Relevance
Cardiac surgeries using cardiopulmonary bypass number more than 1 million annually in the USA and European countries.
Considering that such operations are often performed on elderly patients with comorbid pathologies, we must not forget about the increased risk of complications and deaths. Levosimendan is an inotropic drug that increases the calcium sensitivity of contractile proteins by binding to myocardial troponin C. Its effectiveness in the treatment and prevention of low cardiac output syndrome occurring after cardiac surgery has been demonstrated in small studies.
The relevance of the use of the drug in humane medicine and veterinary medicine
Acute heart failure syndrome (AHF) is defined as a gradual or rapid exacerbation of clinical signs and symptoms of heart failure (HF) requiring urgent therapeutic intervention. This pathological condition is quite common in older people (coronary heart disease, cardiomyopathies), dogs (cardiomyopathies, acquired heart defects) and cats (cardiomyopathies). The frequency of hospitalizations for acute heart failure continues to increase every year and this applies to both humane and veterinary medicine.
The heart failure syndrome is heterogeneous, covering the entire spectrum of patients in whom symptoms may present primarily as congestive AHF or, in later stages of the disease, low cardiac output.
Over the past decade, there has been increased scientific interest in AHF syndrome due to the need for early therapeutic intervention to stabilize the patient and improve symptoms.
In this case it is necessary:
- restore oxygenation and perfusion of internal organs
- avoid or limit damage to the heart, kidneys and other organs
- initiate long-term treatments that will help improve survival and quality of life
Unfortunately, the lack of sufficient knowledge about the causes of AHF does not allow us to develop highly effective methods of specific treatment and improve the survival of such patients. Acute heart failure continues to be a prognostically unfavorable pathology. This applies to low cardiac output AHF syndrome, where low initial systolic blood pressure is an independent predictor of cardiac death.
Heart failure in patients with normal or low systolic blood pressure is generally characterized by a lower left ventricular ejection fraction and more frequent signs of impaired splanchnic perfusion than in those with hypertension. These patients often present with hyponatremia, decreased peripheral body temperature, renal failure, and symptoms of collapse. This population of sick people and small pets (particularly dogs and cats) require inotropes due to the lack of alternative therapeutic options. These patients with acute heart failure have the highest in-hospital mortality rate. Conventional inotropes or inodilators, such as beta-adrenergic agonists and phosphodiesterase inhibitors (PDEs), correct filling pressures and increase cardiac output, improve hemodynamics and symptoms of heart failure. However, survival rates for such patients remain low. Inotropes and inodilators have a positive inotropic effect primarily by increasing cyclic adenosine monophosphate (cAMP) and intracellular calcium concentration in cardiomyocytes, but in severe heart failure their use may be limited by an increase in heart rate, arrhythmias, and a decrease in the effect of their use due to for the phenomenon of beta-adrenergic desensitization (long-term use of appropriate medications neutralizes their therapeutic effect).
There are promising scientific developments regarding the treatment of people and small animals with acute heart failure. Such methods of pharmacotherapy include the use of levosimendan
, the most studied calcium sensitizer, used in several countries for the treatment of acute decompensated heart failure, which has both inotropic and vasodilatory effects. Levosimendan differs from classical inodilators in its ability to improve the contractile efficiency of the myocardium without significantly increasing its oxygen demand, its positive effect on coronary blood flow and its lack of a negative lusitropic effect (myocardial relaxation rate). Several studies have shown significant benefits in improving ejection fraction and clinical outcomes using levosimendan in patients with acute heart failure.
Methods
The phase 3, multicenter, randomized, placebo-controlled LEVO-CTS trial enrolled patients with a left ventricular ejection fraction of 35% or less who underwent cardiac surgery using a cardiopulmonary bypass.
Patients were randomized to receive intravenous levosimendan 0.2 μg/kg/min for 1 hour before surgery, then 0.1 μg/kg/min for 23 hours after surgery, or placebo.
The study's primary endpoints were a composite of death at 30 days, renal replacement therapy at 30 days, perioperative myocardial infarction (up to day 5 after surgery), and circulatory assist device use (up to day 5). , and a two-part composite point (death within 30 days and use of a circulatory assist device within five days).
