Pharmacodynamics and pharmacokinetics
Pharmacodynamics
An anti-inflammatory agent that has a local effect in the intestinal mucosa. Shows activity against Escherichia coli and cocci. Suppresses the synthesis of inflammatory mediators - prostaglandins and leukotrienes. It has antioxidant properties due to the ability to destroy free oxygen radicals. Reduces the risk of relapses in Crohn's disease .
Pharmacokinetics
About 50% of the dose is absorbed in the small intestine. Subject to acetylation in the intestines and liver. 43% protein bound. Metabolites are excreted by the kidneys and intestines. T1/2 0.5-2 hours. Accumulates in persons with chronic renal failure .
Side effects
- paresthesia , headache, sleep disturbance, convulsions, depression , tremor , tinnitus;
- abdominal pain, loss of appetite, heartburn, nausea, vomiting, flatulence , diarrhea , hepatitis, pancreatitis ;
- palpitations, increased blood pressure, tachycardia , shortness of breath;
- hemolytic or aplastic anemia , leukopenia, thrombocytopenia, agranulocytosis, hypoprothrombinemia;
- oliguria or anuria , proteinuria , hematuria, nephrotic syndrome;
- rash, erythema , itching, bronchospasm;
- fever, alopecia , lupus-like syndrome.
Mesalazine
According to the World Health Organization (WHO), adverse effects are classified according to their frequency as follows: very common (>1/10), common (>1/100 and <1/10), uncommon (>1/1000 and < 1/100), rare (>1/10,000 and <1/1000) and very rare (<1/10,000), including isolated reports; frequency unknown - frequency cannot be estimated from available data.
Blood and lymphatic system disorders
Very rarely - pathological indicators of blood cells (aplastic anemia, agranulocytosis, pancytopenia, neutropenia, leukopenia, thrombocytopenia).
Nervous system disorders
Rarely - headache, dizziness.
Very rarely - peripheral neuropathy.
Heart disorders
Rarely - myocarditis, pericarditis.
Respiratory, thoracic and mediastinal disorders
Very rarely - allergic and fibrotic reactions from the lungs (including shortness of breath, cough, bronchospasm, alveolitis, pulmonary eosinophilia, pulmonary infiltrates, pneumonitis).
Gastrointestinal disorders
Rarely - abdominal pain, diarrhea, bloating, nausea, vomiting.
Very rarely - acute pancreatitis.
Renal and urinary tract disorders
Very rarely - renal dysfunction, including acute and chronic interstitial nephritis, renal failure.
Skin and subcutaneous tissue disorders
Rarely - photosensitivity (increased sensitivity of the skin to light).
Very rarely - alopecia.
Musculoskeletal and connective tissue disorders
Very rarely - myalgia, arthralgia.
Immune system disorders
Very rarely - hypersensitivity reactions, for example, allergic exanthema, drug fever, lupus erythematosus syndrome (SLE), pancolitis.
Disorders of the liver and biliary tract
Very rarely - changes in liver function (increased transaminase activity and cholestasis parameters), hepatitis, cholestatic hepatitis.
Reproductive system disorders
Very rarely - oligospermia (reversible).
Photosensitivity (increased skin sensitivity to light)
More severe reactions have been reported in patients with skin diseases such as atopic dermatitis and atopic eczema.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Mesalazine, instructions for use (Method and dosage)
Mesalazine tablets are taken orally, whole, after meals, with a sufficient amount of water.
For ulcerative colitis in the acute stage, adults are prescribed 4 g per day (in several doses). Maximum DM is 6-8 g. Dose for children is 20-30 mg per 1 kg of weight per day, divided into several doses. When switching to maintenance therapy, adults take 2 g per day.
For distal proctitis, drugs are prescribed in the form of suppositories. Since there is no release form for the Mesalazine suppository, Salofalk or Pentasa , 1 g 2 times a day. Duration of treatment is 2-3 months.
The peripheral blood picture, liver and kidney function are monitored monthly during treatment. In individuals with a reduced rate of acetylation in the liver, the risk of adverse reactions increases.
