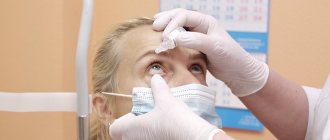
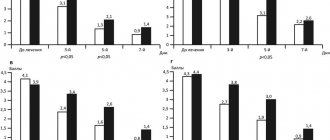
- infectious inflammation of the organs of vision;
glaucoma and cataracts (for corticosteroid drugs);
When using drops for long-term treatment, it is recommended to abandon contact lenses for the duration of therapy, replacing them with glasses for this time.
If, at first glance, there are no contraindications, but when instilled the patient experiences side effects, the use of such drugs should be abandoned, even if, along with improvement, the discomfort and pain intensify.
Pharmacodynamics and pharmacokinetics
The drug is a selective histamine H1 receptor inhibitor that also inhibits the release of inflammatory mediators from mast cells . It has pronounced antiallergic properties.
When applied topically, systemic absorption of the drug is quite low. The maximum concentration of the active substance in plasma is observed after 2 hours. The half-life in plasma is 3 hours. Mainly, the medicine is excreted through the kidneys. About 65% is excreted unchanged.
Instructions for use OPATANOL
Suction
Olopatadine, like other topical drugs, undergoes systemic absorption. However, systemic absorption of topically administered olopatadine is negligible, and plasma concentrations can vary from the lowest quantifiable value (<0.5 ng/mL) to 1.3 ng/mL. These concentrations are 50-200 times lower than the concentrations after the administration of well-tolerated doses of the drug orally.
Metabolism and excretion
When studying pharmacokinetic parameters when prescribing the drug orally, it was revealed that T1/2 of olopatadine in plasma ranges from 8 to 12 hours, and excretion is carried out mainly by the kidneys. Approximately 60-70% of the dose received is found in the urine as the active substance. Two metabolites were also found in urine in low concentrations: monodesmethyl and N-oxide.
Pharmacokinetics in special clinical situations
In pharmacokinetic studies following oral administration of 10 mg olopatadine in young (mean age 21 years) and elderly (mean age 74 years) patients, there were no significant differences between plasma concentrations (AUC), protein binding, and urinary excretion of the parent drug unchanged. and not found in the form of metabolites.
Since olopatadine is excreted by the kidneys mainly in the form of unchanged active substance, impaired renal function causes changes in the pharmacokinetic parameters of olopatadine - in patients with severely impaired renal function (average CC value - 13.0 ml/min), its Cmax in plasma is 2.3 times higher than in healthy people adults. Following oral administration of 10 mg in hemodialysis patients (without urinary excretion), olopatadine plasma concentrations were significantly lower on the day of the procedure than on other days, suggesting that hemodialysis may promote the elimination of olopatadine.
A study was conducted in patients with severe renal impairment in which olopatadine was administered orally. The results obtained indicate a higher plasma concentration of olopatadine in this group of patients. Since plasma concentrations of olopatadine after instillation of the drug Opatanol into the eyes are 50-100 times lower than after oral administration in well-tolerated doses, dose adjustment is not required when prescribed to elderly patients or patients with impaired renal function.
Hepatic metabolism is a less significant route of drug elimination. No dose adjustment is required for liver dysfunction.
Side effects
When using the drug, the following local reactions are possible: lacrimation , conjunctival hyperemia , burning and pain, as well as an unpleasant sensation of a foreign body in the eyes, iritis , keratitis , blurred vision, swelling of the eyelids.
In addition, systemic manifestations such as dizziness , weakness, pharyngitis , sinusitis , headache , nausea, rhinitis , and changes in taste are likely.
The most effective drugs
For your information! If we consider the problem from the point of view of effectiveness and do not take into account the cost of drugs, there is no need to look for a “golden mean” in the form of an inexpensive but effective remedy.
Here are a few high-price products that help well with eye allergies:
- Allergodil . Antihistamine with membrane-stabilizing effect. This means that the drug strengthens the membranes of vascular tissue cells, preventing histamines from penetrating inside and causing inflammatory processes. The product can be taken by adults and children from the age of four. For seasonal allergies, the product is instilled twice a day; if the allergies are non-seasonal, instillations are performed four times a day.
- Visallergol . The drug is distinguished by the fact that it has several mechanisms of action and not only neutralizes the released histamine, but also inhibits the mechanisms that produce such a substance and others like it. It is enough to instill the product once a day, a drop in each eye, and due to the almost complete absence of side effects, Visallergol can be used for long-term treatment (up to four months).
- Visin allergy . A drug from the group of blockers that themselves come into contact with mucosal receptors, limiting their interaction with histamines. This is a fast-acting remedy, the effect of which is observed within the first few minutes, and it ends after 8-10 hours. Therefore, it is enough to instill the drug twice a day.
Analogues of Opatanol
Level 4 ATX code matches: Polinadim
Vizin Alergy
Zaditen
Cromohexal
Lecrolin
Ketotifen
Analogs of Opatanol eye drops that have the same level 4 ATC code:
- Ifiral;
- Allergodil;
- Alergokrom;
- Ketotifen;
- Lastakaft;
- Cromo Sandoz;
- Lecrolin;
- Kromofarm.