Introduction
Heart failure (HF) is a widespread disease. And although great success has been achieved in its treatment, which has improved the quality of life and increased the survival rate of patients, the incidence of complications and mortality in HF remains one of the highest among cardiovascular diseases. Decompensated HF is understood as acute cardiac dysfunction caused by myocardial infarction, heart surgery using cardiopulmonary bypass, pulmonary embolism, supraventricular arrhythmias, hypertensive crisis, heart defects, and chronic, which is based on the natural progression of heart disease and inadequate treatment of chronic heart disease. CHF (CHF). Any decompensated heart failure may be complicated by cardiogenic pulmonary edema, cardiogenic shock, and a significant decrease in cardiac output.
Features of the treatment of acute HF (AHF) depend on its cause and clinical manifestations. Standard therapy includes the administration of diuretics, narcotic analgesics, vasodilators and drugs that increase myocardial contractility.
Recently, in the treatment of CHF, preference is given to ACE inhibitors, b-blockers and aldosterone antagonists. In AHF, the possibility of optimizing treatment with inotropic agents is of particular interest.
In severe heart failure, especially with a significant decrease in ejection fraction, it is possible to administer beta-adrenergic receptor agonists, which lead to an increase in the content of cAMP and calcium inside the cell due to stimulation of these receptor structures. However, isoproterenol and norepinephrine increase tachycardia, myocardial oxygen demand and peripheral vasoconstriction, which is unacceptable in patients with acute myocardial infarction. Dopamine and dobutamine are most often used in practice, since undesirable effects associated with vasoconstriction are observed only when large doses of these drugs are prescribed. In addition, their favorable hemodynamic effect is not accompanied by the development of arrhythmias and an increase in the area of myocardial infarction. When dopamine and dobutamine are administered at a dose of more than 15 mcg/kg/min, slight tachycardia and an increase in myocardial oxygen demand are possible [12].
In the case of AHF, especially with supraventricular arrhythmias, cardiac glycosides (digoxin, strophanthin, corglycone), which also have an inotropic effect, are used. However, their widespread use in patients with myocardial infarction in the early stages is inappropriate due to the risk of toxic reactions, cardiac arrhythmias and death. Moreover, with long-term use, digoxin does not affect the survival of patients with HF.
Phosphodiesterase inhibitors inhibit phosphodiesterase III and IV, affect myosin ATPase, increase cAMP content and promote the entry of calcium into the cell, regardless of its initial concentration. Drugs in this group have inotropic and vasodilating effects and improve diastolic function. However, milrinone and its analogues have also not found widespread practical use due to their arrhythmogenic effects and increased mortality in patients with heart failure.
Therefore, the appearance on the pharmaceutical market in 2000 of a new class of non-glycoside cardiotonic drugs - calcium sensitizers - aroused genuine interest among doctors.
Mechanism of action of levosimendan
Levosimendan is the only calcium sensitizer that has been approved for clinical use and is recommended in more than 30 countries for the short-term treatment of AHF and acute decompensated CHF.
Levosimendan is a pyridazinone dinitrile derivative and has a dual mechanism of action. The main one is considered to be an increase in the sensitivity of the contractile proteins of cardiomyocytes to the intracellular calcium concentration [3, 4].
When the muscle fiber is activated and calcium ions appear in the myoplasm (in the presence of ATP), troponin changes its conformation and moves the tropomyosin filament away, opening up the possibility of connection with actin for the myosin head. The connection of the phosphorylated myosin head with actin leads to a sharp change in the conformation of the bridge and movement of the actin filament by one step, followed by rupture of the bridge. The energy for this process is provided by the disintegration of the macroergic phosphate bond. After this, as a result of a decrease in the local concentration of calcium ions and its disconnection from troponin, tropomyosin again blocks actin, and myosin is again phosphorylated by ATP. Thus, ATP plays a dual role in muscle work, providing energy for both muscle contraction and relaxation.
Levosimendan at the beginning of systole selectively binds to calcium-rich cardiac troponin C, which leads to stabilization of the protein conformation, triggering myofibril contraction. As a result, the connection of myosin cross bridges with actin is prolonged, the number of bonds per unit of time and the force of contraction increase. The effect of levosimendan is reversible and does not affect the process of myocardial relaxation. In diastole, the concentration of calcium ions decreases, which helps to break the bond of levosimendan with cardiac troponin C.
