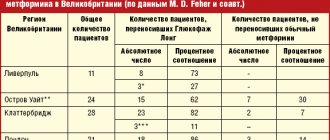
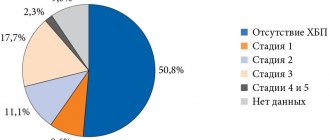
Detailed description of the study
Coagulation factor VIII is an important component of the coagulation cascade. The exact site of production of factor VIII is unknown, but research data indicate that the main source of its synthesis is the liver.
Recent in vivo studies identify the vascular endothelium (the inner lining of blood vessels) and megakaryocytes as a more precise site of production of this protein. This hypothesis is due to the fact that the level of blood clotting factor VIII increases sharply with severe damage to liver cells. Therefore, the source of factor VIII is apparently not the liver parenchyma (the main functioning tissue of organs such as the liver, kidneys, and spleen).
Coagulation factor VIII in blood plasma is presented in a series of forms and is found in a concentration of 0.1-0.2 μg/ml. Almost all factor VIII in plasma is bound to von Willebrand factor, which stabilizes and protects coagulation factor VIII in the bloodstream from destruction by proteases.
The functional activity of blood factor VIII is to ensure the internal mechanism of blood coagulation. The antihemophilic factor, with the participation of calcium ions, forms a complex with coagulation factor IX, which subsequently activates blood coagulation factor X. This process ensures the conversion of prothrombin into thrombin, which, in turn, completes the conversion of fibrinogen into fibrin, the main component of the blood clot.
Lack of activity of blood clotting factor VIII causes the development of hemophilia A, a sex-linked disease (inheritance of a gene located on the sex chromosomes) and manifested by a complex of internal and external bleeding.
For genetic diseases with this type of inheritance, a characteristic feature is the predominant manifestation of the disease in men, while women are only carriers of the defective gene. The incidence of hemophilia A in men is 14:100,000 people. Only isolated cases of the disease are possible among females.
The level of blood factor VIII deficiency can be different, which makes it possible to distinguish between severe, moderate and mild forms of hemophilia. In severe forms, no more than 1% of the active factor is found in the blood plasma of patients, i.e. 1 unit of clotting factor VIII per 1 dl of blood. This condition is manifested by frequent and massive hemorrhages, even with small and unnoticeable injuries. Particularly severe hemorrhages occur in joints and muscles.
The average severity of hemophilia A requires the presence of factor VIII in a volume of 2 to 4 units/dl in the blood plasma. Bleeding in such patients occurs with moderately severe injuries.
In patients with mild hemophilia, 5 to 30 units/dL of factor VIII are found in the plasma. Bleeding occurs during extensive trauma, childbirth, and surgery. The normal level of factor VIII in the blood is from 50 to 180 units/dl.
Acquired deficiency of blood coagulation factor VIII has been described as a rare and extremely severe autoimmune disease. This disorder develops as a result of disruption of the functioning of a person’s own immune system, which is manifested by the formation of antibodies to factor VIII.
This disease can occur in both men and women and causes severe bleeding. Recent research data indicate a possible connection between this condition and other autoimmune disorders (systemic lupus erythematosus, rheumatoid arthritis, ulcerative colitis, etc.), malignant diseases, and the use of certain medications.
The content of factor VIII in healthy individuals increases rapidly with physical effort, stress, or the administration of adrenaline. This phenomenon is transient and is thought to be due to the release of factor VIII from stores. The level of factor VIII also increases in various diseases, such as tumors, autoimmune processes, liver cirrhosis and endocrine disorders.
Structure [edit]
Factor VIII protein consists of six regions: A1-A2-B-A3-C1-C2, and is homologous to factor V.
The A domains are homologous to the A domains of the copper-binding protein ceruloplasmin. [15] C domains belong to the phospholipid-binding discoidin domain family, and the membrane-mediated C2 binding domain. [16]
Activation of factor VIII to factor VIIIa is accomplished by cleavage and release of the B domain. The protein is now divided into a heavy chain consisting of domains A1-A2 and a light chain consisting of domains A3-C1-C2. Both form a complex non-covalently in a calcium-dependent manner. This complex is the procoagulant factor VIIIa. [17]
References
- Hematology. National leadership. Ed. O. A. Rukavitsyna. "GEOTAR-Media". 2022.
