Septic shock is a systemic pathological response to severe infection. It is characterized by fever, tachycardia, tachypnea, and leukocytosis when identifying the source of primary infection. In this case, microbiological blood testing often reveals bacteremia. In some patients with sepsis syndrome, bacteremia is not detected. When arterial hypotension and multiple systemic failure become components of the sepsis syndrome, the development of septic shock is stated.
Types of shock
| Hypovolemic | Cardiogenic | Redistributive |
1. Blood loss:
2. Plasma loss:
3. Fluid loss:
| 1. Acute myocardial infarction (true cardiogenic). 2. Heart rhythm disturbances. 3. Pulmonary embolism (obstructive) 4. Cardiac tamponade | 1. Anaphylactic shock. 2. Septic shock (infectious-toxic). 3. Spinal shock (neurogenic) |
Symptoms
Symptoms of shock:
- decreased blood pressure (may be completely absent);
- increased heart rate or, conversely, the pulse may become “soft”, weak and “thread-like”;
- increased breathing;
- severe weakness;
- pale skin;
- the skin may become pale bluish or pale yellow;
- lack of urine;
- lack of response to pain;
- loss of consciousness.
This condition does not always end in death. Shock can develop within a couple of minutes or hours. The sooner this condition is diagnosed, the greater the chance of saving a person’s life.
Main symptoms of shock:
- Cold, pale, damp skin.
- The “white spot” symptom is a slow filling of the skin capillaries after they are compressed (more than 2 seconds).
- Tachycardia.
- At first there is excitement, then inhibition.
- Decrease in systolic blood pressure less than 100 mm Hg.
- Decrease in pulse pressure less than 20 mm Hg.
- Decreased diuresis (oligo- or anuria).
Shock of any etiology is characterized by a phased development of peripheral circulatory disorders, however, the sequence of phase development is not always traceable. The treatment provided depends on the type of shock and the underlying cause.
In childhood, emergency care by a pediatrician is most often required for redistribution shock (infectious-toxic and anaphylactic).
Urgent measures
- It is necessary, first of all, to stop arterial bleeding by pressing the artery to the bone above the site of injury, applying an arterial tourniquet or twisting it above the site of injury. In this case, you should record the time when the tourniquet was applied.
- Assess the state of vitality of the body (determine the presence and nature of the pulse over the peripheral and central arteries, the degree of depression of consciousness, airway patency, the effectiveness of external respiration function).
- Ensure the correct position of the victim's body. In an unconscious state, he should be turned on his side, his head thrown back and the upper half of his body slightly lowered. A separate position is required for patients with fractures of the spine (on a hard surface) and pelvic bones (with legs bent at the joints and spread apart). It is contraindicated to throw the head back in patients with a cervical spine injury!
- Provide immobilization of injured limbs with standard splints or available material.
- Apply a bandage to the wound. In case of venous or capillary bleeding, a compressive bandage has a hemostatic effect. For open pneumothorax, an adhesive bandage seals the chest.
- Anesthetize the patient. Narcotic and non-narcotic analgesics are used (1% promedol solution, 50% analgin solution).
In patients with decompensated shock, narcotic analgesics can suppress the respiratory center. In addition, excluding the stimulating influence of pain reduces the activity of the adrenal glands; in patients with BCC deficiency, this can lead to threatening hypotension. The high probability of these complications requires continuous monitoring of patients. If it is impossible to provide such supervision (for example, in case of mass arrival of victims, their transportation), for the purpose of pain relief, it is advisable to use a medium for non-inhalation anesthesia - ketamine (2-3 ml of 5% solution, administered intramuscularly). It exhibits an analgesic effect, stabilizes blood pressure and does not suppress the respiratory center. To prevent the unwanted hallucinatory effects of ketamine, it is advisable to combine it with the administration of 1-2 ml of a 0.5% sibazon solution.
10-20 ml of 0.5% novocaine solution is injected into the fracture sites. As part of a specialized team, a nurse performs venipuncture and catheterizes peripheral vessels, assists the doctor with catheterization of arteries and main veins, prepares systems for intravenous infusions, measures arterial and central venous pressure, records an electrocardiogram, provides oxygen therapy and artificial ventilation to the victim, etc.
Infectious-toxic shock
Infectious-toxic shock can occur in patients with meningococcal infection, especially often in the fulminant form of meningococcemia, staphylococcal and fungal sepsis, as well as in other acute infectious diseases with a severe course (diphtheria, scarlet fever and others).
Stages of infectious-toxic shock:
I (compensated) - hyperthermia, consciousness is preserved, the child may be agitated or restless, tachypnea, tachycardia, normal blood pressure.
II (subcompensated) - low-grade or normal body temperature, lethargy, stupor, lethargy, pronounced tachypnea and tachycardia, systolic blood pressure decreases by 30-50% of the initial age level, positive “white spot” symptom, decreased urine output to 25-10 ml /hour.
III (decompensated) – hypothermia, lethargy up to prostration, sharp tachycardia, sharp tachypnea, widespread cyanosis of the skin and mucous membranes, oliguria less than 10 ml/hour or anuria, blood pressure drop to critical, pulse is determined only in large vessels, positive “white spot” symptom " Subsequently, the clinic of the agonal state develops.
At the prehospital stage, the volume of emergency care for patients with ITS depends on the cause and stage of shock and is aimed at combating life-threatening syndromes - hyperthermic, convulsive, etc.
