Cough is a common occurrence in children and adults during seasonal illnesses. Many viral and colds are combined with a cough. This unpleasant phenomenon can be eliminated with the help of folk remedies and pharmacological drugs.
You can find quite a few of these in pharmacies nowadays. It is known that cough can be dry and wet. Depending on the type of cough, you can choose the appropriate drug. Medicines for wet coughs can be harmful for dry coughs, and vice versa. Therefore, you should not choose cough remedies on your own.
Compound
| Pills | 1 table |
| active substances: | |
| butamirate citrate | 4 mg |
| guaifenesin | 100 mg |
| excipients: colloidal silicon dioxide; mannitol; MCC; glyceryl tribehenate; magnesium stearate |
| Drops for oral administration | 1 ml |
| active substances: | |
| butamirate citrate | 4 mg |
| guaifenesin | 100 mg |
| excipients: ethanol 96% - 0.3 g; floral flavor (aroma of alpine flowers) - 0.002 g; purified water - 0.007 g; polysorbate 80 - 0.001 g; liquid licorice extract - 0.003 g; propylene glycol - up to 1 ml |
What kind of medicine is Stoptussin?
The very name of the drug, containing the word “stop,” indicates that it is intended to stop coughing. This medicine is designed for dry cough. The active ingredient guaifenesin can resolve phlegm, neutralizing it. And another substance - butamirate citrate - eliminates sore throat, eliminating the urge to cough.
The drug is available in the form of tablets and tinctures. The tincture contains ethanol and licorice extract. Stoptussin-Fito syrup is also available on sale. It is usually prescribed to children because it has a pleasant taste and aroma. In addition to being an antitussive, the drug has a weak expectorant effect.
Pharmacodynamics
A combined drug that has an antitussive and expectorant effect.
Butamirate has a peripheral local anesthetic effect on the sensitive nerve endings of the bronchial mucosa, which provides an antitussive effect.
Guaifenesin increases the secretion of bronchial glands and reduces the viscosity of mucus. An increase in secretion is caused both by a direct effect on the bronchial glands - stimulation of secretion from the bronchial glands and removal of acidic glycoproteins from acinar cells, and by a reflex pathway, when irritation of afferent parasympathetic fibers of the gastric mucosa occurs and depression of the respiratory center. Increased tone of N.vagus
stimulates the production of bronchial secretions. The mucus produced by the bronchial glands increases the activity of the ciliated epithelium, as a result of which the evacuation of mucus from the bronchi and its coughing are facilitated.
What kind of cough is “Stoptussin-Fito” syrup for?
This medicine is intended for dry cough because it has the ability to stop coughing. The substance quickly penetrates the blood after use, then enters the bronchi, inhibiting secretion, thinning mucus, affecting nerve endings and preventing spasm. The substance is excreted through the kidneys and minimally through the intestines.
If you take the drug with a wet cough, it will create an antitussive effect. Mucus will accumulate in the bronchi, increasing inflammation. Thus, the condition may worsen significantly.
"Stoptussin" is intended only for dry and irritating cough that appears with ARVI, pharyngitis, laryngitis, tracheitis, whooping cough. The drug is able to soften the upper respiratory tract and alleviate the condition. It especially helps with whooping cough, which often affects children. Those parents who have encountered this disease know that children cough all night long. This prevents them from sleeping. And when using this drug, the condition is alleviated, the child can fall asleep peacefully.
Pharmacokinetics
When taken orally, butamirate citrate is quickly and completely absorbed. Plasma protein binding is 94%. It is metabolized to form two metabolites, which also have an antitussive effect. Metabolites are excreted mainly by the kidneys (90%) and only a small part through the intestines. T1/2 - 6 hours.
Guaifenesin, when taken orally, is rapidly absorbed from the gastrointestinal tract. Communication with plasma proteins is insignificant. Guaifenesin is rapidly metabolized to form inactive metabolites that are excreted by the kidneys. T1/2 - 1 hour.
Side effects
If the recommended dosage regimen is followed, patients usually tolerate the drug well. Nausea, vomiting, diarrhea, decreased appetite, stomach pain, dizziness, headache, drowsiness, hives and skin rash may occur. These effects occur in approximately 1% of patients and usually resolve without dose reduction.
The frequency of adverse reactions listed below was determined according to the following criteria: very often (at least 1/10); often (more than 1/100, less than 1/10); uncommon (more than 1/1000, less than 1/100); rare (more than 1/10000, less than 1/1000); very rare (less than 1/10000), including isolated reports.
From the side of the central nervous system:
often - headache.
From the organ of hearing and balance:
often - dizziness.