Mesalazine
Mesalazine
(lat.
mesalazine
) - intestinal anti-inflammatory drug. Salicylic acid derivative.
Mesalazine is a chemical compound
Mesalazine, as a chemical compound, is 5-Amino-2-hydroxybenzoic acid. The empirical formula of mesalazine is C7H7NO3.
Mesalazine is a drug
Mesalazine is the international nonproprietary name (INN) of the drug. According to the pharmacological index, mesalazine belongs to the group “NSAIDs - Salicylic acid derivatives”. According to ATC, mesalazine is included in the group “A07E Intestinal anti-inflammatory drugs” and has the code A07EC02.
Indications for use of mesalazine
Mesalazine is used in the treatment and prevention of inflammatory bowel diseases: ulcerative colitis and Crohn's disease. Mesalazine is effective in the treatment of diverticulitis at a dose of 1.5 g per day. Mesalazine can be prescribed both after antibiotic therapy and along with it (Maev I.V. et al.).
Mesalazine administration and dosage
When taken orally, mesalazine is taken 3 times a day, 400-800 mg 3 for 2-3 months, washed down with plenty of water. Maintenance doses are 500 mg 3 times a day for ulcerative colitis and 1000 mg 4 times a day for Crohn's disease.
Use of mesalazine during pregnancy and breastfeeding
In the first trimester of pregnancy, mesalazine is taken only under strict indications.
In the last 2-4 weeks, the use of mesalazine is prohibited. FDA risk category for the fetus is B. During mesalazine therapy, it is advisable to stop breastfeeding.
Trade names of drugs with the active ingredient mesalazine
The following drugs with the active ingredient mesalazine are (have been) registered in Russia: Mesalazine, Salofalk, Pentasa, Salozinal, Mesacol, 5-ASA, Asacol, Mezavant.
“Instructions for use of the medicinal product for medical use Mezavant”, pdf, extended-release enteric-coated tablets containing 1200 mg of mesalazine, Johnson & Johnson LLC, 11/28/2011.
Mesalazine is one of the most popular drugs for the treatment of gastrointestinal tract in the United States.
Mesalazine is one of the top ten best-selling prescription drugs for digestive disorders in the United States. In this group in 2004, mesalazine was in sixth place in terms of sales (after lansoprazole, esomeprazole, pantoprazole, rabeprazole and omeprazole).
Publications for healthcare professionals regarding the use of mesalazine
- Kazarina A.V. The role of the probiotic Probifor in the treatment of relapse and maintenance of remission of ulcerative colitis. Abstract of dissertation. PhD, 14.00.05 - internal diseases. NMHC named after. N.I. Pirogova, Moscow, 2009.
Mesalazine has contraindications, side effects and application features; consultation with a specialist is necessary.
Back to section
Overdose
Mild overdose is manifested by dizziness, malaise, nausea, vomiting, blurred vision and fever (unfavorable prognostic sign). In severe cases metabolic acidosis , drowsiness, collapse , anuria , convulsions and bleeding develop.
In order to identify an overdose, which is not always clinically manifested, the concentration of salicylates in the blood is determined (above 70 mg% - severe poisoning, above 100 mg% - extremely severe).
Treatment: taking a laxative, gastric lavage, prescribing sorbents, symptomatic therapy, monitoring the acid-base composition. Depending on this, the introduction of sodium bicarbonate or sodium citrate . Alkalinization of urine (if the level of salicylates is more than 40 mg%) is carried out by infusion of sodium bicarbonate. In case of pulmonary edema, perform mechanical ventilation with a mixture of oxygen.
Interaction
When used with Azathioprine and Mercaptopurine, the toxicity of these drugs increases.
Strengthens the hypoglycemic effect of sulfonyl urea , side effects (ulcerogenicity) of GCS .
Enhances the effect of anticoagulants and uricosuric drugs .
Slows down the absorption of cyanocobalamin , reduces the effectiveness of Warfarin , Furosemide , Spironolactone , Rifampicin , and sulfonamides .