Urgent Care:
- for hyperthermia - administer a 50% solution of metamizole (analgin) in combination with a 1% solution of diphenhydramine (diphenhydramine) in a dose of 0.1-0.15 ml per year of life intramuscularly;
- in case of severe psychomotor agitation and convulsions, administer a 0.5% diazepam solution in a single dose of 0.1 ml/kg body weight (no more than 2 ml per administration) intravenously or intramuscularly;
- for ITS that developed against the background of meningococcemia, administer chloramphenicol (chloramphenicol succinate) at a dose of 30 mg/kg intramuscularly;
- for cerebral edema - administer a 1% solution of furosemide (Lasix) at a rate of 1-2 mg/kg intramuscularly (only with systolic blood pressure not lower than 100 mm Hg) or 25% solution of magnesium sulfate 1.0 ml for a year of life;
- for ITS stage I - administer prednisolone at a rate of 3-5 mg/kg intramuscularly;
- for ITS stage II:
- oxygen therapy;
- provide access to the vein;
- administer prednisolone 5-10 mg/kg body weight intravenously, if there is no effect and it is impossible to transport the patient within 30-40 minutes. - repeated administration of prednisolone in the same doses;
- intravenous administration of crystalloid (0.9% sodium chloride solution) and colloid solutions (reopolyglucin, polyglucin, 5% albumin) until blood pressure normalizes (up to 15 ml/kg body weight);
- for ITS stage III:
- oxygen therapy;
- provide access to a vein (preferably two);
- administer prednisolone 10 mg/kg body weight intravenously, if there is no effect, repeat administration in the same doses after 30-40 minutes;
- inject a 0.9% sodium chloride solution intravenously in a single dose of 20 ml/kg until a distinct pulse appears on the radial artery; if there is no effect, repeat the bolus injection at the same dose after 15-20 minutes.
Hospitalization - in the intensive care unit of the hospital.
Causes and pathogenesis of the development of septic shock:
The incidence of sepsis and septic shock has been steadily increasing since the 1930s and is likely to continue to increase. The reasons for this are:
1. Increasing use of invasive devices for intensive care, that is, intravascular catheters, etc.
2. Widespread use of cytotoxic and immunosuppressive drugs (for malignant diseases and transplantations), which cause acquired immunodeficiency.
3. Increase in life expectancy of patients with diabetes mellitus and malignant tumors, who have a high level of predisposition to sepsis.
Bacterial infection is the most common cause of septic shock. In sepsis, the primary foci of infection are often localized in the lungs, abdominal organs, peritoneum, and also in the urinary tract. Bacteremia is detected in 40-60% of patients in a state of septic shock. In 10-30% of patients in a state of septic shock, it is impossible to isolate the culture of bacteria whose action causes septic shock. It can be assumed that septic shock without bacteremia is the result of a pathological immune reaction in response to stimulation by antigens of bacterial origin. Apparently, this reaction persists after pathogenic bacteria are eliminated from the body by the action of antibiotics and other elements of therapy, that is, its endogenization occurs. The endogenization of sepsis may be based on numerous, mutually reinforcing and realized through the release and action of cytokines, interactions of cells and molecules of innate immune systems and, accordingly, immunocompetent cells.
Sepsis, systemic inflammatory response, and septic shock are consequences of an excessive response to stimulation of cells that carry out innate immune responses by bacterial antigens. An excessive reaction of cells of the innate immune system and a secondary reaction of T-lymphocytes and B-cells cause hypercytokinemia. Hypercytokinemia is a pathological increase in the blood levels of agents of autoparacrine regulation of cells that carry out innate immune reactions and acquired immune reactions.
With hypercytokinemia in the blood serum, the content of primary proinflammatory cytokines, tumor necrosis factor-alpha and interleukin-1 increases abnormally. As a result of hypercytokinemia and systemic transformation of neutrophils, endothelial cells, mononuclear phagocytes and mast cells into cellular effectors of inflammation, an inflammatory process devoid of protective significance occurs in many organs and tissues. Inflammation is accompanied by alteration of the structural and functional elements of effector organs.
A critical deficiency of effectors causes multiple systemic failure.
Meningococcal infection
Meningococcemia is one of the forms of generalized meningococcal infection, characterized by an acute onset with a rise in body temperature to febrile levels, general intoxication, hemorrhagic skin rashes, and the development of infectious-toxic shock.
Clinical manifestations of meningococcal infection:
- Often acute onset or sharp deterioration due to nasopharyngitis.
- High, persistent fever with signs of impaired peripheral circulation. Early signs of fulminant toxic progression of meningococcal infection may be a “two-humped” nature of the temperature curve - the first increase in body temperature to 38.5ºC is reduced with the help of antipyretics, the second (after 9-18 hours) - to 39.5-40ºC without the effect of antipyretics.
- Hemorrhagic rash that appears a few hours or 1-2 days after the onset of fever. The typical localization of the rash is the outer surface of the thighs and legs, buttocks, feet, hands, and lower abdomen. Often a hemorrhagic rash is preceded or combined with a polymorphic roseolous or roseolous papular rash with the same localization, less often on the face.
- General cerebral symptoms: intense headache, “cerebral vomiting”, possible disturbances of consciousness, delirium, hallucinations, psychomotor agitation, convulsions.
- Meningeal syndrome. It usually appears later, against the background of advanced symptoms of meningococcemia.
Threatening syndromes in generalized forms of meningococcal infection are:
- infectious-toxic shock develops during the hyperacute course of meningococcemia. As a rule, symptoms of shock occur simultaneously or after the appearance of a hemorrhagic rash. However, ITS can occur without a rash, so all children with infectious toxicosis should have their blood pressure measured;
- cerebral edema with brainstem dislocation. It manifests itself as impaired consciousness, hyperthermia, severe meningeal symptoms (sometimes their absence in the terminal stage of the disease), convulsive syndrome and unusual changes in hemodynamics in the form of relative bradycardia and a tendency to increase blood pressure. In the terminal stage of cerebral edema there is absolute bradycardia and respiratory arrhythmia.
Urgent Care:
- Ensure patency of the upper respiratory tract, oxygen therapy.
- Administer chloramphenicol (chloramphenicol succinate) at a dose of 25-30 mg/kg intramuscularly.
- Intravenous (if not possible, intramuscular) administration of glucocorticoids in terms of prednisolone 5-10 mg/kg or dexamethasone 0.6-0.7 mg/kg body weight; if there is no effect and it is impossible to transport the patient, it is necessary to re-introduce hormones in the same doses (for ITS stage III, the dose of corticosteroids can be increased 2-5 times).