- Clinical guidelines for the diagnosis and treatment of hemophilia. The recommendations were approved at the IV Congress of Russian Hematologists (April 2018). A team of authors led by Academician V.G. Savchenko. Authors: Zozulya N.I., Kumskova M.A., Polyanskaya T.Yu., Svirin P.V.
- Sheyda Khalilian, Majid Motovali-Bashi, Halimeh Rezaie. "Factor VIII: Perspectives on Immunogenicity and Tolerogenic Strategies for Hemophilia A Patients". Int J Mol Cell Med. 2022 Winter; 9(1): 33–50. doi: 10.22088/IJMCM.BUMS.9.1.33
- Sebastien Lacroix-Desmazes, Jan Voorberg, David Lillicrap, David W. Scott, Kathleen P. Pratt. "Tolerating Factor VIII: Recent Progress. Front Immunol" 2019; 10: 2991.doi: 10.3389/fimmu.2019.02991
- João A. Abrantes, Alexander Solms, Dirk Garmann, Elisabet I. Nielsen, Siv Jönsson, Mats O. Karlsson. “Relationship between factor VIII activity, bleeds and individual characteristics in severe hemophilia A patients” Haematologica. May 2022; 105(5): 1443–1453.doi: 10.3324/haematol.2019.217133
Pollution scandal[edit]
Main article: Contaminated hemophilia blood products
In the 1980s, some pharmaceutical companies such as Baxter International and Bayer caused controversy by continuing to sell contaminated Factor VIII after new heat-treated versions became available. [24] Under pressure from the FDA, the unheated product was withdrawn from US markets but sold to countries in Asia, Latin America and some European countries. The product was contaminated with the HIV virus and the issue was discussed by Bayer and the US Food and Drug Administration (FDA). [24]
In the early 1990s, pharmaceutical companies began producing products based on recombinant synthesized factors, which now prevent almost all forms of disease transmission during replacement therapy.
Links[edit]
- ^ abc GRCh38: Ensembl Issue 89: ENSG00000185010 - Ensembl May 2022
- ^ abc GRCm38: Ensembl release 89: ENSMUSG00000031196 - Ensembl, May 2022
- "Human PubMed Reference:". National Center for Biotechnology Information, US National Library of Medicine
. - "Mouse PubMed link:". National Center for Biotechnology Information, US National Library of Medicine
. - Toole JJ, Knopf JL, Wozney JM, Sultzman LA, Buecker JL, Pittman DD, Kaufman RJ, Brown E, Shoemaker C, Orr EC (1984). "Molecular cloning of cDNA encoding human antihemophilic factor." Nature
.
312
(5992):342–47. Bibcode: 1984Natur.312..342T. DOI: 10.1038/312342a0. PMID 6438528. - Truett MA, Blacher R, Burke RL, Caput D, Chu C, Dina D, Hartog K, Kuo CH, Masiarz FR, Merryweather JP (October 1985). "Characterization of the polypeptide composition of human factor VIII:C and the nucleotide sequence and expression of human kidney cDNA." DNA
.
4
(5): 333–49. DOI: 10.1089/dna.1985.4.333. PMID 3935400. - Antonarakis SE (July 1995). "Molecular genetics of the coagulation factor VIII gene and hemophilia A." Thrombosis and hemostasis
.
74
(1): 322–28. DOI: 10.1055/s-0038-1642697. PMID 8578479. - ^ ab "NIH: F8 - blood clotting factor VIII". National Institutes of Health.
- "Entrez Gene: Clotting factor F8 VIII, procoagulant component (hemophilia A)".
- Jenkins PV, Rawley O, Smith OP, O'Donnell JS (June 2012). "Elevated Factor VIII Levels and Risk of Venous Thrombosis". British Journal of Hematology
.
157
(6):653–63. DOI: 10.1111/j.1365-2141.2012.09134.x. PMID 22530883. - Jump up
↑ Milne DB, Nielsen FH (March 1996).
"Effect of a low-copper diet on copper status indicators in postmenopausal women". American Journal of Clinical Nutrition
.
