewer, which makes it possible to determine the presence of changes not only in the lobar and segmental branches, but also in a number of subsegmental arteries. Pathological changes, in addition to the presence of “old” thrombotic material, may include local thickening of the vessel wall, narrowing at the mouth of the vessels and along them, occlusions, intravascular structures in the form of membranes and bridges. If changes are detected in several branches of the pulmonary arteries, we can conclude that there is a high probability of thromboembolic nature of PH. It is important to note that the resolution of modern CT scanners is limited and does not allow the determination of very thin membranous and stringy structures in the lumen of the LA, especially if the size of the object does not exceed 2-3 mm. In some cases, calcification of the “old” thrombotic material develops, and CT can be invaluable in determining the location of the calcification. CT makes it possible to detect not only stenotic changes in the vessels of the lungs, but also disturbances in the perfusion of lung tissue by the nature of the contrast of the parenchyma. In some cases, parenchymal enhancement is so uneven that mosaic enhancement is detected on scans. A clearly defined mosaic pattern of segments usually indicates a good prognosis for surgical treatment. Contrasting exclusively in the root zones is not a true mosaic and is often observed in microvascular forms of PH. By providing detailed images of the lung parenchyma, CT allows the diagnosis of other lung diseases. In addition to the state of the arterial bed, CT can provide comprehensive information about all intrathoracic structures, which is important for confirming the diagnosis and developing a surgical treatment plan. Before performing the operation, the condition of the pulmonary parenchyma, bronchial tree, and pulmonary veins should be taken into account.
Angiographic diagnostics
The main objectives of angiographic diagnosis are to determine the severity of pulmonary hypertension, clarify the nature of the lesion in the pulmonary bed through angiopulmonography, and identify/exclude coronary disease. Carrying out catheterization in isolation without high-quality angiopulmonography in a patient with clear signs of CTEPH is inappropriate. This study should provide clear information to doctors to resolve the issue of the patient’s operability and the severity of his condition. Hemodynamic criteria for post-embolic pulmonary hypertension, identified during catheterization of the right heart, are: mean pulmonary artery pressure (PAP) above 25 mm Hg. Art., pulmonary artery wedge pressure (PAWP) ≤ 15 mm Hg. Art., PVR > 2 units. according to Wood (160 dyn. sec cm-5) in the presence of multiple stenotic and/or occlusive lesions of the branches of the pulmonary artery of various calibers.
Non-invasive diagnostics
Electrocardiography (ECG) reveals signs of hypertrophy and overload of the right ventricle, dilatation and hypertrophy of the right atrium (p-pulmonale), deviation of the electrical axis of the heart to the right.
Chest X-ray allows you to clarify the etiology of PH: identify interstitial lung diseases, acquired and congenital heart defects, and also judge the severity of PH. The main radiological signs of PH are bulging of the trunk and left branch of the pulmonary artery, which form the second arch in the direct projection along the left contour of the heart, expansion of the roots of the lungs, and enlargement of the right parts of the heart. In patients with post-embolic pulmonary hypertension, signs can be identified that indicate the presence of blood clots in the large branches of the pulmonary artery (PA) - dilation of the trunk and main branches of the PA, a symptom of deformation and shortening of the root. A specific symptom is the depletion of the pulmonary pattern in the area of impaired blood supply.
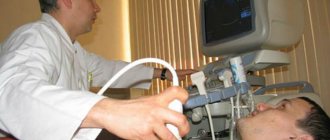
Echocardiography (EchoCG) - Ultrasound of the heart is considered the most valuable non-invasive method for diagnosing PH, as it not only allows one to assess the level of pressure in the pulmonary artery, but also provides important information about the cause and complications of PH. Using this method, it is possible to exclude damage to the mitral and aortic valves, myocardial disease, congenital heart defects with blood shunting, leading to the development of PH. Unfortunately, the method does not allow differentiating post-embolic pulmonary hypertension from other forms of precapillary PH. The exception is rare cases of the presence of massive thrombi in the trunk and main branches of the pulmonary artery in the immediate vicinity of the bifurcation.
Functional classification of pulmonary hypertension
| Class I | There is no restriction on physical activity. Ordinary physical activity does not cause shortness of breath, weakness, chest pain, or dizziness |
| Class II | some decrease in physical activity. Ordinary physical activity is accompanied by shortness of breath, weakness, chest pain, dizziness |
| Class III | severe limitation of physical activity. Little physical activity causes shortness of breath, weakness, chest pain, dizziness |
| Class IV | inability to perform any physical activity without the above clinical symptoms. Shortness of breath or weakness may be present even at rest, discomfort increases with minimal exertion |