Features of the composition and pharmacological action of Rumalon
The instructions indicate that the main active component of the drug is a glycosaminoglycanopeptide complex; additional ingredients include purified water and metacresol. Rumalon is produced in ampoules of 1-2 ml, each package contains 25 units.
The active component of the drug is included in the subgroup of biogenic stimulants of animal origin. When used, a temporary protective effect is observed; most enzyme substances that destroy the cartilaginous surfaces of joints are suppressed.
The medication inhibits catabolism, improves the production of sulfated mucopolysaccharides, and stabilizes metabolic processes in hyaline tissues. The drug is based on an extract from the cartilage and bone marrow of young animals.
Rumalon helps restore cartilage cells due to the active production of collagen and glycosaminoglycan, which are building materials for tissues. Therapy reduces the effect of enzymatic substances that destroy cartilage, increases the production of secretions used to reduce joint friction, and improves the nutritional processes of articular and cartilaginous elements.
When therapy is started in a timely manner, the medication slows down the development of osteoarthritis, which provokes changes on the outer side of the cartilage of the joint and thinning of the cartilaginous plate.
RUMALON (solution)
condition, so hope remained only for Rumalon, which I want to talk about.
This drug is a chondoprotector and, in short, is designed to reduce inflammation, restore the structure of cartilage tissue, and thereby relieve pain. Its action is also aimed at ensuring that the old cartilage can regenerate and recover.
Rumalon is a natural preparation and CONSISTS of a natural extract of animal (calf) cartilage.
It is sold in almost every pharmacy, I also ordered from Pharmacy. ru, and at a local online pharmacy (for grandma). On average, with all the discounts, it cost me 2,200 - 2,300 rubles.
The cardboard package contains 25 ampoules of 1 ml each. They are located 5 pieces in five blister packs.
The reverse side is covered with thick film. I didn’t find a place for a tear, so I simply opened it from the edge with a knife and pulled it all the way.
The ampoules fit very tightly and securely in the cells; they endured the rather long journey home well and none of them broke.
The ampoules are made of dark glass; the label contains all the necessary information about volume, expiration dates, etc. There is a white break ring on the neck.
The Rumalon solution is golden brown in color and, as the nurse told me, has a characteristic odor. I, however, did not feel it, since I personally did not open the ampoule and did not draw the contents into the syringe.
Detailed INSTRUCTIONS are included with the product. I think that no one will inject themselves with Rumalon just like that, but only as prescribed by a doctor, so the treatment regimen specifically for you may be slightly different.
For example, I was prescribed to inject Rumalon DAILY and immediately from a dose of 1 ml. Well, plus forced days off, since I went to my clinic for all these procedures.
For very elderly people (as my grandmother was prescribed), it is better to start injecting this solution exactly according to the instructions and every other day. The body is already weak, blood pressure and all that. You never know, although Rumalon has practically no SIDE EFFECTS as such - only allergic reactions, including anaphylactic shock.
Intramuscular injections of Rumalon are painless and quick. Apart from the needle prick, I didn’t feel anything else, only sometimes I felt a barely noticeable warmth (a lot depends on the nurses). It also did not cause bumps and bruises, a large number of which I received after Voltaren and Mydocalm. I don’t know whether it depends on the drug or on the technique of those who inject…
I noticed the first positive change about Rumalon only after 3 WEEKS of injections (15 injections). Only by the end of the third did I clearly understand that my back had stopped hurting by 90% - the constant severe pain was gone and I was able to look at the world calmly, and not through a veil of torment. I no longer wanted to constantly change positions, since only with constant movement I could previously reduce the discomfort.
During the 4th week, I could experience a slight nagging REGULAR back pain after several hours on my feet, for example, or long cleaning, or a rare change of position (long sitting, standing). That is, that terrible pain in the lumbar and thoracic region left me, taking with it the eternal terrible migraines that began after being affected by protrusions of the cervical spine.
The FIFTH week of injections did not give much, in fact, it simply consolidated the result. After moderate loads, my back also began to ache slightly, but it was tolerable, and any ointment/gel simply helped.
Unfortunately, I still can’t see if the condition of my cartilage in my spine has changed even a little. An MRI costs a lot of money, and you won’t get a free referral from us. Moreover, now I feel many times better than when I went to the doctor in the spring. As the neurologist said, if a pain relapse occurs, then go back to her, again injections, physio and, possibly, a free referral for an MRI. My case is most likely not curable and requires annual maintenance courses of therapy.