In addition, levosimendan has vasodilatory and anti-ischemic effects associated with the opening of ATP-dependent potassium channels in the smooth muscles of the vascular wall, which may be due to hyperpolarization of cardiomyocytes under the influence of activation of potassium channel ATPase or blockade of endothelin-1 release. Due to the expansion of veins and arteries, preload and afterload are reduced, and pressure in the pulmonary circulation decreases. In vitro, levosimendan and its active metabolite OR-1896 highly selectively block phosphodiesterase III without affecting phosphodiesterase IV. In this respect, levosimendan differs significantly from milrinone, which inhibits phosphodiesterase III and IV. It should be noted that it is with the inhibition of phosphodiesterase IV that the entry of calcium into the cell is associated, regardless of its initial content.
Pharmacokinetics
Levosimendan has linear pharmacokinetics. The drug is well absorbed, its bioavailability is 85%. It is quickly distributed throughout the body and quickly eliminated. The half-life is about 60 minutes, the degree of binding to blood albumin is 98% [2].
Levosimendan is almost completely metabolized in the liver, with only a small amount of unchanged substance excreted in urine and feces. Metabolism in the liver occurs due to conjugation with glutathione.
Basically, the metabolites are inactive and are excreted in urine and feces. Only 5% of levosimendan is converted to the active metabolite OR-1896. The latter is formed only after 24 hours of constant infusion of the drug; its concentration continues to increase over the next four days. The degree of binding of the metabolite to blood proteins is 42%. The half-life can reach 75–80 hours. OR-1896 is excreted unchanged in the urine and, to a lesser extent, in the feces. HF does not affect the pharmacokinetic parameters of levosimendan itself and its metabolites [4].
Cytochrome P-450 enzyme systems are not involved in the biotransformation of levosimendan and its metabolites. When liver function is impaired, the formation and excretion of active metabolites does not change significantly. In renal failure, the elimination of metabolites is slightly reduced.
The dose-dependent inotropic effect of levosimendan has been confirmed in experiments on animals, in healthy individuals, in patients with impaired contractility of the left ventricular myocardium, and in patients who have undergone cardiac surgery [20].
Adverse reactions
The most common adverse reactions occurring in patients with heart failure during intravenous infusion of levosimendan are headache (up to 6%), arterial hypotension (up to 5.5%), nausea and dizziness (less than 2%). The following cardiac arrhythmias were observed in patients taking levosimendan: tachycardia - 2.4%, atrial fibrillation - 1.4%, extrasystole - 1.3%, ventricular tachycardia - 1%, atrial flutter - 0.9%. A meta-analysis of 792 ECGs obtained during Holter monitoring in 386 patients in 10 studies did not reveal significant differences between levosimendan and placebo in the incidence of ventricular arrhythmias, supraventricular arrhythmias, atrioventricular block and bradyarrhythmias [1].
It was found that 83% of all adverse reactions developed during the administration of the drug and 17% after completion of treatment. The majority of adverse reactions (98%) were reported during the first 3 days of levosimendan infusion.
In large comparative studies, when levosimendan was administered in doses of 0.05–0.2 mg/kg/min during the day, the heart rate increased by no more than 4-6 beats per minute, and the decrease in blood pressure (BP) did not exceeded 10 mmHg. Art. [2, 3]. The number of cases of severe tachycardia, arterial hypotension, myocardial ischemia and ventricular arrhythmias did not increase noticeably.
According to several double-blind, controlled studies, in groups of patients taking levosimendan, a decrease in the concentration of potassium, red blood cells and hemoglobin was recorded. This is explained by hemodilution occurring against the background of vasodilation. There was no evidence of bone marrow suppression, bleeding or hemolysis. Before starting levosimendan administration, it is recommended to determine the level of potassium in the blood [13].
Levosimendan does not differ from placebo in terms of its effect on blood glucose levels and the effectiveness of hypoglycemic drugs in patients with diabetes mellitus.
Adverse reactions of levosimendan are dose-dependent, which makes it possible not to abandon treatment if the drug is poorly tolerated, but to use lower doses.
Levosimendan tolerance
The effect of levosimendan on ejection fraction, stroke volume, heart rate and pulmonary artery wedge pressure (PAWP) is clearly evident already at 5 minutes of intravenous administration, and reaches a maximum after 30 minutes [19].
