Today, more than 90% of the inhabitants of our planet are susceptible to herpes virus infections every year. In addition, 20% of those infected subsequently experience severe illness, leading to serious illness.
Only once having penetrated the human body, the herpes virus becomes its permanent resident. Over time, this infection, if left untreated, progresses and can lead to disability or death.
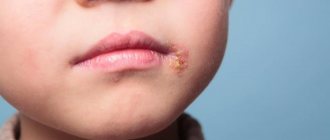
One of the popular viruses, type I virus affects the area of the lips, nose, certain areas of the skin on the face, oral mucosa, conjunctiva of the eyes, and also, in extreme cases, causes inflammation of the brain and its membranes. Type II virus manifests itself in the human genital area, causes intrauterine infection and acquires threatening disseminated forms.
Efficiency of using Vitagerpavak
Currently, the field of study and direct treatment of herpes diseases is developing significantly. About a year and a half ago in our country, medical scientists developed and introduced a new effective vaccine aimed at combating infections caused by viruses of the Herpesviridae family.
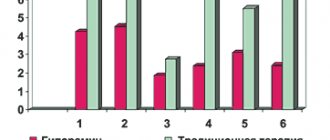
This vaccine, the name of which sounds like “Vitagerpavak,” was studied in the deepest sense for 5 years and, as a result of many studies conducted, was found to be reliable and highly effective: 63% of subjects after the procedure were completely free from infection, in 27% symptoms appeared much less frequently, and only in 8% of patients the frequency of disease manifestations remained the same.
No side effects have been identified when using the Vitagerpavac vaccine. Toxic reactions and other adverse effects on the human body are not observed.
Adverse reactions of Zostavax and Shingrix vaccines
The most common side effects of the drugs (which can affect more than 2 in 10 people) are reactions at the injection site, such as pain, redness and swelling.
In rare cases, other manifestations of a general reaction may be observed.
These are chills, fever, muscle pain, fatigue, headache, digestive system upset (nausea, vomiting, diarrhea and stomach pain).
Most of these effects last 2 to 3 days, and usually do not require special treatment.
Who is recommended for the Vitagerpavak vaccination?
- Patients who have exacerbations of chronic infections caused by Herpesviridae viruses three or more times over the course of one year.
- Elderly people.
- HIV-infected patients (stages I and II).
Today there is a fairly large number of various drugs, creams, ointments aimed at treating infections caused by Herpesviridae viruses. However, many treatments are not always effective, and the virus often returns again.
Prevention of Varicella Zoster in children
The American vaccines described above are generally used to vaccinate patients over 50 years of age.
Young people, as well as children, are offered the drug Varivax as an alternative as a method of preventing Human herpesvirus 3.
Varivax is a vaccine intended for active immunization of children 12 months and older.
Directions for use: Varivax is administered in the form of 0.5 ml by subcutaneous injection into the outer shoulder or thigh area.
These are the most optimal areas for administering such drugs.
Intramuscular or intravenous administration of the vaccine is not recommended.
The vaccine is intended for subcutaneous administration only.
Evidence suggests that inadvertent intramuscular administration of Varivax, while not increasing side effects, does produce a short-term immune response.
Viravax is not recommended for use in patients who are hypersensitive to gelatin or neomycin.
The vaccine is also contraindicated in cases of active febrile infection, tuberculosis, immunodeficiency, and leukemia.
Immunosuppressive therapy and pregnancy are also contraindications to the administration of the drug.
Like other known vaccines, Virovax cannot provide 100% protection for all patients against naturally acquired Human herpesvirus 3.
But, nevertheless, it showed impressive results in research.
Clinical trials assessed efficacy 6 weeks after a single dose in individuals under 12 years of age and 6 weeks after a second dose in older patients.
In the first case, the effectiveness of the vaccine was 86%, in the second - 98%.
Vaccination may be considered as a preventive method for patients with immunodeficiencies.
When the benefits outweigh the risks (eg, asymptomatic HIV, IgG deficiency, congenital neutropenia, chronic granulomatous disease).
If it is impossible to vaccinate with Virovax, in Russian pharmacies it is possible to purchase an analogue of the drug Varilrix.
The composition of the vaccine is absolutely identical; the medicine is produced in the UK.
British vaccines are distinguished by their effectiveness and reduced risk of side effects.
Where and how is vaccination against herpes carried out?
An excellent way to effectively combat herpes is the Vitagerpavac vaccination procedure.
In the network of multidisciplinary clinics “Policlinika.ru” you can take a course of vaccination against herpes virus infection. The course of this treatment involves administering 5 injections to the patient. Injection therapy is carried out at intervals of a week (or 10 days, in the case of complicated herpes infection). A repeat course of 5 injections is indicated after 6 months. This drug involves intradermal administration.