Reviews about Mesalazine
Aminosalicylates are considered as first-line drugs in the treatment of mild and moderate forms of ulcerative colitis . There are often reviews from patients suffering from this disease. For mild cases, patients took Mesalazine, and for moderate severity of the disease, doctors prescribed Salofalk.
The dose, depending on the severity of the disease, was increased to 4.0 g/day and even to 6.0 g/day. After the process subsided, patients took the drug for maintenance treatment. In children, Pentasa was most often used, granules of which can be mixed with food, which is convenient for children. With long-term use, adverse reactions were observed: nausea, pain in the right hypochondrium, bloating.
In severe cases of this disease, hormones provide a quick result, but a third of patients become hormonal dependent - after corticosteroids, aminosalicylates are ineffective.
Effective domestic mesalazine in the treatment of ulcerative colitis
Ulcerative colitis (UC) is a chronic autoimmune disease and is characterized by erosive and ulcerative lesions of the colon mucosa (COMC). In the pathogenesis of UC, activation of opportunistic microflora and reduction of the barrier function of SOTK play an important role. The resulting microbial antigens interact with the intestinal immune system, activate T and B lymphocytes and initiate the synthesis of autoantibodies. A major role in the development of inflammation in inflammatory bowel diseases (IBD) belongs to pro-inflammatory cytokines: tumor necrosis factor alpha (TNF-α), interleukins (IL) 1β, 12β, 17, 23, etc., causing damage to the cellular tissue. Genetic predisposition increases the risk of developing UC several times. 168 gene loci leading to the development of UC and Crohn's disease (CD) are already known. On this basis, attempts are being made to create diagnostic panels for IBD [1].
The incidence of IBD in northern European countries and North America has increased approximately 6-fold over the past 40 years. The peak incidence occurs between 20 and 30 years of age; both men and women are equally affected. According to the latest epidemiological surveys, the prevalence of UC on average reaches 500 cases per 100 thousand population [2].
The social significance of UC is due to its predominant distribution among young people, its chronic progressive course, the need for frequent hospital treatment, the development of systemic extraintestinal manifestations, and a decrease in working capacity and quality of life. In 25–37%, the course of UC in the first 5 years is accompanied by intestinal bleeding, perforation, toxic megacolon and other severe complications requiring surgical intervention [3, 4].
Drug therapy for UC is carried out according to the recommendations of the European Consensus (European Crohn's Colitis Organization, ECCO) 2022 [5]. In our country, clinical recommendations of the Russian Gastroenterological Association and the Russian Association of Coloproctologists for the diagnosis and treatment of UC and CD are used [6, 7]. Treatment tactics depend on the prevalence of inflammation in the colon, the severity of the attack, the presence of extraintestinal manifestations and complications.
The first-line drug for the treatment of UC is mesalazine or 5-aminosalicylic acid (5-ASA) [8]. The mechanism of action of 5-ASA is to inhibit the metabolism of arachidonic acid, inflammatory mediators, primarily leukotrienes and pro-inflammatory interleukins, neutralize free oxygen radicals and suppress the production of antibodies by B lymphocytes. In severe cases of UC, glucocorticosteroids and genetically engineered biological drugs (GEBPs) are used. When a positive response is achieved, glucocorticosteroids are gradually discontinued and the patient is transferred to 5-ASA drugs to induce and maintain remission. In case of ineffectiveness of hormonal therapy, biologically active drugs are used.
Currently, the government of the Russian Federation has set pharmaceutical companies the task of creating domestic modern drugs that will solve the problem of completely replacing imported drugs. The first domestic drug of mesalazine, Kansalazine, appeared in Russia.
In its structure, the Cansalazine tablet is a polymer matrix with mesalazine, which provides long-term continuous release of the active substance throughout the gastrointestinal tract from the duodenum to the rectum, regardless of pH values.
The purpose of this work is to study the clinical effectiveness of the drug Cansalazine in patients with mild to moderate UC in the acute phase through an open prospective clinical non-comparative study.