With intravenous infusion of the drug for 48 hours, tolerance to its hemodynamic effect was not recorded, and the positive effect on stroke volume and PAWP persisted for two days after stopping the infusion. The phenomenon of withdrawal after abrupt cessation of levosimendan administration for 24 hours and up to 14 days was not observed [17, 18].
Drug interactions
When prescribing levosimendan with nitrates (isosorbide-
5-mononitrate), calcium antagonists (felodipine), beta-blockers (carvedilol) and ACE inhibitors (captopril) no pharmacological interactions were identified [14–17]. All data on the effectiveness and safety of levosimendan in heart failure were obtained from clinical studies in which patients, in addition to this drug, received diuretics, ACE inhibitors, nitrates and digoxin. No significant drug interactions were reported in these clinical studies.
Key clinical studies of levosimendan
RUSSLAN research
This double-blind, placebo-controlled study, which was conducted in Russia and Latvia, included 504 patients with myocardial infarction with HF [11–13]. Patients with radiologically confirmed left ventricular failure who required the use of drugs with a positive inotropic effect were included in the study on the 5th day after the occurrence of acute myocardial infarction, subject to signing informed consent.
Exclusion criteria:
- systolic blood pressure below 90 mm Hg. Art.;
- right ventricular infarction;
- myocardial rupture;
- cardiac tamponade;
- severe mitral valve insufficiency;
- ventricular tachycardia;
- atrial fibrillation;
- renal and liver failure;
- the need for invasive intervention and therapy with inotropic drugs before the study.
The patients were divided into 5 groups of approximately 100 people each, who received:
- levosimendan at a loading dose of 6 mcg/kg followed by an infusion of 0.1 mcg/kg/min;
- levosimendan at a loading dose of 12 mcg/kg followed by an infusion of 0.2 mcg/kg/min;
- levosimendan at a loading dose of 24 mcg/kg followed by an infusion of 0.2 mcg/kg/min;
- levosimendan in a loading dose of mcg/kg followed by infusion of 0.1 mcg/kg/min;
- placebo.
The duration of drug administration was 6 hours: in the first 10 minutes, levosimendan was administered as a bolus, then as an infusion. In addition, patients received all therapy indicated for acute myocardial infarction and HF.
The primary endpoint of the study was the incidence of clinically significant cases of arterial hypotension and myocardial ischemia, secondary endpoints were death and progression of heart failure. Patients were observed for 180 days.
The study obtained the following results. The incidence of myocardial ischemia and arterial hypotension did not differ significantly between groups, amounting to 10.8% in the levosimendan group and 13.4% in the placebo group. As the dose was increased, a dose-dependent increase in the frequency of these events was observed.
Progression of heart failure and mortality within 24 hours were observed more often in the placebo group than in the levosimendan group. The overall risk of death and progression of heart failure after a 6-hour infusion was 2% in the levosimendan group and 5.9% in the placebo group, and after 24 hours it was 4 and 8.8%, respectively. There was no relationship between the dose of the drug used and mortality. Serious adverse reactions were reported in 52 patients - 14.7% in the placebo group versus 9.2% in the levosimendan group.
The results of the study confirmed the safety of the use of levosimendan for the treatment of HF in patients with acute myocardial infarction. The administration of the drug led to a decrease in weakness, shortness of breath and the need for other medications necessary for the treatment of heart failure. Treatment with levosimendan reduced the risk of death and progressive heart failure.
In the placebo group, mortality after 14 days was 19.6%, and after 180 days - 31.4%, while in the levo-simendan group it was 11.7% (p = 0.031) and 22.6% (p = 0.054) respectively. The risk of all-cause mortality within 14 days was reduced by 44% in patients receiving levosimendan. The effect persisted over 6 months of follow-up—a 33% reduction in the risk of overall mortality.
Thus, intravenous infusion of levosimendan in doses of 0.1–0.2 mcg/kg/min for 6 hours is quite well tolerated and effective in the treatment of patients with acute myocardial infarction complicated by left ventricular failure. At the indicated doses, levosimendan did not increase the incidence of clinically significant hypotension and myocardial ischemia. Its use in the indicated doses significantly reduced mortality, and the effect achieved in the first 2 weeks after the start of treatment was maintained for 6 months.