Prevention and treatment of herpes virus infection with the help of the Vitagerpavak vaccination is your unique opportunity to forget about such an unpleasant disease as herpes once and for all. Our experienced specialists conduct a detailed examination of each patient with complaints of herpes virus infection and prescribe an individual course of comprehensive treatment.
Features of a person’s immune status, his indications or contraindications for the vaccination procedure - these and a number of other reasons are important components in the plan and outcome of treatment of infections caused by viruses of the Herpesviridae family.
That is why our doctors treat each of their patients with care and attention, use an individual approach in their practice that takes into account all the characteristics of their body, accurately diagnose and draw up an adequate treatment plan.
In addition to the vaccination procedure itself, you will receive a professional consultation from a doctor who will give some simple tips on maintaining the overall favorable state of your health and ways to effectively combat herpesvirus infections.
Contraindications to the use of herpes vaccines
Like any medications, vaccines have their own indications for use and contraindications.
Zostavax and Shingrix are not prescribed to patients with the following conditions:
- ARI/ARVI (respiratory diseases)
Any respiratory disease (including open tuberculosis) or other active infections that are accompanied by fever are a contraindication for vaccination.
The vaccine can be administered to patients with an extremely mild course of acute respiratory infections/ARVI, which are not accompanied by severe symptoms.
The decision to delay vaccination depends on the severity of symptoms and the etiology of the disease.
- Immunodeficiency
Vaccination is not carried out for patients with severe forms of immunodeficiency:
- acquired immunodeficiency syndrome (AIDS)
- Bruton's disease (agammaglobulinemia)
- human immunodeficiency virus (HIV)
- hypogammaglobulinemia
- IgA deficiency
- severe combined immunodeficiency (SCID)
- neoplastic processes
In addition, vaccination is not prescribed to patients undergoing chemotherapy and treatment with corticosteroid drugs.
- Pregnancy
Vaccination with Zostavax and Shingrix is contraindicated for use during pregnancy.
The main reason is the lack of adequate studies that could show the effect of drugs on the fetus and the ongoing pregnancy.
- Breastfeeding (lactation)
The manufacturer recommends that vaccination be prescribed to patients during lactation with extreme caution.
Many experts recommend avoiding vaccinations during breastfeeding or temporarily stopping breastfeeding.
Nothing is known about the possibility of the active ingredients passing into breast milk.
- Hypersensitivity to gelatin and neomycin
The vaccines do not contain preservatives, but do contain residual amounts of neomycin.
Therefore, these drugs are not recommended for use in patients with hypersensitivity to neomycin.
Also not recommended for patients allergic to gelatin.
Intramuscular or intravenous administration of smallpox vaccine is not recommended.
The vaccine is intended for subcutaneous administration only, preferably in the shoulder area.
Russian vaccines against herpes
Vitagerpavak and Gerpovax are the most popular .
Both vaccines are analogues of each other, containing antibodies from herpes virus types 1 and 2.
As additional substances, the medications contain gelato, sucrose, and gentamicin.
The action of the drugs is aimed at stimulating cellular immunity and increasing the body's resistance to viral infections.
Vaccines have been shown to reduce the severity of the clinical symptoms of herpes and provide long-lasting immunity.
In addition, vaccination with these drugs increases the duration of remission.
Vitagerpavac is a polyvalent herpes vaccine.
Recommended for women planning pregnancy and who have a history of previous herpes therapy.
This vaccine is especially recommended as a prevention against genital herpes.
It is also recommended to administer the drug to patients who have a high titer of antibodies to Herpes simplex.
Even in the absence of symptoms of a viral infection.
Side effects include slight redness in the injection area.
Other adverse reactions may include: fever, chills, muscle soreness, worsening symptoms of herpes.
In general, the herpes vaccine Vitagerpavac is well tolerated and does not cause significant deterioration in well-being.
Side effects
Most often, after vaccination, negative general phenomena were noted, accompanied by weakness and fever (up to 37.5 ° C), which went away on their own.
In some cases, during the first day after administration of the vaccine , the formation of local reactions was observed, expressed by skin hyperemia at the injection site with a diameter of up to 2 cm and a weak, fast-passing burning sensation .
In the event of more serious symptoms of a general and local nature or the development of a relapse of herpes, vaccination is temporarily stopped. Repeated administration of the drug can be carried out 14 days after the disappearance of all negative clinical symptoms.
| (7.92 KB in archive, MS Word format) | “Laboratory diagnostics” No. 3(7) 2012 |
| WHEN PUBLISHING MATERIALS, A LINK TO THE SOURCE IS MANDATORY!!! | |
| SUBSCRIBE TO THE MAGAZINE | |
The use of herpetic vaccine for the treatment and prevention of relapses of herpetic infection
Ph.D. Shitikova G. S., Petrovsky S. V., Abrosimova Yu. Yu.