- Introduce a 50% solution of metamizole (analgin) 0.1 ml/year of life intramuscularly + 1% solution of diphenhydramine (diphenhydramine) at a dose of 0.1-0.15 ml per year of life intramuscularly (or 2% solution Suprastin solution – 2 mg/kg intramuscularly) + 2% papaverine solution 0.1 ml/year of life).
- For agitation, convulsive syndrome - 0.5% diazepam solution in a single dose of 0.1 ml/kg body weight (no more than 2 ml per administration) intravenously or intramuscularly, for intractable convulsions again in the same dose .
- In case of severe meningeal syndrome - intramuscular administration of a 1% furosemide solution at the rate of 1-2 mg/kg intramuscularly or 25% magnesium sulfate solution 1.0 ml/year of life intramuscularly.
Call the resuscitation team for yourself!!! Hospitalization in the intensive care unit of the nearest hospital. If meningococcal infection is suspected, hospitalization is indicated .
Intestinal toxicosis with exicosis is a pathological condition that is a complication of acute intestinal infections (AI) due to exposure to toxic products and significant fluid losses on the body, which leads to disruption of hemodynamics, water-electrolyte metabolism, acid-base reserve, and the development of secondary endogenous intoxication.
In the pathogenesis of toxicosis with exicosis, the leading role is played by dehydration, which leads to a deficiency in the volume of extra- (and in severe cases, intra-) cellular fluid and the volume of circulating blood. The degree of exicosis (dehydration) determines the choice of therapeutic tactics and affects the prognosis.
Severity of dehydration
| Signs | Degrees of dehydration | |||
| I degree | II degree* | III degree | ||
| Underweight | Up to 5% | 6-9 % | More than 10% | |
| BCC deficiency | More than 10% | 11-20 % | More than 20% | |
| General state | good | Excitation | Lethargic, lethargic, unconscious | |
| Chair | Infrequent (4-6 times a day) | Up to 10 times a day | Frequent (more than 10 times a day), watery | |
| Vomit | One-time | Repeated (3-4 times a day) | Multiple | |
| Thirst | Moderate | Sharply expressed | Refusal to drink | |
| Skin fold | Spreads out quickly | Unfolds slowly (≤2 sec) | Unfolds very slowly (>2 sec) | |
| Mucous membranes | Moist or slightly dry | Dry | Very dry, bright | |
| Cyanosis | ||||
| Eyeballs | Without features | Soft | They're sinking | |
| Voice | Without features | Weakened | Aphonia | |
| Heart sounds | Loud | Slightly muted | Deaf | |
| Heart rate | Norm | Moderate tachycardia | Severe tachycardia | |
| Diuresis | Saved | Reduced | Significantly reduced | |
| Blood plasma electrolytes | Norm | Hypokalemia | Hypokalemia | |
| CBS | Norm | Compensated acidosis | Decompensated acidosis | |
| Rehydration method | Oral | Parenteral | ||
- II degree of exicosis is divided into IIA and IIB (with loss of body weight 6-7% and 8-9%, respectively).
Measures for dehydration
When starting to rehydrate children with exicosis, it is necessary to determine which route (orally and/or parenterally) and what volume of fluid should be administered to the child, at what speed to administer, and what solutions to use. Oral rehydration is carried out for exicosis of I, IIA degrees, parenteral - for exicosis of IIB and III degrees.
Outpatient treatment is possible (in the absence of other contraindications, see below) for grade I exicosis with oral rehydration.
Oral rehydration therapy
Indications: exicosis I, IIA degrees.
- The necessary solutions are glucose-salt (rehydron, oralit, gastrolit, glucosalan). In children under 3 years of age, it is advisable to combine glucose-saline solutions with salt-free solutions (tea, water, rice water, etc.) in a ratio of 1:1 - for severe watery diarrhea, 2:1 - for loss of fluid mainly through vomiting, 1: 2 – with loss of fluid with perspiration. The administration of saline and salt-free solutions alternates.
The amount of liquid for each hour of administration is divided into ½ teaspoon - 1 tablespoon (depending on age) every 5-10 minutes. If there is vomiting, rehydration does not stop, but is interrupted for 5-10 minutes and then continues again.
Features of the introduction of solutions at the 1st stage (the first 6 hours from the start of treatment):
- for grade I dehydration: 50 ml/kg for 6 hours. + 10 ml/kg b.w. after each loose stool or vomiting;
- for degree IIA dehydration: 80 ml/kg for 6 hours + 10 ml/kg b.w. after each loose stool or vomiting.
At the 2nd stage, maintenance therapy is carried out in the amount of ongoing fluid losses administered at this stage (80-100 ml/kg per day until the loss stops).
Shock. Etiology. Pathogenesis. Classification
The term "shock", meaning a blow, shock, shock in English and French, was accidentally introduced in 1743 by a now unknown translator into English of a book by Louis XV's army consultant Le Dran to describe the condition of patients after a gunshot injury. Until now, this term has been widely used to describe the emotional state of a person when exposed to unexpected, extremely strong mental factors, without implying specific damage to organs or physiological disorders. In clinical medicine, shock means a critical condition, which is characterized by a sharp decrease in organ perfusion, hypoxia and metabolic disorders. This syndrome is manifested by arterial hypotension, acidosis and rapidly progressive deterioration in the functions of vital body systems. Without adequate treatment, shock quickly leads to death.
Acute short-term hemodynamic disturbances can be a transient episode when there is a violation of vascular tone, reflexively caused by sudden pain, fear, the sight of blood, stuffiness or overheating, as well as cardiac arrhythmia or orthostatic hypotension due to anemia or hypotension. This episode is called collapse and in most cases resolves on its own without treatment. Due to a transient decrease in blood supply to the brain, syncope - a short-term loss of consciousness, which is often preceded by neuro-vegetative symptoms: muscle weakness, sweating, dizziness, nausea, darkening of the eyes and tinnitus. Characterized by pallor, low blood pressure, bradycardia or tachycardia. The same thing can develop in healthy people at high ambient temperatures, since heat stress leads to a significant dilation of skin vessels and a decrease in diastolic blood pressure. Longer hemodynamic disorders always pose a danger to the body.