LIDO Study
This double-blind, comparative study was conducted in 11 Western European countries [9, 10]. It included 203 patients hospitalized for HF with low cardiac output (ejection fraction less than 35%, cardiac index less than 2.5 l/min/m2, PAWP more than 15 mmHg), requiring invasive monitoring of parameters central hemodynamics and intravenous administration of an inotropic drug.
The study did not include persons under 21 years of age, patients with heart valve stenosis, restrictive or hypertrophic cardiomyopathy, sustained ventricular tachycardia or ventricular fibrillation, or systolic blood pressure below 85 mmHg. Art., heart rate more than 120 beats per minute, renal and liver failure, as well as taking b-adrenergic receptor agonists or phosphodiesterase inhibitors.
The study had an original design. Patients were divided into two groups: the first group received levosimendan (a loading dose of 24 mcg/kg administered over 10 minutes, followed by an infusion of 0.1 mcg/kg/min) and dobutamine placebo; the second group was prescribed dobutamine at an initial dose of 5 mcg/kg/min and levosimendan placebo. The drugs were administered over 24 hours; if the effect was insufficient, the rate of administration was doubled 2 hours after the start of the infusion. In case of undesirable reactions, the infusion was interrupted for 30 minutes, followed by the resumption of drug administration at a dose reduced by 2 times.
The primary endpoint was improvement in hemodynamic parameters within 24 hours. Secondary end points were: changes in hemodynamic parameters after 24 hours, number of days in hospital without parenteral drug administration in the first month, time to death or decompensation of heart failure, mortality 31 days after randomization , mortality within 180 days.
The LIDO study found that a 24-hour infusion of levosimendan led to a significant improvement in hemodynamic parameters compared with dobutamine in patients with acute decompensated CHF (28% vs. 15%; p = 0.022). Overall mortality in patients receiving levosimendan for 31 days was significantly lower than in the placebo group - 8 versus 17%
(p = 0.049), which corresponds to a 57% risk reduction. This effect persisted when observation was extended to 180 days - 26 versus 38% (p = 0.029), reducing the risk of overall mortality by 43%.
It was shown that levosimendan was less likely than dobutamine to cause adverse reactions, such as rhythm disturbances (3.9 vs. 13%; p = 0.023), myocardial ischemia, and chest pain (0 cases vs. 7; p = 0.013). Combined use with beta-blockers did not weaken the hemodynamic effect of levosimendan.
CASINO Research
This randomized, double-blind, placebo-controlled study was planned to include 600 patients with decompensated NYHA functional class IV CHF and a left ventricular ejection fraction of less than 35% [7, 8]. Hospitalized patients were randomized within 48 hours into 3 groups that received:
- levosimendan at a loading dose of 16 mcg/kg administered over 10 minutes, followed by an infusion of 0.2 mcg/kg/min over 24 hours;
- dobutamine;
- placebo.
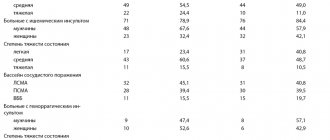
The primary endpoint was mortality at 1, 6 and 12 months. After enrollment of 299 patients, the study was stopped early due to the apparent superiority of levosimendan. The results of the study are presented in table.
Mortality at 1 month was 6.1% in the levosimendan group, 12.8% in the dobutamine group, and 8.2% in the placebo group; after 6 months – 15.3, 39.6 and 24.7%, respectively. Thus, the CASINO study provided data indicating a positive effect of short-term levosimendan infusions on mortality in acute decompensated CHF.
REVIVE I Study
A double-blind, placebo-controlled study involving 100 patients examined the effectiveness of 24-hour parenteral administration of levosimendan in patients with acute decompensated CHF [6]. Hospitalized patients with a left ventricular ejection fraction less than 30% and dyspnea at rest that persisted after parenteral diuretic administration were randomized into two groups. The first group received levosimendan at a loading dose of 6–12 mcg/kg administered over 10 minutes, followed by an infusion of 0.1 mcg/kg/min over 50 minutes (later, if well tolerated, the dose was doubled); the second group was given a placebo.
The main goal of the study was to assess the clinical condition of patients in the first 5 days during and after levosimendan infusion.
According to the study results, clinical improvement in the levosimendan group occurred earlier (6 hours after the start of the infusion, persisted for 5 days) and significantly more often than in the placebo group. This study was preliminary to the ongoing REVIVE II study, as the results obtained from it were used to refine the protocol.