Research Institute of Vaccines and Serums and Enterprise for the Production of Bacterial Preparations, St. Petersburg, Russia
The results of complex therapy of chronic recurrent herpetic infection in patients with clinical manifest form of the disease are presented. The complex therapy regimen included the prescription of drugs with antiviral effects, immunomodulators, interferon, multivitamins and the use of infrared laser. Two weeks after the disease entered the remission stage, a course of vaccination with the cultural inactivated vaccine “Gerpovax” was prescribed. Positive dynamics of treatment were noted. Clinical trials have shown that the vaccine stimulates the resistance mechanisms of the human body and provides protection to the patient from relapses of herpes infection (HI) over a 1.5-3 year observation period.
In Russia and the CIS, about 20 million people are infected annually with various forms of herpetic infection, which allows us to consider GI an important medical and social problem of modern healthcare. Herpes infection caused by herpes simplex viruses can occur in the form of an opportunistic infection or have an independent meaning. This disease is characterized by polymorphism of clinical manifestations, a persistent nature of the course with the manifestation of infection against the background of immunosuppression. A persistent virus can be activated under any unfavorable conditions. The most common causes are stress, pregnancy, injury, sun exposure, infection, and hypothermia. According to the serological survey (RSS), the prevalence of HI among pregnant women is 14–19% in European countries, 22–36% in the USA, and 35% in Russia. The mortality rate from neonatal herpes is 50–70%; Only 15% of children remain healthy [1].
The spectrum of clinical manifestations of HI is significantly diverse. The most typical forms of herpetic infection, depending on the clinical picture and localization of the pathological process, in addition to genital herpes, are: herpetic lesions of the mucous membranes of the gastrointestinal tract, lesions of the eyes, skin, nervous system, generalized herpes simplex. There are also atypical forms of the course of HI - they are more common in people with immunodeficiency conditions. The widespread prevalence of HI, the severity of the course and numerous complications make timely and effective treatment of this disease necessary. This is very important, since at present there is no longer any doubt that the herpes simplex virus (HSV) plays the role of a cofactor in cervical carcinogenesis.
Today, the main directions of treatment and prevention of herpesvirus infections are chemotherapy, immunotherapy, or complex treatment with a combination of these methods, with correction of the patient’s immune status, as well as the use of local drugs. Currently, a number of effective chemotherapeutic drugs with antiherpetic activity have been created [2]. Clinical experience with their use has shown that Acyclovir, Arbidol, Foscarnet, Valaciclovir, Famciclovir, while quickly and effectively stopping acute manifestations of GI, do not prevent recurrence of chronic herpetic infection.
To increase the effectiveness of treatment, it is necessary to include immunobiological drugs in treatment regimens. However, it is not always possible to avoid relapse of the disease; therefore, it is necessary to continue treatment during the inter-relapse period in order to consolidate the therapeutic effect and correct residual immune disorders. For the final stage of treatment, it is recommended to use a herpetic vaccine, which gives a pronounced anti-relapse effect. In order to prevent relapses of GI, including genital herpes, in the phase of clinical and immunological remission, the antiherpetic cultural inactivated dry vaccine “Gerpovax” was used, which was developed and produced at the enterprise for the production of bacterial preparations of the St. Petersburg Research Institute of Vaccines and Serums using improved technology. The Herpovax vaccine is prepared on the basis of reproductive and immunologically competent strains of herpes simplex viruses I (US) and II (VN) antigenic types provided by prof. I. F. Barinsky. The substrate for obtaining the vaccine is a primary culture of chicken fibroblast cells.
Conditions have been developed for increasing the reproductive activity of seed strains of HSV, purifying the vaccine and stabilizing semi-finished products. The herpetic vaccine was inactivated with formaldehyde. The specific response obtained in the immune sera of laboratory animals was determined by the neutralization index, which should be at least 1.5 - 2.0 lg TCD50/0.1 ml. Control of sterility, absence of foreign contaminants, harmlessness, authenticity and specific activity was carried out in accordance with approved regulatory documentation. The Herpovax vaccine is purified from ballast proteins. The protein content in the semi-finished product is no more than 80 μg/ml; bovine serum albumin content – no more than 0.5 µg/ml. The vaccine is non-toxic and does not contain human albumin, which, in our opinion, generally reduces the allergenicity of the drug. Available in dry form, 0.3 ml in an ampoule (1 dose - 0.2 ml). The dry vaccine is stored for up to 2.5 years at temperatures from 2 to 8 0C, is easily transported and retains immunological activity and therapeutic effectiveness. The dissolved vaccine is an opalescent liquid from yellowish-red to red, without sediment or inclusions.