From the digestive organs:
often - anorexia, nausea, abdominal pain, vomiting, diarrhea; very rarely - bitter taste in the mouth, heartburn, feeling of heaviness in the epigastrium (tablets).
For the skin and subcutaneous fat:
very rarely - exanthema, urticaria (tablets, oral drops), itching, hot flashes (tablets).
Additionally for tablets
From the respiratory system:
very rarely - shortness of breath.
From the SSS side:
rarely - chest pain; very rarely - tachycardia, palpitations.
Other:
very rarely - pain around the eyes.
Additionally for oral drops
From the kidneys and urinary tract:
very rarely - urolithiasis.
Stoptussin, drops 10ml (Ivex, CZECH REPUBLIC)
Compound.
| active substances: | |
| butamirate citrate | 4 mg |
| guaifenesin | 100 mg |
| excipients: colloidal silicon dioxide; mannitol; MCC; glyceryl tribehenate; magnesium stearate |
| Drops for oral administration | 1 ml |
| active substances: | |
| butamirate citrate | 4 mg |
| guaifenesin | 100 mg |
| excipients: ethanol 96% - 0.3 g; floral flavor (aroma of alpine flowers) - 0.002 g; purified water - 0.007 g; polysorbate 80 - 0.001 g; liquid licorice extract - 0.003 g; propylene glycol - up to 1 ml |
Description of the dosage form.
Pills:
flat-cylindrical, white or almost white with a chamfer and a dividing line.
Drops for oral administration:
transparent viscous liquid from yellow to yellowish-brown.
Pharmachologic effect. Expectorant, antitussive.
Pharmacodynamics.
A combined drug that has an antitussive and expectorant effect.
Butamirate has a peripheral local anesthetic effect on the sensitive nerve endings of the bronchial mucosa, which provides an antitussive effect.
Guaifenesin increases the secretion of bronchial glands and reduces the viscosity of mucus. An increase in secretion is caused both by a direct effect on the bronchial glands - stimulation of secretion from the bronchial glands and removal of acidic glycoproteins from acinar cells, and by a reflex pathway, when irritation of afferent parasympathetic fibers of the gastric mucosa occurs and depression of the respiratory center. Increased tone of N.vagus
stimulates the production of bronchial secretions. The mucus produced by the bronchial glands increases the activity of the ciliated epithelium, as a result of which the evacuation of mucus from the bronchi and its coughing are facilitated.
Pharmacokinetics.
When taken orally, butamirate citrate is quickly and completely absorbed. Plasma protein binding is 94%. It is metabolized to form two metabolites, which also have an antitussive effect. Metabolites are excreted mainly by the kidneys (90%) and only a small part through the intestines. T1/2 - 6 hours.
Guaifenesin, when taken orally, is rapidly absorbed from the gastrointestinal tract. Communication with plasma proteins is insignificant. Guaifenesin is rapidly metabolized to form inactive metabolites that are excreted by the kidneys. T1/2 - 1 hour.
Indications.
● dry cough and irritating cough of various etiologies (including infectious and inflammatory diseases of the upper and lower respiratory tract).
Additionally for oral drops
● relief of cough in the pre- and postoperative period.
Contraindications.
● increased sensitivity to the components of the drug;
● myasthenia gravis;
● children under 12 years of age - for tablets, up to 6 months - for drops for oral administration;
● I trimester of pregnancy;
● breastfeeding period.
Use during pregnancy and breastfeeding.
The drug Stoptussin® should not be used in the first trimester of pregnancy. If it is necessary to use it in the 2nd and 3rd trimesters, you should make sure that the expected benefit to the mother outweighs the potential risk to the fetus.
There is no reliable information about the penetration of butamirate and guaifenesin into breast milk. It is necessary to stop breastfeeding while using the drug Stoptussin®.
Side effects.
If the recommended dosage regimen is followed, patients usually tolerate the drug well. Nausea, vomiting, diarrhea, decreased appetite, stomach pain, dizziness, headache, drowsiness, hives and skin rash may occur. These effects occur in approximately 1% of patients and usually resolve without dose reduction.
The frequency of adverse reactions listed below was determined according to the following criteria: very often (at least 1/10); often (more than 1/100, less than 1/10); uncommon (more than 1/1000, less than 1/100); rare (more than 1/10000, less than 1/1000); very rare (less than 1/10000), including isolated reports.
From the side of the central nervous system:
often - headache.
From the organ of hearing and balance:
often - dizziness.
From the digestive organs:
often - anorexia, nausea, abdominal pain, vomiting, diarrhea; very rarely - bitter taste in the mouth, heartburn, feeling of heaviness in the epigastrium (tablets).