Causes of shock
Shock occurs when the body is exposed to super-strong irritants and can develop as a result of various diseases, injuries and pathological conditions. Depending on the cause, hemorrhagic, traumatic, burn, cardiogenic, septic, anaphylactic, blood transfusion, neurogenic and other types of shock are distinguished. There may also be mixed forms of shock caused by a combination of several reasons. Taking into account the pathogenesis of changes occurring in the body and requiring certain specific therapeutic measures, four main types of shock are distinguished
Hypovolemic shock occurs with a significant decrease in blood volume as a result of massive bleeding or dehydration and is manifested by a sharp decrease in venous return of blood to the heart and severe peripheral vasoconstriction.
Cardiogenic shock occurs when there is a sharp decrease in cardiac output due to impaired myocardial contractility or acute morphological changes in the heart valves and interventricular septum. It develops with normal bcc and is manifested by overflow of the venous bed and pulmonary circulation.
Redistribution shock is manifested by vasodilation, a decrease in total peripheral resistance, venous return of blood to the heart and an increase in the permeability of the capillary wall.
Extracardiac obstructive shock occurs due to the sudden occurrence of an obstruction to blood flow. Cardiac output drops sharply despite normal blood volume, myocardial contractility and vascular tone.
Pathogenesis of shock
Shock is based on generalized perfusion disturbances, leading to hypoxia of organs and tissues and disorders of cellular metabolism ( Fig. 15. 2. ). Systemic circulatory disorders are a consequence of decreased cardiac output (CO) and changes in vascular resistance.
Primary physiological disorders that reduce effective tissue perfusion are hypovolemia, heart failure, impaired vascular tone, and obstruction of large vessels. With the acute development of these conditions, a “mediator storm” develops in the body with the activation of neuro-humoral systems, the release into the systemic circulation of large quantities of hormones and pro-inflammatory cytokines, affecting vascular tone, vascular wall permeability and CO. In this case, the perfusion of organs and tissues is sharply disrupted. Acute severe hemodynamic disorders, regardless of the reasons that caused them, lead to the same type of pathological picture. Serious disturbances of central hemodynamics, capillary circulation and critical disruption of tissue perfusion with tissue hypoxia, cell damage and organ dysfunction develop.
Hemodynamic disorders
Low CO is an early feature of many types of shock, except for redistribution shock, in which in the initial stages the cardiac output may even be increased. CO depends on the strength and frequency of myocardial contractions, venous blood return (preload) and peripheral vascular resistance (afterload). The main reasons for a decrease in CO during shock are hypovolemia, deterioration in the pumping function of the heart and increased arteriolar tone. The physiological characteristics of various types of shock are presented in table. 15.2 .
In response to a decrease in blood pressure, the activation of adaptive systems increases. First, reflex activation of the sympathetic nervous system occurs, and then the synthesis of catecholamines in the adrenal glands increases. The content of norepinephrine in plasma increases 5-10 times, and the level of adrenaline increases 50-100 times. This enhances the contractile function of the myocardium, increases cardiac activity and causes a selective narrowing of the peripheral and visceral venous and arterial beds. Subsequent activation of the renin-angiotensin mechanism leads to even more pronounced vasoconstriction and the release of aldosterone, which retains salt and water. The release of antidiuretic hormone reduces urine volume and increases its concentration.
In shock, peripheral vasospasm develops unevenly and is especially pronounced in the skin, abdominal organs and kidneys, where the most pronounced decrease in blood flow occurs. Pale and cool skin observed during examination and pallor of the intestine with weakened pulse in the mesenteric vessels visible during surgery are clear signs of peripheral vasospasm.
Constriction of the blood vessels of the heart and brain occurs to a much lesser extent compared to other zones, and these organs are provided with blood longer than others due to a sharp limitation of the blood supply to other organs and tissues. The metabolic rates of the heart and brain are high, and their reserves of energy substrates are extremely low, so these organs do not tolerate prolonged ischemia. Neuroendocrine compensation of a patient in shock is primarily aimed at providing the immediate needs of vital organs - the brain and heart. Sufficient blood flow in these organs is maintained by additional autoregulatory mechanisms as long as blood pressure exceeds 70 mmHg. Art.
Centralization of blood circulation is a biologically appropriate compensatory reaction. In the initial period, it saves the patient’s life. It is important to remember that initial shock reactions are adaptation reactions of the body aimed at survival in critical conditions, but beyond a certain limit, they begin to be pathological in nature, leading to irreversible damage to tissues and organs. Centralization of blood circulation, which persists for several hours, along with protection of the brain and heart, is fraught with a mortal danger, although more distant. This danger lies in the deterioration of microcirculation, hypoxia and metabolic disorders in organs and tissues.
Correction of central hemodynamic disturbances during shock includes intensive infusion therapy aimed at increasing blood volume, the use of drugs that affect vascular tone and myocardial contractility. Only in case of cardiogenic shock is massive infusion therapy contraindicated.
Disorders of microcirculation and tissue perfusion
The microvasculature (arterioles, capillaries and venules) is the most important link in the circulatory system in the pathophysiology of shock. It is at this level that nutrients and oxygen are delivered to organs and tissues, and metabolic products are also removed.