SURVIVE Study
This large, randomized, double-blind trial is currently underway in Europe, Russia and Israel. By April 2004, 722 patients had already been included. In total, the study plans to include 1,300 patients. In hospitalized patients with AHF, ejection fraction less than 30%, shortness of breath at rest, PAWP more than 18 mmHg. Art. and the need for parenteral administration of inotropic drugs, overall mortality for 31 and 180 days, the number of days out of hospital for 180 days, clinical condition and safety of therapy will be assessed.
Other studies
A number of studies examine the question of the anti-ischemic effect of levosimendan. The drug was administered through a catheter installed in the coronary artery directly into the area with impaired myocardial contractility, but no significant changes in hemodynamics were detected. This experiment confirms the opinion that when levosimendan acts on “stunned” myocardium, the sensitivity of myofibrils to calcium increases.
In a small randomized trial, patients with acute myocardial infarction after successful angioplasty were administered levosimendan 24 mcg/kg over 10 minutes. During the administration of the drug, the number of hypokinetic segments decreased and the overall contractility of the myocardium increased without increasing its oxygen demand [4].
In the experiment, a ligature was placed on the coronary artery and levosimendan was infused. At the same time, the size of myocardial infarction decreased [5]. However, the anti-ischemic effect of levosimendan has not been specifically studied in clinical studies.
In a placebo-controlled study in 151 patients without decompensated CHF, a significant, dose-dependent increase in the level of norepinephrine in the blood occurred during the administration of levosimendan, which is explained by an increase in sympathetic activity in response to pronounced vasodilation. However, the level of adrenaline did not change noticeably. When using small doses of the drug, there was a tendency to reduce the content of atrial natriuretic peptide and blood renin levels.
In several studies, levosimendan was administered postoperatively to patients undergoing cardiac surgery using cardiopulmonary bypass. The data obtained indicated a significant increase in stroke volume and a decrease in peripheral resistance. The patients tolerated the drug well; it did not affect the oxygenation of arterial blood and did not have an arrhythmogenic effect.
Currently, the use of levosimendan for myocardial infarction is permitted in a number of European countries, including Russia. Considering the beneficial effect of this drug on hemodynamic parameters, clinical symptoms and mortality in AHF and acute decompensation of CHF, the safety and good tolerability of the drug proven in studies, the issue of expanding the indications for its use is being considered.
results
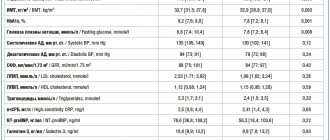
Of the 882 patients randomized, 849 were included in the intention-to-treat analysis.
- The four-part endpoint was met in 105 of 428 patients (24.5%) in the levosimendan group and 103 of 421 patients (24.5%) in the control group (odds ratio, 1.00; 99% CI, 0.66-1.54; P=0.98).
- The two-part primary endpoint was met in 56 patients (13.1%) in the study group, compared with 48 (11.4%) receiving placebo (adjusted odds ratio, 1.18; 96% CI, 0.76-1.82; P=0.45).
- The incidence of adverse drug events did not differ significantly between groups.
Pharmacology and mechanism of action of levosimendan (Simdax)
The chemical name of the drug is [(R)-[[4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl]hydrazone]propanedinitrile]. Simdax is the levorotatory isomer of racemic simendan, a pyridazinone dinitrile derivative. Simendan is a racemic compound of 2 enantiomers: dextrosimendan and levosimendan.
Levosimendan
belongs to the so-called group of “calcium sensitizers” (“calcium ion sensitizers”), which include several other substances that have the ability to increase the sensitivity of myofilaments to calcium ions, which leads to an increase in myocardial contractility without increasing the concentration of intracellular cAMP or intracellular calcium concentrations, which may be due to some phosphodiesterase inhibitory effects. This concept of therapy, as it appears at the current stage of development of humane and veterinary cardiology, is associated with the least amount of side effects, a decrease in proarrhythmogenic potential, and also has a beneficial effect on myocardial oxygen consumption compared to traditional inotropes or inodilators.