The objectives of the study were: a) to determine the effectiveness of Cansalazine in stopping the acute phase of inflammation; b) achieving stable positive dynamics during ulcerative colitis; c) assessment of the frequency of side effects (headache, nausea, effect on hematopoiesis, hepatotoxicity, allergic reactions).
Material and research methods
The study included 31 patients with UC. Men and women met with equal frequency. The average age of the patients was 39 ± 7.5 years (18–60 years). The duration of the disease ranged from 6 months to 5 years and averaged 3.4 ± 1.6 years.
The criteria for inclusion in the study were: patients with mild to moderate UC aged 18–60 years, a verified diagnosis of “ulcerative colitis, chronically relapsing course, acute stage, mild or moderate severity, form: proctosigmoiditis, left-sided ulcerative colitis” (confirmed by clinical laboratory and instrumental studies), written consent for inclusion in the study.
Exclusion criteria: patient age under 18 and over 60 years; diagnosis of "Crohn's disease, gastric and duodenal ulcers" in the anamnesis, blood diseases, pregnancy, lactation, burdened allergic history, drug intolerance, salicylate intolerance, acute infectious disease, chronic diseases of the cardiovascular, neuroendocrine systems, renal, liver failure, oncological history of diseases, taking medications that have a pronounced effect on hemodynamics, liver function, taking immunosuppressants for 3 months or systemic glucocorticosteroids for 1 month before inclusion in the study, drug addiction, alcoholism, smoking more than 10 cigarettes per day, taking systemic antibiotics for treatment of nonspecific ulcerative colitis for 7 days before the study, treatment with probiotics, antidiarrheal drugs for 7 days before the study.
The extent of lesions in UC was assessed according to the Montreal classification. They distinguished: a) proctitis (inflammation limited to the rectum); b) left-sided colitis - spread of inflammation to the left flexure of the colon (including proctosigmoiditis); c) total and subtotal UC - total UC with retrograde ileitis.
The severity of the UC attack was determined using the Mayo index, which includes clinical manifestations, endoscopic changes in the UC and the general condition of the patient (Table 1). Mayo index values were interpreted as 0–1 point—remission of UC; 2–5 points – minimal activity; 6–9 points – moderate activity; 10–12 points – high activity.
Endoscopic changes included in the Mayo index were assessed using the Schroeder scale (Table 2).
The design of the examination included clinical tests with determination of C-reactive protein (CRP), colonoscopy (CS) with histological examination of biopsy specimens.
In addition, IL-1β, IL-4, TNF-α were determined in blood serum. The concentration of calprotectin was examined in feces. Fecal calprotectin (FCP) is produced by polymorphonuclear non-neutrophils and can serve as a marker of inflammation in the intestine [10]. An enzyme-linked immunosorbent assay was used to study IL and FCP.
Among the examined patients, patients with left-sided UC predominated (20 patients - 64.5%). 10 patients (32.4%) had total UC and 1 patient (3.2%) had UC in the form of proctitis. In table Figure 3 shows the distribution of patients according to the degree of inflammation depending on the location of ulcerative colitis.
From the table Table 3 shows that in the study group, UC of moderate severity predominated - 23 patients (73.4%) and only 8 (26.6%) had minimal activity of autoimmune inflammation.
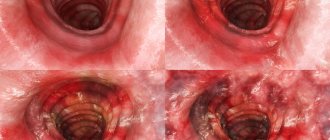
Patients with left-sided or total UC of moderate severity complained of diarrhea up to 5–6 times a day, during the day and at night, mixed with scarlet blood, urgency, tenesmus, low-grade fever in the evenings, weakness, decreased ability to work, and weight loss by 2–3 kg. Half of the patients had systemic extraintestinal manifestations: peripheral arthropathy, and one had aphthous stomatitis. Colonofibroscopy revealed ulcerative colitis of moderate activity (the mucosa is hyperemic, granular, edematous, with multiple superficial erosions, microabscesses, single ulcers covered with fibrin. Contact bleeding is insignificant. The folding is moderately smoothed. There is no vascular pattern, which corresponded to 2 points on the Schroeder scale (Fig. . 1).