The developing spasm of arterioles and precapillary sphincters during shock leads to a significant decrease in the number of functioning capillaries and a slowdown in the speed of blood flow in the perfused capillaries, ischemia and tissue hypoxia. Further deterioration of tissue perfusion may be associated with secondary capillary pathology. The accumulation of hydrogen ions, lactate and other products of anaerobic metabolism leads to a decrease in the tone of arterioles and precapillary sphincters and an even greater decrease in systemic blood pressure. In this case, the venules remain narrowed. Under these conditions, the capillaries become overfilled with blood, and albumin and the liquid part of the blood intensively leave the vascular bed through pores in the walls of the capillaries (“capillary leak syndrome”). Thickening of blood in the microcirculatory bed leads to an increase in blood viscosity, while the adhesion of activated leukocytes to endothelial cells increases, red blood cells and other formed elements of blood stick together and form large aggregates, peculiar plugs, which further worsen microcirculation until the development of sludge syndrome.
Vessels blocked by the accumulation of blood cells are switched off from the bloodstream. The so-called “pathological deposition” develops, which further reduces the bcc and its oxygen capacity and reduces the venous return of blood to the heart and, as a result, causes a drop in CO and a further deterioration in tissue perfusion. Acidosis, in addition, reduces the sensitivity of blood vessels to catecholamines, preventing their vasoconstrictor effect and leads to atony of the venules. Thus, a vicious circle is closed. A change in the ratio of the tone of the precapillary sphincters and venules is considered a decisive factor in the development of the irreversible phase of shock.
An inevitable consequence of slowing capillary blood flow is the development of hypercoagulation syndrome. This leads to disseminated intravascular thrombus formation, which not only increases capillary circulation disorders, but also causes the development of focal necrosis and multiple organ failure.
Ischemic damage to vital tissues consistently leads to secondary damage that maintains and aggravates the shock state. The resulting vicious circle can lead to a fatal outcome.
Clinical manifestations of impaired tissue perfusion are cold, moist, pale cyanotic or marbled skin, prolongation of capillary refill time over 2 seconds, temperature gradient over 3 °C, oliguria (urination less than 25 ml/hour). To determine the capillary refill time, squeeze the tip of the nail plate or the pad of the toe or hand for 2 seconds and measure the time during which the pale area regains its pink color. In healthy people this happens immediately. If microcirculation deteriorates, blanching lasts for a long time. Such microcirculation disorders are nonspecific and are a constant component of any type of shock, and the degree of their severity determines the severity and prognosis of shock. The principles of treatment of microcirculation disorders are also not specific and practically do not differ for all types of shock: elimination of vasoconstriction, hemodilution, anticoagulant therapy, disaggregant therapy.
Metabolic disorders
Under conditions of reduced perfusion of the capillary bed, adequate delivery of nutrients to tissues is not ensured, which leads to metabolic disorders, dysfunction of cell membranes and cell damage. Carbohydrate, protein, and fat metabolism are disrupted, and the utilization of normal energy sources—glucose and fatty acids—is sharply inhibited. In this case, pronounced catabolism of muscle protein occurs.
The most important metabolic disorders in shock are the destruction of glycogen, a decrease in dephosphorylation of glucose in the cytoplasm, a decrease in energy production in mitochondria, disruption of the sodium-potassium pump of the cell membrane with the development of hyperkalemia, which can cause atrial fibrillation and cardiac arrest.
The increase in plasma levels of adrenaline, cortisol, glucagon that develops during shock and the suppression of insulin secretion affect the metabolism in the cell by changes in the use of substrates and protein synthesis. These effects include increased metabolic rate, increased glycogenolysis and gluconeogenesis. A decrease in tissue glucose utilization is almost always accompanied by hyperglycemia. In turn, hyperglycemia can lead to a decrease in oxygen transport, disruption of water-electrolyte homeostasis and glycosylation of protein molecules with a decrease in their functional activity. Significant additional damaging effects of stress hyperglycemia during shock contribute to the deepening of organ dysfunction and require timely correction while maintaining normoglycemia.
Against the background of increasing hypoxia, oxidation processes in tissues are disrupted, their metabolism proceeds along the anaerobic pathway. At the same time, acidic metabolic products are formed in significant quantities, and metabolic acidosis develops. The criterion for metabolic dysfunction is a blood pH level below 7.3, a base deficiency exceeding 5.0 mEq/L and an increase in the concentration of lactic acid in the blood above 2 mEq/L.
An important role in the pathogenesis of shock belongs to the disturbance of calcium metabolism, which intensively penetrates into the cytoplasm of cells. Elevated intracellular calcium levels increase the inflammatory response, leading to intense synthesis of potent mediators of the systemic inflammatory response (SIR). Inflammatory mediators play a significant role in the clinical manifestations and progression of shock, as well as in the development of subsequent complications. Increased production and systemic distribution of these mediators can lead to irreversible cell damage and high mortality. The use of calcium channel blockers improves survival in patients with various types of shock.
The action of pro-inflammatory cytokines is accompanied by the release of lysosomal enzymes and free peroxide radicals, which cause further damage - “sick cell syndrome”. Hyperglycemia and an increase in the concentration of soluble products of glycolysis, lipolysis and proteolysis lead to the development of hyperosmolarity of the interstitial fluid, which causes the transition of intracellular fluid into the interstitial space, dehydration of cells and further deterioration of their functioning. Thus, cell membrane dysfunction may represent a common pathophysiological pathway for various causes of shock. And although the exact mechanisms of cell membrane dysfunction are unclear, the best way to eliminate metabolic disorders and prevent the irreversibility of shock is the rapid restoration of blood volume.
Inflammatory mediators produced during cellular damage contribute to further disruption of perfusion, which further damages cells within the microvasculature. Thus, a vicious circle is completed - impaired perfusion leads to cell damage with the development of systemic inflammatory response syndrome, which in turn further worsens tissue perfusion and cell metabolism. When these excessive systemic responses persist for a long time, become autonomous and cannot be reversed, multiple organ failure syndrome develops.
In the development of these changes, the leading role belongs to tumor necrosis factor (TNF), interlekins (IL-1, IL-6, IL-8), platelet activating factor (PAF), leukotrienes (B4, C4, D4, E4), thromboxane A2, prostaglandins (E2, E12), prostacyclin, interferon gamma. The simultaneous and multidirectional action of etiological factors and activated mediators during shock leads to endothelial damage, disruption of vascular tone, vascular permeability and organ dysfunction.