Rumalon may not act instantly, but methodically and persistently, and by the end of the course it brought me into relative order, relieved my back pain almost completely, and provided a long-term cumulative effect. It also has a natural composition, did not cause any allergies in me, and I endured the injections, which were not painful at all, with dignity). The only downside is the considerable price... it’s very expensive! Getting sick is always expensive, of course. But I don’t recommend skimping on Rumalon!
Contraindications, indications for treatment
The instructions recommend the use of Rumalon for degenerative lesions of the joints:
- for arthrosis, spondylosis, coxarthrosis;
- meniscopathies – pathologies of cartilage in the knee joints, accompanied by functional disorders and pain;
- spondyloarthrosis, gonarthrosis;
- chondromalacia – necrosis of cartilage cells.
The medicine is contraindicated in patients:
- with individual intolerance to the component composition;
- rheumatoid arthritis, thrombophlebitis;
- tendency to spontaneous bleeding.
Rumalon is not prescribed to pregnant and lactating women and minor patients. During lactation, the medication can be used provided that the newborn is transferred to artificial nutrition.
Particular caution and supervision by the attending physician is needed during treatment with the drug in patients with blood clotting disorders, diabetes mellitus, renal, and hepatic pathologies. The same requirements apply to women planning pregnancy and overweight or obese patients.
Discussion
To previous article
Adverse reactions
Therapeutic procedures can provoke non-standard responses of the body, manifested by:
- dizziness, cephalgia, erythema;
- nettle fever, eczema, maculopapular rash with itching or swelling;
- dyspeptic disorders, nausea with vomiting;
- allergies, anaphylaxis, Quincke's edema;
- painful sensations in the joints - the disorder does not require discontinuation of the medication and goes away spontaneously;
- local bleeding - in the area of drug administration.
The occurrence of side effects during therapy with Rumalon requires additional consultation with a doctor. The patient must describe in detail the deviations that have arisen, and the specialist must select a more suitable drug.
Research results
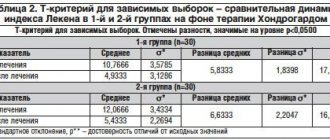
The drugs Artradol and Artracam are well tolerated, both in monotherapy and in combination therapy.
The occurrence of undesirable side reactions was observed during monotherapy with Artradol in 10%, and with Artracam in 9.99%. During a course of combination therapy, an increase in undesirable side reactions was revealed to 3.32%. No serious adverse events were identified. The association of adverse events with drug intake was possible or probable. To correct undesirable side effects, dietary measures were carried out; if gastralgia developed, NSAIDs were discontinued and antispasmodics were prescribed (once in all groups). When nausea occurred, the duration of which did not exceed 10 hours, metoclopramide was prescribed. The appearance of flatulence and facial redness did not require medication. Synovitis was controlled by prescribing NSAIDs in adequate doses. There was no need to discontinue therapy in the observation groups.
The need for NSAIDs in the observation groups decreased: after two weeks in 24 (80%) patients in group 2, in 23 (76.7%) in group 3, in 27 (90%) in group 4. After 1 month, the need for regular NSAID use continued to decrease. 27 (90%) in group 2 refused to take non-steroidal drugs, 28 (93.3%) in group 3, and 29 (96.7%) in group 4.
While monitoring patients with comorbid pathology, we did not identify any negative drug interaction reactions, which did not require changing the recommendations of specialists in the relevant field.
Nuances of Rumalon therapy
The instructions recommend intramuscular injections according to a specific scheme:
- the first day - 0.3 ml;
- the second – 0.5 ml;
- the remaining 1.5 months - 1 ml three times a week.
The frequency of treatment procedures is limited to two courses per year. For complex pathologies, therapy is repeated up to 4 times within 12 months, and can last for several years in a row.
If the recommended injection volume is accidentally exceeded, symptoms of overdose occur. Patients develop hemorrhages at the injection site, signs of allergy with redness, itching, swelling and skin rashes. Treatment consists of suppressing symptoms after stopping the medication.
Instructions from the manufacturer and interaction features
The instructions indicate the following nuances:
- therapeutic procedures with Rumalon are stopped when signs of an allergic reaction appear;
- the medication is prohibited for use by minors, pregnant and lactating women;
- Before using the medicine, a diagnostic examination and confirmation of the presumptive diagnosis is necessary.