A histological examination of SBTC biopsies revealed lymphoplasmacytic infiltration of the lamina propria, deformation of the crypts, and a decrease in the number of goblet cells (Fig. 2).
Clinical blood tests confirmed iron deficiency anemia (HB 95 ± 5 g/l, serum iron 7.1 ± 3.2 μmol/l), moderate leukocytosis up to 12 ± 1.6 thousand with a band shift up to 13 ± 2%, ESR 27 ± 6 mm/hour; increase in CRP to 9 ± 2 mg/l. Moderate hypoproteinemia was also noted (total protein 63 ± 2 g/l when the norm was over 67 g/l) with an albumin level of 33 ± 1 g/l. All patients had an increase in TNF-α (98.4 ± 13.6 pg/ml), IL-1β (81.9 ± 7.5 pg/ml with a normal value of up to 50 pg/ml) and a decrease in IL-4 to 36 .7 ± 2.2 pg/ml (normal 50 pg/ml). In stool tests, a positive reaction to occult blood was observed, and the FCP on average reached 540 ± 150 μg/g (the norm is up to 50 μg/g). As a result, the UC activity index on the Mayo scale was 7 ± 1 point.
The study group also included a patient with UC in the form of catarrhal-hemorrhagic proctitis of moderate severity, who had no effect from treatment with rectal microenemas of mesalazine.
In 26.4% of patients with UC with total or left-sided lesions of minimal activity, the frequency of loose stools did not exceed 3-4 times a day, but with streaks of blood and urgency. Body temperature remained within normal limits, weight loss was not noted, or it did not exceed 1 kg. Clinical blood tests showed no inflammatory changes (CRP ≤ 5 mg/L), iron deficiency anemia was limited to mild severity (HB 110 ± 8 g/L, serum iron 9 ± 1 μmol/L). In the stool analysis, a positive reaction to occult blood was noted, the FCP increased to an average of 360 ± 50 μg/g. Levels of TNF-α and IL-1β were elevated in all patients and averaged 87.3 ± 11.4 pg/ml and 78.9 ± 13.5 pg/ml, respectively. IL-4 levels decreased to an average of 35.7 ± 1.4 pg/ml.
One third of the patients in this group had peripheral arthropathy in the form of intermittent pain in large joints. When performing colonofibroscopy, signs of catarrhal-hemorrhagic colitis were observed with slight contact vulnerability of the somatic tissue and the absence of a vascular pattern, which corresponded to 2 points on the Schroeder scale. Histological examination showed slight lymphoplasmacytic infiltration and a decrease in the number of goblet cells. The UC activity index in patients in this group was 4 ± 1 points according to Mayo.
All patients included in the study were prescribed the drug Cansalazine orally in tablets, at a dose of 3 g/day (2 tablets × 3 times a day), as well as rectally - in microenemas in the form of a suspension, 2 g, at night. Also, all patients received antibacterial therapy in the form of infusions of metronidazole 1500 mg/day.
The safety and tolerability of Cansalazine were assessed by dynamic monitoring of clinical symptoms of UC and the possible development of adverse events (nausea, headache, etc.); using laboratory parameters, including the study of alanine and aspartic aminotransferases and other liver tests.
All studies were performed before treatment was prescribed; control clinical tests were carried out on the 15th and 30th days of therapy. Repeated colonofibroscopy was performed a month after the start of treatment.
Statistical analysis was performed using Microsoft® Office Excel 2003; Statisticav. 6.0; Primerof Biostatistics Version 4.03 by StantonA. Glantz 1998. Student's t-test was used to determine the significance of differences between means in normally distributed populations.