Persistence or progression of shock may result from either ongoing perfusion defects, cellular damage, or a combination of both. Since oxygen is the most labile vital substrate, its inadequate delivery by the circulatory system forms the basis of the pathogenesis of shock, and timely restoration of tissue perfusion and oxygenation often completely stops the progression of shock.
Thus, the pathogenesis of shock is based on deep and progressive disorders of hemodynamics, oxygen transport, humoral regulation and metabolism. The interrelation of these disorders can lead to the formation of a vicious circle with complete depletion of the body’s adaptive capabilities. Preventing the development of this vicious circle and restoring the body's autoregulatory mechanisms is the main task of intensive care for patients with shock.
Stages of shock
Shock is a dynamic process that begins with the action of the aggression factor, which leads to systemic circulatory disorders, and, as the disorders progress, ends with irreversible damage to organs and death of the patient. The effectiveness of compensatory mechanisms, the degree of clinical manifestations and the reversibility of the resulting changes make it possible to distinguish a number of successive stages in the development of shock.
Preshock stage
Shock is usually preceded by a moderate decrease in systolic blood pressure, not exceeding 20 mm Hg. Art. from normal (or 40 mm Hg if the patient has arterial hypertension), which stimulates the baroreceptors of the carotid sinus and aortic arch and activates the compensatory mechanisms of the circulatory system. Tissue perfusion is not significantly affected and cellular metabolism remains aerobic. If the influence of the aggression factor ceases, then compensatory mechanisms can restore homeostasis without any therapeutic measures.
Early (reversible) stage of shock
This stage of shock is characterized by a decrease in systolic blood pressure below 90 mmHg. Art. , severe tachycardia, shortness of breath, oliguria and cold clammy skin. At this stage, compensatory mechanisms are independently unable to maintain adequate CO and satisfy the oxygen needs of organs and tissues. Metabolism becomes anaerobic, tissue acidosis develops, and signs of organ dysfunction appear. An important criterion for this phase of shock is the reversibility of the resulting changes in hemodynamics, metabolism and organ functions and a fairly rapid regression of developed disorders under the influence of adequate therapy.
Intermediate (progressive) stage of shock
This is a life-threatening critical situation with a systolic blood pressure level below 80 mmHg. Art. and pronounced but reversible organ dysfunction with immediate intensive treatment. This requires artificial pulmonary ventilation (ALV) and the use of adrenergic drugs to correct hemodynamic disorders and eliminate organ hypoxia. Prolonged deep hypotension leads to generalized cellular hypoxia and critical disruption of biochemical processes, which quickly become irreversible. It is on the effectiveness of therapy during the first so-called “golden hour” that the patient’s life depends.
Refractory (irreversible) stage of shock
This stage is characterized by severe disorders of central and peripheral hemodynamics, cell death and multiple organ failure. Intensive therapy is ineffective, even if the etiological causes are eliminated and blood pressure temporarily increases. Progressive multiorgan dysfunction usually leads to irreversible organ damage and death.
Diagnostic testing and monitoring for shock
Shock does not leave time for an orderly collection of information and clarification of the diagnosis before starting treatment. Systolic blood pressure during shock is most often below 80 mmHg. Art. , but shock is sometimes diagnosed at higher systolic blood pressure if there are clinical signs of a sharp deterioration in organ perfusion: cold skin covered with clammy sweat, mental status changes from confusion to coma, oligo- or anuria, and insufficient skin capillary refill. Rapid breathing during shock usually indicates hypoxia, metabolic acidosis and hyperthermia, and hypoventilation indicates depression of the respiratory center or increased intracranial pressure.
Diagnostic tests for shock also include a clinical blood test, determination of electrolytes, creatinine, blood clotting parameters, blood group and Rh factor, arterial blood gases, electrocardiography, echocardiography, and chest radiography. Only carefully collected and correctly interpreted data helps make the right decisions.
Monitoring is a system for monitoring the vital functions of the body, capable of quickly notifying about the occurrence of threatening situations. This allows you to start treatment on time and prevent the development of complications. To monitor the effectiveness of shock treatment, monitoring of hemodynamic parameters, heart, lung and kidney activity is indicated. The number of controlled parameters must be reasonable. Monitoring for shock must necessarily include recording of the following indicators:
- Blood pressure, using intra-arterial measurement if necessary;
- heart rate (HR);
- intensity and depth of breathing;
- central venous pressure (CVP);
- pulmonary artery wedge pressure (PAWP) in severe shock and unknown cause of shock;
- diuresis;
- blood gases and plasma electrolytes.
To approximate the severity of shock, you can calculate the Algover-Burri index, or, as it is also called, the shock index - the ratio of the pulse rate per minute to the value of systolic blood pressure. And the higher this indicator, the greater the danger to the patient’s life. The inability to monitor any of the listed indicators makes the correct choice of therapy difficult and increases the risk of developing iatrogenic complications.
Central venous pressure
Low central venous pressure is an indirect criterion of absolute or indirect hypovolemia, and its rise above 12 cm of water. Art. indicates heart failure. Measuring central venous pressure and assessing its response to a low fluid load helps to select a fluid therapy regimen and determine the appropriateness of inotropic support. Initially, the patient is given a test dose of liquid over 10 minutes: 200 ml at an initial CVP below 8 cm aq. Art. ; 100 ml – with a central venous pressure within 8-10 cm aq. Art. ; 50 ml – with a central venous pressure above 10 cm aq. Art. The reaction is assessed based on the rule “5 and 2 cm aq. Art. ": if the central venous pressure increases by more than 5 cm, the infusion is stopped and the question of the advisability of inotropic support is decided, since such an increase indicates a breakdown of the Frank-Starling contractility regulation mechanism and indicates heart failure. If the increase in central venous pressure is less than 2 cm water. Art. – this indicates hypovolemia and is an indication for further intensive fluid resuscitation without the need for inotropic therapy. Increase in central venous pressure in the range of 2 and 5 cm aq. Art. requires further infusion therapy under the control of hemodynamic parameters.