For the skin and subcutaneous fat:
very rarely - exanthema, urticaria (tablets, oral drops), itching, hot flashes (tablets).
Additionally for tablets
From the respiratory system:
very rarely - shortness of breath.
From the SSS side:
rarely - chest pain; very rarely - tachycardia, palpitations.
Other:
very rarely - pain around the eyes.
Additionally for oral drops
From the kidneys and urinary tract:
very rarely - urolithiasis.
Interaction.
Guaifenesin enhances the analgesic effect of paracetamol and acetylsalicylic acid, the effect of alcohol, sedatives, hypnotics and general anesthetics on the central nervous system, and the effect of muscle relaxants.
When determining the concentration of vanillylmandelic acid and 5-hydroxyindoleacetic acid using nitrosonaphthol as a reagent, false positive results may be obtained. Therefore, treatment with guaifenesin should be discontinued 48 hours before urine collection for this test.
Method of administration and dose.
Inside
, after meal.
Pills
The tablets are taken whole, without chewing, with liquid (water, tea, fruit juice).
For adults and children over 12 years of age, the drug is dosed depending on the patient’s body weight: less than 50 kg - 1/2 tablet. 4 times a day; 50–70 kg - 1 tablet. 3 times a day; 70–90 kg - 1.5 tablets each. 3 times a day; more than 90 kg - 1.5 tablets. 4 times a day.
The interval between doses should be 4–6 hours.
Drops for oral administration
The appropriate number of drops is dissolved in 100 ml of liquid (water, tea, fruit juice).
Dosage depending on the patient’s body weight: less than 7 kg - 8 drops 3-4 times a day; 7–12 kg – 9 drops 3–4 times a day; 12–20 kg - 14 drops 3 times a day; 20–30 kg – 14 drops 3–4 times a day; 30–40 kg – 16 drops 3–4 times a day; 40–50 kg - 25 drops 3 times a day; 50–70 kg - 30 drops 3 times a day; - more than 70 kg, 40 drops 3 times a day.
The interval between doses should be 6–8 hours.
Drinking plenty of fluids during the course of treatment enhances its effectiveness. When used in children weighing less than 7 kg, a dose reduction may be possible due to the fact that the child does not drink all 100 ml of the prepared mixture; however, the total concentration of the drug should not be exceeded.
If there is no positive effect, you should consult a doctor.
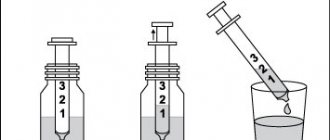
For precise dosing of the drug, you can use a dosing syringe (if supplied) (Fig. 1).
The scale on the syringe shows the number of drops in the measured dose.
Instructions for using the dosing syringe
1. Unscrew the cap of the bottle (counterclockwise).
2. Place the syringe in the adapter on the neck of the bottle in a vertical position (Fig. 2).
3. The bottle with the syringe placed in it should be turned upside down. It is necessary to hold the syringe tightly and, by pulling out the piston, draw the required amount of the drug (Fig. 3).
4. If necessary, measure a dose of 40 drops, take 10 and 30 drops alternately.
5. Turn the bottle upside down into a vertical position.
6. Carefully remove the syringe with the drug from the bottle adapter.
7. Dissolve the collected dose of the drug in water, tea or fruit juice.
8. Screw on the bottle with the drug (clockwise).
9. After use, rinse the syringe with warm water.
Overdose.
Symptoms:
in case of overdose, signs of the toxic effects of guaifenesin predominate - drowsiness, muscle weakness, nausea, vomiting, urolithiasis.
Treatment:
in case of overdose, consult a doctor. Guaifenesin does not have a specific antidote. Gastric lavage, activated charcoal, and symptomatic therapy (support of cardiovascular, respiratory and renal functions, electrolyte balance) are prescribed.
Special instructions.
If symptoms persist, a change in treatment should be considered.
The drug should not be prescribed for productive cough in patients suffering from long-term or chronic cough (including those caused by smoking), bronchitis or emphysema.
Impact on the ability to drive a car or perform work that requires increased speed of physical and mental reactions.
The drug Stoptussin® may have an adverse effect on the ability to drive vehicles and engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions, due to the fact that it can cause dizziness and other side effects.
Release form.
Pills.
10 tablets each in a blister made of aluminum foil and PVC film; 2 blisters in a cardboard box.
Drops for oral administration.
10, 25 or 50 ml in dark glass bottles with PE droppers, caps with tamper evident. 1 fl. in a cardboard box.
50 ml in dark glass bottles with PE droppers, tamper evident caps and a PE syringe for dosing. 1 fl. in a cardboard box.
Manufacturer.