Research results
Positive dynamics were observed by the end of the 2nd week of taking the drug Cansalazine. In all patients, the frequency of stool decreased, the admixture of blood in the stool disappeared, and tenesmus stopped. Blood tests showed a decrease in the level of leukocytes and band shift, ESR, and CRP level. After 30 days from the start of therapy, in all patients with UC, regardless of the severity of inflammation and the localization of the process, the frequency of stools decreased to 1–2 times a day, the admixture of blood in the stool, tenesmus and urgency disappeared. In clinical blood tests, all parameters returned to normal. PCF values decreased to 260 ± 38 μg/g. With an average degree of UC activity, the cytokine profile improved: the TNF-α index decreased from 98.4 ± 13.6 pg/ml to 63.4 ± 10.5 pg/ml (p < 0.05); IL-1β decreased from 81.9 ± 7.5 pg/ml to 40.1 ± 1.5 pg/ml (p < 0.05); IL-4 values increased from 36.7 ± 2.2 pg/ml to 43.8 ± 1.8 pg/ml (p < 0.05) (Fig. 3). In patients with an average degree of activity of UC, the endoscopic index according to Schroeder decreased to 1 point (the mucosa is pale pink, smooth, shiny, with single small pseudopolyps; there is no contact bleeding; folding is pronounced; the vascular pattern is preserved, rebuilt) - incomplete remission of ulcerative colitis (Fig. . 4).
Histological examination revealed a slight lymphoplasmacytic infiltration, the number of goblet cells remained reduced, and the shape of the crypts improved (Fig. 5). The clinical activity index on the Mayo scale averaged 3 ± 1 point.
In patients with mild UC, during colonofibroscopy, there were no signs of inflammation of the vascular system, but areas of a blurred vascular pattern remained. A histological examination revealed signs of minimal colitis, in the form of moderate lymphoplasmacytic infiltration of the somatic tissue, and a decrease in the number of goblet cells. The clinical activity index on the Mayo scale did not exceed 1 point. FCP remained elevated and averaged 240 ± 24 μg/g. The levels of pro-inflammatory cytokines decreased (TNF-α level decreased from 87.3 ± 11.4 pg/ml to 57.3 ± 9.5 pg/ml (p < 0.05); IL-1β level decreased from 78.9 ± 13.5 pg/ml to 45.3 ± 2.5 pg/ml (p < 0.05)) and the level of anti-inflammatory cytokine increased - IL-4 increased from 35.7 ± 1.4 pg/ml to 45.8 ± 2.3 pg/ml (p < 0.05) (Fig. 6). As inflammation of the colon mucosa subsided, extraintestinal manifestations of UC disappeared in patients: peripheral arthropathy and aphthous stomatitis.
Thus, all patients achieved a significant positive effect from Cansalazine in relieving the acute phase of UC.
Side effects: one patient with UC with left-sided lesions and a moderate degree of activity developed nausea and vomiting on the 3rd day of treatment with Cansalazine. The drug was discontinued and the patient was excluded from the study. Nausea and vomiting stopped within 24 hours without prescribing additional drug therapy. According to the classification, the severity of the described side effects was assessed as moderate, i.e. the complications affected daily activity, but did not lead to a permanent loss of work activity and resolved on their own without additional therapy.
Discussion
According to the data obtained, the drug Cansalazine (the active substance is mesalazine), when prescribed to patients with UC at a dose of 3 g per day for 1 month, proved its effectiveness: 96.7% of patients experienced clinical and endoscopic remission with the restoration of laboratory parameters characteristic of inflammation to normal. In all patients, during repeated histological examinations of the biopsy specimens of the STC, lymphoplasmacytic infiltration remained, but to a lesser extent. The persistence of autoimmune inflammation of SOTC was confirmed by a moderate increase in the concentration of PCP and an altered profile, primarily of TNF-α and IL-4.
All patients tolerated therapy with Cansalazine well, no allergic reactions were noted. Side effects of the drug were recorded in 1 patient in the form of nausea and vomiting, which completely resolved after discontinuation of the drug.
When analyzing laboratory blood parameters throughout the entire course of treatment, in no case were changes in liver tests recorded, which indicates the absence of Cansalazine hepatotoxicity. After completion of the clinical trial, all patients were recommended to maintain remission of UC with maintenance therapy with Cansalazine at a dose of 3 g per day in combination with local therapy under the supervision of a gastroenterologist.