63
(3): 358–64. DOI: 10.1093/ajcn/63.3.358. PMID 8602593. - "19th WHO Model List of Essential Medicines" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- Gitschier J, Wood WI, Goralka TM, Wion KL, Chen EY, Eaton DH, Vehar GA, Kaplun DJ, Lawn RM (November 1984). "Characteristics of the human factor VIII gene." Nature
.
312
(5992): 326–30. Bibcode: 1984Natur.312..326G. DOI: 10.1038/312326a0. PMID 6438525. - Levinson W, Kenwrick S, Lakich D, Hammonds G, Gitschier J (May 1990). "A transcribed gene in an intron of the human factor VIII gene." Genomics
.
7
(1): 1–11. DOI: 10.1016/0888-7543 (90) 90512-S. PMID 2110545. - Villoutreix BO, Dahlback B (June 1998). "Structural investigation of human coagulation factor V domains using molecular modeling". Protein Science
.
7
(6): 1317–25. DOI: 10.1002/pro.5560070607. PMC 2144041. PMID 9655335. - ↑
Macedo-Ribeiro S, Bode W, Huber R, Quinn-Allen MA, Kim SW, Ortel TL, Bourenkov GP, Bartunik HD, Stubbs MT, Kane WH, Fuentes-Prior P (November 1999).
"Crystal structures of the membrane-binding domain C2 of human coagulation factor V". Nature
.
402
(6760):434–39. Bibcode: 1999Natur.402..434M. DOI: 10.1038/46594. PMID 10586886. - Thorelli E, Kaufman RJ, Dahlback B (June 1998). "The C-terminal region of the Factor V B domain is critical for the anticoagulant activity of Factor V". Journal of Biological Chemistry
.
273
(26):16140–45. DOI: 10.1074/jbc.273.26.16140. PMID 9632668. - Jump up
↑ Kumar V, Abbas A, Aster J (2005).
Robbins and Cothran Pathological Basis of Disease
(9th ed.). Pennsylvania: Elsevier. item 655. ISBN 978-0-8089-2450-0. - ^ ab Kaushansky K., Lichtman M., Beutler E, Kipps T., Prchal J., Seligson U. (2010). Williams Hematology
(8th ed.). McGraw-Hill. ISBN 978-0-07-162151-9. - Fischer, Katelin; Pendu, Ronan; van Schooten, Karina J.; van Dijk, Karin; Denis, Cecile V.; van den Berg, H. Marijke; Post, Peter J. (August 25, 2009). "Models for predicting factor VIII half-life in severe hemophilia patients: different approaches for blood group O and non-O patients". PLoS ONE
.
4
(8):e6745. DOI: 10.1371/journal.pone.0006745. ISSN 1932-6203. - Hollestelle MJ, Geertzen HG, Straatsburg IH, van Gulik TM van Mourik JA (February 2004). "Expression of Factor VIII in Liver Disease". Thrombosis and hemostasis
.
91
(2):267–75. DOI: 10.1160/th03-05-0310. PMID 14961153. - Jump up
↑ Rubin R, Leopold L (1998).
Hematological pathophysiology
. Madison, CT: Fence Creek Publishing. ISBN 1-889325-04-X. - Losier J (2004). "Overview of factor VIII inhibitors". CMEonHemophilia.com. Archived from the original on 2008-12-16. Retrieved January 7, 2009.
- ^ ab Bogdanich V., Koli E (May 22, 2003). "2 Ways Bayer Drug Distributed in the 80s: A Riskier Path Overseas". New York Times
. Retrieved January 7, 2009. - Tuddenham EG, Trabold NC, Collins JA, Hoyer LW (1979). "Properties of factor VIII coagulant obtained by immunoadsorption chromatography." J Lab Clin Med
.
93
: 40–53.CS1 maint: several names: list of authors (link)
4.What can be done at home for hemophilia?
To prevent bleeding and improve your well-being, patients with hemophilia can recommend the following:
- Ask your doctor about how to manage bleeding if you have hemophilia;
- Maintain a healthy weight. Additional stress on joints due to excess weight can cause bleeding in hemophilia;
- Choose forms of physical activity with caution. It is better to give preference to swimming and other sports that do not put unnecessary stress on the joints;
- Consult your doctor before taking any medications. And do not take aspirin, ibuprofen or other non-steroidal anti-inflammatory drugs because they affect blood clotting.