It must be emphasized that CVP is an unreliable indicator of left ventricular function, since it depends primarily on the condition of the right ventricle, which may differ from the condition of the left. More objective and broader information about the condition of the heart and lungs is provided by monitoring hemodynamics in the pulmonary circulation. Without its use, the hemodynamic profile of a patient with shock is incorrectly assessed in more than a third of cases. The main indication for catheterization of the pulmonary artery in shock is an increase in central venous pressure during infusion therapy. The response to the introduction of a small volume of fluid when monitoring hemodynamics in the pulmonary circulation is assessed according to the rule “7 and 3 mm Hg. Art. "
Hemodynamic monitoring in the pulmonary circulation
Invasive monitoring of blood circulation in the pulmonary circulation is performed using a catheter installed in the pulmonary artery. For this purpose, a catheter with a floating balloon at the end (Swan-Gans) is usually used, which allows you to measure a number of parameters:
- pressure in the right atrium, right ventricle, pulmonary artery and pulmonary artery, which reflects the filling pressure of the left ventricle;
- SV by thermodilution method;
- partial pressure of oxygen and oxygen saturation of hemoglobin in mixed venous blood.
Determination of these parameters significantly expands the possibilities of monitoring and assessing the effectiveness of hemodynamic therapy. The resulting indicators allow:
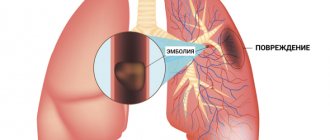
- differentiate cardiogenic and non-cardiogenic pulmonary edema, identify pulmonary embolism and rupture of the mitral valve leaflets;
- assess blood volume and the state of the cardiovascular system in cases where empirical treatment is ineffective or is associated with an increased risk;
- adjust the volume and rate of fluid infusion, doses of inotropic and vasodilator drugs, and the value of positive end-expiratory pressure during mechanical ventilation.
Decreased oxygen saturation of mixed venous blood is always an early indicator of inadequate cardiac output.
Diuresis
A decrease in diuresis is the first objective sign of a decrease in blood volume. Patients with shock must have a permanent urinary catheter installed to monitor the volume and rate of urination. When carrying out infusion therapy, diuresis should be at least 50 ml/hour. During alcohol intoxication, shock can occur without oliguria, since ethanol inhibits the secretion of antidiuretic hormone.
Treatment of shock
Depending on the reasons that caused such a negative response from the body, the doctor will take various measures. Of course, there is a general set of actions for any type of shock, since it is aimed at maintaining the last strength of the body.
Thus, in case of traumatic shock, the pain syndrome should first be relieved. If necessary, administer hemostatic drugs, perform reposition, and apply a bandage.
In case of bleeding, a temporary stop is carried out, followed by ligation and replenishment of the circulating blood volume. In case of anaphylactic shock, glucocorticosteroids and antihistamines are immediately administered, and artificial ventilation is performed.
Next, resuscitation is carried out and emergency medications are used. After stabilization of the patient's condition, maintenance therapy and intensive care are carried out.
Anatomical and functional features of the cardiovascular system
From the left ventricle, blood enters the arterial system of the systemic circulation. Arteries contain 15% of the total circulating blood volume. These vessels carry blood to the tissues. The terminal sections of the arteries end in arterioles (resistance vessels). They perform the function of distributing blood in tissues. Thus, an increase in the tone of the arterioles (their spasm) prevents the flow of blood into the capillary pool. Tissue ischemia occurs, and blood flows into the venous system through arteriovenous anastomoses. A decrease in arteriolar tone, on the contrary, increases their lumen and enhances blood supply to tissues.
Natural vasoconstrictors (vasoconstrictors) are:
- adrenalin,
- norepinephrine,
- serotonin,
- angiotensin-2.
Under stress, the concentration of catecholamines (adrenaline, norepinephrine) in the blood increases sharply. They cause spasm of arterioles; a phenomenon of centralization of blood circulation occurs with a decrease in peripheral blood flow. The vasodilating effect is exerted by “acidic” metabolites (lactates, pyruvate, adenylic and inosinic acids), bradykinin, histamine, acetylcholine, a number of medications (neuroleptics, alpha-adrenolytics, peripheral vasodilators, ganglion blockers, etc.), some exogenous poisons, etc. Their action causes the phenomenon of decentralization of blood circulation (opening of the lumen of arterioles and redistribution of blood from the central vessels to the periphery, into the capillary bed).
Capillaries are an extensive network of the smallest vessels in the body, with a total length of 90,100,000 kilometers. About 20-25% of the capillaries function simultaneously, in which the transition of oxygen and nutrients from the blood to the tissues and the removal of “waste” metabolic products from them occurs. Periodically, with an interval of several tens of seconds, other capillaries open, where the blood is redistributed (vasomotion effect). Capillaries contain 12% of all circulating blood. However, in some pathological conditions this volume can increase several times.
The waste blood flows from the capillaries into the venous system. Veins play the role of a blood reservoir, since they contain the bulk of it (70%). They, unlike arteries, are able to change their volume, affecting the flow of blood to the heart.
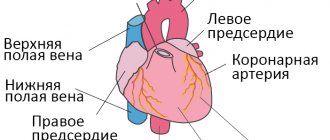
The most important hemodynamic indicator of the venous system is central venous pressure (CVP). This is the pressure that blood exerts on the walls of the vena cava and the right atrium. It is an integral indicator of circulating blood volume, vascular tone and pumping function of the heart. CVP is measured with a phlebotonometer. Normally it is 60-120 mm of water column.
Central venous pressure decreases when:
- blood loss;
- excessive loss of water (hypohydration);
- decreased tone of arterioles and veins.