Levosimendan binds in a calcium-dependent manner to the N-terminal domain of cardiac troponin C, which has higher affinity at high calcium concentrations and lower affinity at low calcium concentrations. By stabilizing the calcium-cardiac troponin C complex, levosimendan inhibits the effect of troponin I and increases the rate of association of the actin-myosin cross-bridge complex. This positive inotropic effect is achieved without increasing intracellular calcium concentrations or significantly increasing myocardial oxygen demand.
The beneficial therapeutic effect of levosimendan on the symptoms of acute heart failure is also associated with its vasodilatory effect on the systemic arterial, coronary, pulmonary, renal, intestinal, and cerebral circulation. Simdax also improves systemic venous circulation, in particular in the portal vein. The vasodilatory effect of levosimendan is mediated by adenosine triphosphate, acting on potassium and calcium channels in the muscle cells of the vascular wall. Membrane hyperpolarization induced by the opening of potassium channels blocks calcium entry and activates sodium-calcium exchange, which leads to a decrease in intracellular calcium concentration and causes vasodilation.
Clinical studies of the effectiveness of levosimendan
Acute heart failure
Several clinical trials have shown a beneficial effect of levosimendan on short-term hemodynamic and clinical signs in sick patients with AHF. Cardiac output from the use of the drug (24-hour infusion in patients with FC III-IV HF) increases by an average of 40%, and pulmonary capillary wedge pressure decreases by 30%. Infusion of levosimendan, compared with dobutamine, was characterized by higher rates of therapeutic efficacy. An interesting fact is that the use of beta blockers enhances the hemodynamic effects of levosimendan, but reduces the hemodynamic effects of dobutamine. Levosimendan significantly reduces mortality
in patients with acute heart failure, compared with the use of dobutamine as an inotrope.
It is important to note that the inodilator effects of levosimendan are maintained during concomitant use of beta blockers. Levosimendan significantly improves clinical symptoms in humans, dogs and cats with acute heart failure.
In patients with decompensated heart failure awaiting heart transplantation, levosimendan improves renal function within 3 months of use of the pharmacological drug.
Levosimendan improves coronary circulation, reduces myocardial oxygen consumption, and improves perfusion of ischemic myocardium. This effect was proven in clinically healthy animals and people, but later these properties of the drug were confirmed in patients with congestive heart failure, as well as in people with acute myocardial infarction with left ventricular dysfunction.
In patients undergoing elective coronary artery bypass grafting (CABG), levosimendan increases cardiac output
and cardiac stroke volume and reduces systemic vascular resistance without increasing myocardial oxygen consumption or particularly increasing myocardial utilization of nutrient substrates.
Diastolic heart failure
In preclinical studies in animals and humans, levosimendan improves left ventricular myocardial diastolic function, and its inotropic effect is associated with an increase in the rate of relaxation and a decrease in relaxation time of the left ventricular myocardium, thereby improving cardiac diastolic function.
In severe heart failure patients with limited left ventricular filling, levosimendan improved both systolic and diastolic left ventricular filling functions, increased stroke volume and cardiac output while reducing pulmonary capillary wedge pressure.
Levosimendan in the treatment of peripartum cardiomyopathy
The use of new drugs in patients with rare diseases is always associated with certain difficulties. There are few clinical observations regarding the use of levosimendan in patients with peripartum cardiomyopathy. Acute heart failure is a life-threatening condition that, in very rare cases, develops during or after childbirth. These clinical reports show the high effectiveness of levosimendan in improving heart function, which was associated with both an improvement in the symptoms of AHF and positive dynamics of hemodynamic and echocardiographic parameters characterizing the systolic and diastolic function of the left ventricle. Simdax
caused a sustained increase in cardiac output and left ventricular stroke volume and, accordingly, accelerated the recovery of patients with peripartum cardiomyopathy.
Right-sided heart failure and cardiogenic shock
Levosimendan
also used to restore impaired left ventricular function in patients after cardiac surgery and in patients who have developed cardiogenic shock after heart transplantation. In patients with acute respiratory distress syndrome, pulmonary hypertension and right ventricular cardiac dysfunction, the effectiveness of levomisendan was not high. In septic shock, the use of levosimendan turned out to be completely justified; in sick patients, while using the drug, there was a decrease in the mean pressure in the pulmonary artery, the index of vascular resistance in the pulmonary vessels and the end-systolic volume of the right ventricle, an increase in cardiac output, right ventricular ejection fraction and mixed venous saturation blood oxygen.