- Organize your living space to avoid injuries and accidents as much as possible.
Diagnosis and prognosis
In addition to laboratory tests, anamnestic data will help establish the diagnosis: a tendency to prolonged bleeding and thrombotic complications in the patient and his relatives, as well as a positive Rumpel-Leede sign (the appearance of pinpoint hemorrhages distal to the tourniquet applied to the shoulder). The prognosis for Hageman factor deficiency is favorable in most cases; treatment is not required. This condition requires correction only in connection with surgical interventions. In preparation for surgery, a transfusion of small doses of fresh frozen plasma may be prescribed.
The period of removal of donor factor XII is 48–56 hours. Also, in the presence of this coagulopathy, more attention should be paid to the prevention of thrombotic complications: preventive compression of the lower extremities, ultrasound monitoring of the condition of the veins of the lower extremities and pelvis, especially with prolonged bed rest. In the postoperative period, for the prevention of thrombosis, it is necessary to prescribe low-molecular-weight heparins, and for the treatment of bleeding, it is necessary to avoid the use of fibrinolysis inhibitors, such as aminocaproic and tranexamic acid.
Sources
- Panteleev M. A., Ataullakhanov F. I. Blood coagulation: biochemical foundations // Clinical oncohematology. 2008. No. 1. URL: https://cyberleninka.ru/article/n/svertyvanie-krovi-biohimicheskie-osnovy
- Litvitsky P.F. Pathology of the hemostatic system // Issues of modern pediatrics. 2014. No. 2. URL: https://cyberleninka.ru/article/n/patologiya-sistemy-gemostaza
- Shulutko A.M., Krylov A.Yu., Prosolov N.V., Petrovskaya A.A. Perioperative management of a patient with factor XII deficiency (Hageman disease) //Moscow Surgical Journal. 2015. No. 4 (44). URL: https://www.mossj.ru/journal/MOSSJ_2015/MXG_2015_04.pdf
Further reading[edit]
- Gitschier J (1991). "Molecular basis of hemophilia A". Annals of the New York Academy of Sciences
.
614
(1 Virginia process): 89–96. Bibcode: 1991NYASA.614...89G. DOI: 10.1111/j.1749-6632.1991.tb43694.x. PMID 1902642. - White GC, Shoemaker CB (January 1989). "The factor VIII gene and hemophilia A." Blood
.
73
(1): 1–12. PMID 2491949. - Antonarakis S.E., Kazazian H.H., Tuddenham E.G. (1995). "Molecular etiology of factor VIII deficiency in hemophilia A". Human mutation
.
5
(1): 1-22. DOI: 10.1002/humu.1380050102. PMID 7728145. - Lenting PJ, van Mourik JA, Mertens K (December 1998). "Life cycle of coagulation factor VIII in terms of its structure and function". Blood
.
92
(11):3983–96. DOI: 10.1182/blood.V92.11.3983. PMID 9834200. - Saenko E.L., Ananyeva N., Kuyavskaya D., Schwinn H., Josic D., Shima M., Hauser K.A., Pipe S. (August 2002). "Molecular defects in coagulation factor VIII and their impact on factor VIII function" (PDF). Vox Sanguinis
.
83
(2):89–96. DOI: 10.1046/j.1423-0410.2002.00183.x. HDL: 2027.42 / 74861. PMID 12201837. - Lollar P (August 2002). "Molecular characterization of the immune response to factor VIII". Vox Sanguinis
. 83. 83 Supplement 1: 403–08. DOI: 10.1111/j.1423-0410.2002.tb05342.x. PMID 12617176. - Fay PJ (March 2004). "Activation of factor VIII and mechanisms of cofactor action". Blood Reviews
.
18
(1): 1–15. DOI: 10.1016/S0268-960X(03)00025-0. PMID 14684146. - Lavigne-Lissalde G, Schved JF, Granier C, Villard S (October 2005). "Anti-Factor VIII Antibodies: 2005 Update." Thrombosis and hemostasis
.
94
(4): 760–69. DOI: 10.1160/TH05-02-0118. PMID 16270627. - Fang X, Wang L, Wang X (2007). "Protein structure and action of factor VIII". Thrombosis study
.
119
(1):1–13. DOI: 10.1016/j.thromres.2005.12.015. PMID 16487577.