Basic vaccination regimen with inactivated vaccine: 0.2 ml of vaccine is injected intradermally into the flexor surface of the forearm. The vaccination cycle consists of 5 injections, which are carried out at intervals of 2-3 days. The main course includes 2 cycles with 10-day breaks between them. For a lasting preventive effect, repeated courses of vaccination are necessary after 6-12 months. There are other vaccine administration regimens.
According to clinical trials of the herpetic vaccine, its therapeutic effect is accompanied by increased cellular immunity reactions (specific killer activity of lymphocytes, blast transformation reactions, delayed leukocyte migration, increased levels of serum interferon, etc.) [2].
The therapeutic effect of the inactivated cultural vaccine “Gerpovax” was confirmed by us together with clinicians in the process of selective observation and complex treatment of patients suffering from various forms of recurrent herpetic infection. 2391 patients with various forms of HI were under observation. Clinical manifestations of herpes occurred in individuals aged 28 to 45 years, and the predominant form of infection was genital herpes, which affected 44% of those observed. Slightly fewer of those who sought medical help were patients with labial herpes (35%), ophthalmic herpes, skin rashes and other forms of GI. Treatment of patients was comprehensive and aimed at increasing the duration of remission. This was achieved by using chemotherapy drugs, immunomodulators, interferon drugs, multivitamins and using an infrared laser for treatment. Two weeks after the disease entered the remission stage, treatment with a herpetic vaccine began. The effectiveness of treatment was assessed by reducing the clinical manifestations of herpetic infection, prolonging the period of remission and the absence of the HSV viral antigen in tests from the patient according to PCR data 3, 6 and 12 months after therapy [3].
Clinical trials have shown that the vaccine stimulates the human body’s resistance mechanisms to this infection and provides protection for the patient from relapses over a 1.5-3 year observation period. In case of ophthalmoherpes, the use of the vaccine ensures cure of patients in 62% of cases, prolongation of the inter-relapse period and reduction in the severity of clinical manifestations in 28% of cases [4].
CONCLUSIONS. Summing up the results of the use of “Gerpovax” in the complex treatment of patients with GI, we can assume that the vaccine provides a gradual increase in the duration of remission, alleviation of the course of the disease, and possible complete disappearance of the symptoms of herpetic infection. Successful treatment of herpes is possible!
Literature:
1. Isakov V.A. and others. Modern therapy of herpesvirus infections / V.A. Isakov,
S.A. Selkov, L.K. Monshetova, G.M. Chernakova. – St. Petersburg. – M., 2004.
2. Barinsky I.F., Kasparov A.A., Lazarenko L.A.. // Biopreparations. – 2002. – T. 6, No. 2 – P. 18-21.
3. Shitikova G.S. and others. “Gerpovax” – ten years of production and application in healthcare practice / G.S. Shitikova, Yu.Yu. Abrosimova, O.V. Zhunko, M.V. Potapchuk // Materials of the All-Russian scientific conference with international participation, dedicated to the 100th anniversary of the founding of the “Immunopreparat” branch of the FSUE “NPO Microgen” of the Ministry of Health and the SRRF. – M., 2005. – Part I – pp. 213-215.
4. Shitikova G.S. and others. The use of a vaccine for the treatment of patients with recurrent herpes virus infection / G.S. Shitikova, Yu.Yu. Abrosimova, L.V. Andrianova // Materials of the III interdisciplinary scientific and practical conference “Urogenital infections and reproductive health: clinical and laboratory diagnostics and therapy.” – M., 2010. – P. 83-84.
Contraindications
The use of Vitagerpavac is contraindicated in:
- observed relapses of herpes infection that are in the active stage ( vaccination can be carried out 14 days after the cessation of clinical symptoms; for manifestations of ophthalmic herpes after 30 days);
- pregnancy;
- acute diseases of infectious and non-infectious etiology ( vaccination can be carried out 30 days after complete recovery);
- high personal sensitivity to Gentamicin and other aminoglycosides ;
- malignant neoplasms;
- exacerbation of chronic diseases ;
- pathologies that are in the stage of decompensation.
special instructions
vaccination course must be completed in special medical institutions ( dispensary , hospital , clinic ) by medical personnel trained for this purpose.
The first intravenous injection can be carried out only in the stage of complete remission , after at least 5 days have passed after the disappearance of all clinical symptoms of the previous manifestation of herpes .
If severe general and/or local negative reactions the vaccination of the vaccine should be stopped, with possible resumption of vaccination only if their clinical symptoms completely disappear.
All episodes of severe general and/or local negative reactions should be carefully investigated and reflected in the appropriate documents.
All procedures for preparing the vaccine and its further use must be carried out under aseptic conditions .
The prepared solution (dissolved vaccine ) should be pink and slightly opalescent.
The drug is considered unsuitable for use if the integrity of the bottle is damaged, the inscriptions on the label are smeared, color changes, storage conditions are not observed and the expiration date has expired.
vaccine solution cannot be stored.