Pills
Teva Czech Enterprises s.r.o., Czech Republic, Ostravska 29, 747 70, Opava-Komarov.
Holder of the registration certificate: Teva Czech Enterprises s.r.o., Czech Republic.
Address for receiving claims: 119049, Moscow, st. Shabolovka, 10, building 2 (Business).
Tel/fax/35/36.
Drops for oral administration
Teva Czech Enterprises s.r.o., Czech Republic, Ostravska 29/305, 747 70, Opava-Komarov.
Registration Certificate Holder: Teva Pharmaceutical Enterprises Ltd., Israel.
Address for receiving claims: 119049, Moscow, st. Shabolovka, 10, building 2.
Tel.; fax/36.
Conditions for dispensing from pharmacies.
Over the counter.
Directions for use and doses
Inside
, after meal.
Pills
The tablets are taken whole, without chewing, with liquid (water, tea, fruit juice).
For adults and children over 12 years of age, the drug is dosed depending on the patient’s body weight: less than 50 kg - 1/2 tablet. 4 times a day; 50–70 kg - 1 tablet. 3 times a day; 70–90 kg - 1.5 tablets each. 3 times a day; more than 90 kg - 1.5 tablets. 4 times a day.
The interval between doses should be 4–6 hours.
Drops for oral administration
The appropriate number of drops is dissolved in 100 ml of liquid (water, tea, fruit juice).
Dosage depending on the patient’s body weight: less than 7 kg - 8 drops 3-4 times a day; 7–12 kg – 9 drops 3–4 times a day; 12–20 kg - 14 drops 3 times a day; 20–30 kg – 14 drops 3–4 times a day; 30–40 kg – 16 drops 3–4 times a day; 40–50 kg - 25 drops 3 times a day; 50–70 kg - 30 drops 3 times a day; - more than 70 kg, 40 drops 3 times a day.
The interval between doses should be 6–8 hours.
Drinking plenty of fluids during the course of treatment enhances its effectiveness. When used in children weighing less than 7 kg, a dose reduction may be possible due to the fact that the child does not drink all 100 ml of the prepared mixture; however, the total concentration of the drug should not be exceeded.
If there is no positive effect, you should consult a doctor.
For precise dosing of the drug, you can use a dosing syringe (if supplied) (Fig. 1).
The scale on the syringe shows the number of drops in the measured dose.
Instructions for using the dosing syringe
1. Unscrew the cap of the bottle (counterclockwise).
2. Place the syringe in the adapter on the neck of the bottle in a vertical position (Fig. 2).
3. The bottle with the syringe placed in it should be turned upside down. It is necessary to hold the syringe tightly and, by pulling out the piston, draw the required amount of the drug (Fig. 3).
4. If necessary, measure a dose of 40 drops, take 10 and 30 drops alternately.
5. Turn the bottle upside down into a vertical position.
6. Carefully remove the syringe with the drug from the bottle adapter.
7. Dissolve the collected dose of the drug in water, tea or fruit juice.
8. Screw on the bottle with the drug (clockwise).
9. After use, rinse the syringe with warm water.
How many days to take Stoptussin tablets
The tablets should be taken without chewing and washed down with water. The dosage is calculated as follows: if the patient’s weight is less than fifty kilograms, then half a tablet is taken. If your weight is from fifty to seventy kilograms, then you need to take one tablet. And if you weigh more than seventy, then you need to take one and a half tablets.
The tablets are taken three times a day. The duration of the course is prescribed by the doctor individually and depends on the severity of the process. Typically, the average duration of treatment is two weeks.
special instructions
If symptoms persist, a change in treatment should be considered.
The drug should not be prescribed for productive cough in patients suffering from long-term or chronic cough (including those caused by smoking), bronchitis or emphysema.
Impact on the ability to drive a car or perform work that requires increased speed of physical and mental reactions.
The drug Stoptussin® may have an adverse effect on the ability to drive vehicles and engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions, due to the fact that it can cause dizziness and other side effects.
Manufacturer
Pills
Teva Czech Enterprises s.r.o., Czech Republic, Ostravska 29, 747 70, Opava-Komarov.
Holder of the registration certificate: Teva Czech Enterprises s.r.o., Czech Republic.
Address for receiving claims: 119049, Moscow, st. Shabolovka, 10, building 2 (Business).
Tel/fax/35/36.
Drops for oral administration
Teva Czech Enterprises s.r.o., Czech Republic, Ostravska 29/305, 747 70, Opava-Komarov.
Registration Certificate Holder: Teva Pharmaceutical Enterprises Ltd., Israel.
Address for receiving claims: 119049, Moscow, st. Shabolovka, 10, building 2.
Tel.; fax/36.