This reduces the volume of blood flowing to the heart and, accordingly, reduces cardiac output. With negative CVP values, there is a danger of circulatory arrest. Venous pressure increases when:
• heart failure (left and right ventricular);
• excessive infusion of blood and other fluids;
• obstruction of blood flow from the right ventricle of the heart (pulmonary embolism).
When the CVP is more than 150-160 mm Hg. against the background of left ventricular failure, patients may develop pulmonary edema. An integral indicator of the hemodynamics of the arterial part of the vascular system is blood pressure (systolic, diastolic, pulse and average). Systolic and diastolic blood pressure are measured with a tonometer using the Korotkoff method. Pulse blood pressure is the difference between systolic and diastolic.
The value of blood pressure is influenced by the volume of cardiac output and the resistance of peripheral vessels (arterioles). This dependence is directly proportional. Therefore, you can increase a patient’s blood pressure in the following ways:
- introduce vasoconstrictor agents (adrenaline solution, mezaton, etc.);
- increase the volume of circulating blood (by transfusion of polyglucin, refortan, etc.);
- improve the functional ability of the myocardium (by introducing cardiac glycosides, etc.).
The total blood volume in humans is about 7% of body weight: in men 70 ml/kg, in women - 65 ml/kg. The circulating blood volume (CBV) is somewhat smaller, since part of the blood does not participate in the circulation, being in the vascular depot. BCC can be measured by injecting a known concentration of a substance into the bloodstream, for example, Evans blue or polyglucin, and determining the degree of its dilution. So, measurements of central venous pressure, blood pressure, cardiac output and blood volume in patients make it possible to identify the characteristics of circulatory disorders and carry out adequate corrective therapy.
Acute cardiovascular failure
Acute cardiovascular failure is the inability of the heart and blood vessels to provide blood supply to body tissues adequate to metabolic needs, which leads to dysfunction of cells and their death.
The causes of the pathology can be mechanical trauma, bleeding, burns, excessive loss of water and salts by the body, intoxication with exogenous and endogenous poisons, the action of microorganisms and their toxins, immediate hypersensitive reaction to allergens, coronary heart disease, arrhythmias, disorders of neurohumoral regulation of vascular tone, etc. .d.
Acute heart failure is a violation of the pumping function of the heart. It can develop as a result of both cardiac disorders (heart disease) and secondary, under the influence of extracardiac factors (infection, intoxication). Acute heart failure occurs in the left and right ventricular types.
Acute left ventricular failure is the inability of the left ventricle to pump blood from the pulmonary circulation to the large circulation. Most often it occurs with myocardial infarction, mitral heart disease, stenosis of the left atrioventricular orifice, stenosis and insufficiency of the aortic valve, hypertension, coronary vascular sclerosis, acute pneumonia.
Patients experience impaired blood circulation in the coronary vessels (which occurs only during diastole and is intermittent) and cardiac output decreases. During systole, not all the blood is pushed into the aorta, but some remains in the left ventricle. Therefore, during diastole, the pressure in it increases, which leads to stagnation of blood in the left atrium. The right ventricle, which retains its function, continues to pump blood into the pulmonary vessels, which are not able to accommodate such a volume. Hydrostatic pressure in the vessels of the pulmonary circulation increases; The liquid part of the blood passes into the lung tissue.
Patients experience suffocation (first during physical exertion, and then at rest). Subsequent attacks of suffocation are accompanied by a cough producing pink sputum. This condition is called cardiac asthma. With a further increase in hydrostatic pressure in the capillaries of the pulmonary circulation (over 150-200 mm Hg), the liquid part of the blood penetrates into the lumen of the alveoli. Pulmonary edema occurs. There are interstitial and alveolar pulmonary edema.
With interstitial edema, serous fluid is released from the congestive vessels of the small circle and infiltrates all lung tissues, including the peribronchial and perivascular spaces.
With alveolar edema, not only plasma penetrates into the lumen of the alveoli, but also erythrocytes, leukocytes, and platelets. When breathing, liquid mixes with air; A large amount of foam is formed, which disrupts the flow of oxygen into the blood. Circulatory hypoxia (due to heart failure) is accompanied by hypoxic hypoxia (due to impaired oxygen supply).
The patient's condition deteriorates sharply. He takes a forced (sitting) position. Shortness of breath increases (30-35 breaths per minute), which often turns into suffocation. Acrocyanosis occurs. Consciousness is clouded, psychomotor agitation is observed (due to hypoxia of the central nervous system). The breath is squeaky, with the release of pink foam. Multiple moist rales of different sizes are heard in the lungs, which can be heard at a distance (the “boiling samovar” symptom).
There are two forms of pulmonary edema: with high blood pressure (hypertension, aortic valve insufficiency, with damage to the structures and vessels of the brain) and with normal or low blood pressure (with extensive myocardial infarction, acute myocarditis, severe mitral or aortic heart disease, severe pneumonia).
Shock
Shock is a pathological condition of the body that occurs when it is exposed to excessive irritants and is manifested by a violation of systemic circulation, microcirculation and metabolic processes in cells.
Shock occurs when the body responds to aggression by mobilizing its own defenses. The universal response to stress is stimulation of the sympathetic nervous system and the hypothalamus-adrenal glands with the release of large amounts of catecholamines and other vasoactive substances into the blood. These mediators excite the receptors of peripheral vessels, causing their narrowing, while simultaneously promoting the expansion of life-support vessels.
Centralization of blood circulation occurs: it is advisable, from the position of the body, to limit the perfusion of the skin, abdominal organs, and kidneys to ensure normal blood supply to such vital organs and systems as the central nervous system, myocardium, and lungs. However, the influence of shock factors (pain, hypovolemia, damage to tissues and organs, accumulation of toxic metabolites in the blood), blockade of microcirculation due to vascular spasm and microthrombosis, and prolonged tissue ischemia leads to hypoxic damage and death of cells of internal organs. Multiple organ failure syndrome develops.