Consequently, the domestic drug Kansalazine has a pronounced anti-inflammatory effect in patients with mild and moderate UC, not inferior in effectiveness to foreign analogues.
Conclusion
In 96.7% of patients with mild and moderate UC, clear positive dynamics were achieved as a result of taking Cansalazine for 1 month at a dose of 3 g/day.
Cansalazine has a pronounced anti-inflammatory effect in patients with UC, stops the acute attack of the disease and promotes the development of remission of the disease.
Side effects in the form of nausea and vomiting were recorded in 1 patient with UC with a left-sided lesion of moderate activity, and therefore the patient was transferred to another form of mesalazine.
Thus, the domestic drug Cansalazine can be recommended for the treatment of mild and moderate UC both during exacerbation and for maintaining remission.
Literature
- Jostins L., Ripke S., Weersma RK et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease // Nature. 2012; 491:119–124.
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M., Chernoff G., Benchimol EI, Panaccione R., Ghosh S., Barkema HW, Kaplan GG Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review // Gastroenterology. 2012; 142:46–54.
- Shelygin Yu. A., Blagodarny L. A. Handbook of coloproctology. M.: Publishing house "Litterra", 2014. pp. 460–522.
- Langholz E., Munkholm P., Davidsen M., Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years // Gastroenterology. 1994. Vol. 107. P. 3–11.
- Magro F., Gionchetti P., Eliakim R., Ardizzone S., Armuzzi A., de Acosta MB, Burisch J., Gecse KB, Hart AL, Hindryckx P., Langner C., Limdi JK, Pellino G., Zagurowicz E., Raine T., Harbord M., Rieder F. For the European Crohn's and Colitis Organization // Journal of Crohn's and Colitis. 2022, 649–670. DOI: 10.1093/ecco-jcc/jjx008.
- Clinical recommendations of the Russian Gastroenterological Association and the Association of Coloproctologists in Russia for the diagnosis and treatment of ulcerative colitis // Coloproctology. 2022, No. 1 (59).
- Khatkov I. E., Parfenov A. I., Knyazev O. V., Mikhailyants G. S., Atroshchenko A. O., Ruchkina I. N. Inflammatory bowel diseases in the practice of a therapist and surgeon. M.: VITA Press, 2022. pp. 12–50.
- Li W., Zhang ZM, Jiang XL Once daily vs multiple daily mesalazine therapy for mild to moderate ulcerative colitis: a meta-analysis // Colorectal Dis. 2016, Jul; 18(7):O214–223. DOI: 10.1111/codi.13393.
- Schroeder KW, Tremaine WJ, Ilstrup DM Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study // N. Engl. J. Med. 1987; 317:1625–1629.
- Moein S., Qujeq D., Tabari MV, Kashifard M., Hajiantilaki K. Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: From laboratory to clinic // Caspian J Intern Med. 2017; 8 (3): 178–182. DOI: 10.22088/cjim.8.3.178.
I. N. Ruchkina, Doctor of Medical Sciences O. V. Knyazev, Doctor of Medical Sciences I. E. Trubitsyna, Doctor of Medical Sciences N. A. Fadeeva, Candidate of Medical Sciences E. A. Dobrolyubova S. V. Belousov S. G. Khomeriki, Doctor of Medical Sciences, Professor A. I. Parfenov1, Doctor of Medical Sciences, Professor
GBUZ MKSC im. A. S. Loginova DZM, Moscow
1 Contact information
Effective domestic mesalazine in the treatment of ulcerative colitis / I. N. Ruchkina, O. V. Knyazev, I. E. Trubitsyna, N. A. Fadeeva, E. A. Dobrolyubova, S. V. Belousov., S. G. Khomeriki , A. I. Parfenov
For citation: Attending physician No. 2/2018; Page numbers in the issue: 32-37 Tags: intestinal diseases, inflammation, exacerbation