Improvement in joint mobility, suppression of pain and other therapeutic effects are recorded 14-21 days from the start of treatment. The results obtained last for several months after completion of the procedures.
Rumalon improves the effect of individual drugs during joint therapy, the list includes:
- indirect anticoagulants;
- antiplatelet agents;
- fibrinolytics.
The combination with them requires regular checking of blood clotting speed. Combining Rumalon with NSAIDs allows you to reduce the dosage of the latter medications.
Osteoarthritis
The problem of osteoarthritis in the Russian Federation is one of the most pressing, occupying a leading position among all diseases of the musculoskeletal system in terms of prevalence. Epidemiological studies conducted over the past 3-5 years show that verified osteoarthritis by clinical and radiological manifestations is present in no less than 13% of the population of various ages, and its prevalence increases significantly in the elderly. This disease negatively affects performance, social activity, and self-care at home, significantly worsening the quality of life.
Previously, osteoarthritis was called a degenerative disease; now, according to leading experts, it is a chronic inflammatory process that affects not only cartilage tissue, but also the joint capsule, tendons, ligaments, and subchondral bone. Of particular interest is damage to the shoulder joints, and it should be noted that the opinion on the etiopathogenesis and clinical manifestations of this pathology is ambiguous.
Currently, the definition of “humeroscapular periarthritis” is not used; in our study, in accordance with the modern classification, patients with the following nosology related to periarticular lesions of the shoulder joints were included:
- Tendinitis of the rotator cuff muscles and bicipital tendonitis M 75.2;
- Calcific tendinitis M 75.3;
- Subacromial (impigment) collision syndrome M 75.1;
- Retractile capsulitis M 75.0;
Purpose of the study: to evaluate the effectiveness, safety and tolerability of the drugs “Artradol” and “Artracam” in patients with periarticular lesions of the shoulder joints (humeral periarthritis).
Materials and methods: the study included 120 patients with periarticular lesions of the shoulder joints, namely:
- Rotator cuff tendinitis and bicipital tendinitis – 34 people
- Calcific tendinitis – 38 people
- Subacromial (impigment) impingement syndrome – 27 people
- Retractile capsulitis – 21 people
We observed over time 47 (39.17%) men and 73 (60.83%) women, whose average age was 54.5 ± 8.37 years in the range from 45 to 75 years. There were 32 (26.66%) working in observation groups. There are 11 (9.17%) unemployed people of working age, 77 (64.17%) pensioners.
In the first (control) observation group, no chondroprotective drugs were prescribed, including Artradol and Artracam.
In the second group, every other day Artradol was administered intramuscularly, the dry substance was dissolved in 1 ml of water for injection. The first three injections contained a dose of 0.1 g, starting with the fourth injection, the dose increased to 0.2 g.
In the third group, Artracam was prescribed as a chondroprotector; the contents of one sachet were dissolved by patients in 200 ml of water and taken orally once a day for 6 weeks.
In the fourth group, a combined prescription of “Artradol” and “Artracam” was carried out according to the scheme of alternating intramuscular administration of “Artradol” 0.2 mg and “Artracam” 1 sachet orally every other day.
In the study, 35 0.1 mg ampoules of Artradol and 20 sachets of Artracam were prescribed per course of treatment per patient.
The study included patients with periarticular lesions of the shoulder joints (diagnosis of glenohumeral periartitis according to the ACR criteria of 1987). They had radiographic stage II or III of osteoarthritis of the acromioclavicular joint according to Kellgren-Lawrence. All patients signed informed consent.
The initial exclusion criteria were patients with secondary gonarthrosis, infectious arthritis, systemic inflammatory diseases, gout, pseudogout, Paget's disease, intra-articular fractures, ochranosis, acromegaly, hemochromatosis, Wilson's disease, primary chondromatosis, chondrocalcinosis, concomitant severe diseases (uncontrolled arterial hypertension, unstable angina pectoris, cardiovascular failure, diabetes mellitus type 1. Severe liver and kidney diseases), with a stomach or duodenal ulcer within the last month, with bleeding or a tendency to bleeding, with a history of thrombophlebitis, pregnancy, lactation, as well as body mass index more than 40 kg/m2. Also, individuals included in the study were not required to undergo intra-articular injections of any drugs within 6 weeks before the start of the study. Patients with known hypersensitivity to chondroitin sulfate or glucosamine sulfate were excluded.
The observed patients had a pronounced need to take non-steroidal anti-inflammatory drugs (NSAIDs) (within 30 days over the last 3 months). At the beginning of the study, NSAIDs were not discontinued; patients continued taking this group of drugs in accordance with the individual presence of contraindications, followed by a dose reduction or discontinuation of NSAIDs based on their well-being. So, they took: Nimesulide in a daily dose of 200 mg in the form of sachets or Nise tablets, Ketoprofen intramuscularly 100 mg or 2 capsules (200 mg) per day, Meloxicam tablets or injections 15 mg per day, Ibuprofen 1200 mg-2400 mg in capsules per day, Airtal 1 tablet 2 times a day or 1 sachet 2 times a day, Naysilat 600 mg 2 times a day in the form of tablets, strictly 1 hour before meals, Ibuklin 1 tablet 3 times a day before meals or 2-3 hours after food without chewing.
Assessing the need for NSAIDs, we emphasize that 97.5% of the patients included in the study took NSAIDs regularly. As mentioned above, the personalized selection of NSAIDs was controlled, taking into account contraindications and predicted unwanted side effects. The optimal dose of NSAIDs was selected, since in more than 13.33% it was unreasonably overestimated or underestimated.
During the therapy period, intra-articular injections of glucocorticoids, hyaluronic acid preparations and any other drugs were not performed. Also excluded from the list of prescriptions were drugs that have chondroprotective properties, such as anticoagulants, antiplatelet agents, fibrinolytics, and physiotherapy procedures were not performed.
Patients included in the study suffered from comorbid diseases. We identified 58 patients with verified nosologies, which accounted for 48.3% of all examined. Note that hypertension was diagnosed in 51 (42.5%), spondyloarthrosis of the thoracic and lumbosacral spine - in 29 (24.2%), atherosclerosis - in 21 (17.5%), discirculatory encephalopathy 2 - in 4 (3.3%), coronary heart disease – in 3 (2.5%), chronic obstructive bronchitis – 2 (1.6%), chronic cholecystitis outside the acute stage – 2 (1.6%), duodenal ulcer (history of exacerbations 1 and 2 years ago, respectively) - 2 (1.6%), chronic pyelonephritis, chronic renal failure stage I - 1 (0.8%), type 2 diabetes mellitus in the compensated stage - 1 (0. 8%). In 62 patients, comorbid conditions were not diagnosed, which amounted to 51.6%.
Patients were prescribed supportive pharmacotherapy for concomitant pathology: diuretics in combination with ACE inhibitors, as well as beta-blockers for the treatment of hypertension, NSAIDs and chondroprotectors for the treatment of spondyloarthrosis, statins for the correction of dyslipidemia, nootropics for patients with dyscirculatory encephalopathy, choleretic drugs and hepatoprotectors - for patients with chronic cholecystitis, proton pump inhibitors - for patients with peptic ulcer disease. Treatment of comorbid conditions was coordinated with specialists in the profile of concomitant pathology.
In the observed patients, initially, using a visual analog scale (VAS, mm), pain at night in bed, pain while sitting or lying down, pain in an upright position, pain when moving the upper limbs (active and passive) and pain on palpation were determined.
Safety assessment was carried out according to the following indicators: frequency and nature of adverse events that developed during the observation period, their relationship with the study drug (no connection, unlikely, possible, probable, definite, unknown). An adverse event was considered to be any medical event that occurred to the patient during participation in the study, regardless of connection with the therapy. The criterion for early exclusion of a patient from their study was the development of serious adverse events (events leading to death, threatening the patient’s life, and also requiring surgical intervention and additional therapy). All cases of adverse events were recorded in the study chart and primary documentation, and were also sent to the study sponsor. During the study, blood test parameters were monitored: clinical (hemoglobin, leukocytes, ESR) and biochemical (AST, ALT), urine analysis. During the open study, patients were excluded according to the following criteria: ineffectiveness of therapy for the underlying disease - persistence or worsening of pain requiring treatment adjustment, serious adverse events, refusal to participate in the study, as well as violation of the protocol.
Analogs
The occurrence of intolerance to the drug or side effects requires its replacement. The list of popular analogues is presented:
- Alflutop;
- Arthroi;
- Bishofite;
- Gialganom Phidias;
- Glucosamine-Chondroitin;
- Incenoy;
- KONDROnova;
- Movex Comfort;
- Piaskledin 300;
- Traumemel S;
- Chondroitin Complex;
- The goal of T.
The product has no complete structural analogues.