All Opatanol analogues have their own application characteristics, so it is not recommended to use them without consulting a specialist.
Reviews about treatment with drops
Since childhood, I have had to pollen, which for some reason went away by about 20 years old, and ten years later it returned again.
I don’t believe in traditional methods and in the fact that it is enough just not to leave the house during periods of flowering plants.
I live in an area where it is too hot in the summer with the windows closed, and if you open them, pollen particles are sure to enter the room. Allergodil helps .
As soon as the flowering period begins, I buy a bottle of this medicine and during this entire dangerous period for me I do not complain about allergies .”
Nikolai Konstantinovich, Saratov.
“
seven-year-old child is allergic to some indoor flowers. Moreover, this manifests itself not only during the flowering period, but also in cases where such plants are nearby at any time of the year.
When dealing with such a problem, the allergist advised that at the first signs of such a reaction, drop opatanol into the eyes.
And now this medicine is always in our home medicine cabinet in case our son accidentally ends up near such plants.”
Nadezhda Chernova, Moscow.
Opatanol price, where to buy
In most cases, the price of Opatanol eye drops is considered very high. However, if the product helps, patients believe that such a cost is justified. The price of Opatanol is on average 360 rubles.
- Online pharmacies in RussiaRussia
ZdravCity
- Opatanol eye drops 0.1% 5mlS.A.
Alcon-Couvreur nv RUR 484 order
Inexpensive means
When choosing anti-allergy drops, you cannot focus only on the cost of drugs , but, on the other hand, in the situation with drugs, the principle “more expensive is better” does not always work.
There are several inexpensive remedies that help quite well, and one of them is cromohexal .
Know! This remedy not only eliminates allergy symptoms, fights its pathogens and blocks histamines, but can also be used for prevention.
The main course of treatment consists of four instillations daily, one or two drops are instilled each time.
An alternative to these drops is polynadim . The drug contains the substance diphenhydramine, which has an antiallergic effect by blocking histamine receptors.
Another active component of the drops, naphazoline, has a vasoconstrictor effect, normalizing blood flow in the organs of vision.
The product is instilled every three hours on the first day and then two to three times a day until the main signs of allergic reactions disappear.
Opatanol (olopatadine 0.1%) – eye drops with a dual antiallergic mechanism of action
YF Maychuk Moscow Helmholtz Institute of Ophthalmology Histamine receptor activation and degranulation of mast cells are the main pathogenic mechanism of allergic eye diseases. Objective of this article – review the currently available literature regarding new topical antiallergic agent – olopatadine hydrochlorid 0.1% ophthalmic solution (Opatanol®, registered in Russia trade name of Alcon Laboratories Inc., USA). Opatanol is indicated for the treatment of the signs and symptoms of allergic conjunctivitis that include itching, redness, tearing, lid swelling, conjunctival hyperemia and chemosis. Relevant research of laboratory animal model, clinical study in the conjunctival antigen challenge model and clinical trials confirmed unique combined dual mechanisms of action – antihistaminic and mast cell stabilizing proterties, with non–perturbation of mast cell membranes. In comparative clinical trials with many antiallergic agents Opatanol demonstrated superior efficacy and greater tolerability. Medical and social significance of eye allergies Treatment of allergic eye diseases remains a serious problem in ophthalmological practice. This is primarily due to the wide prevalence of ocular allergies, the variety of causative factors, persistent course, and the risk of visual impairment. The increase in the incidence of allergies, which is becoming an epidemic in economically developed countries, served as the basis for the creation in 2003 of the “Program for the Prevention of Allergy and Bronchial Asthma” in the form of a joint project of the World Allergy Organization (WOA) and the World Health Organization (WHO) [46]. The SAI report (2005) emphasizes that the incidence of allergies is increasing worldwide [1]. Over the past 40 years, the prevalence of allergy diseases in Western countries has increased 2–3 times. This growth has coincided with changes in both the environment and people's lifestyles. If in 2000 MH Friedlender indicated that the population affected by allergic conjunctivitis (AK) was 15% and called eye allergies a common problem not only in ophthalmology, but also in general practice [30], then in 2003–2006. the incidence of AK in the population is said to be 20% [25], 20–21% [19]. According to recent data, from 20% [1, 3] to 30% [18] of the population suffer from various allergic diseases mediated by immunoglobulin E (IgE). L. Bielory (2006) believes that from 30 to 50% of the US population suffer from some form of allergy [18]. However, among patients with hay fever, AK was detected in 91.2% [4], with allergic rhinitis - 75% [18], and atopic keratoconjunctivitis - in 25% of patients with atopic dermatitis [28]. MB Abelson believes that ocular clinical signs of allergy are present in approximately 50% of patients [7]. In general, one should agree with the opinion of L. Bielory (2006) [18] about insufficient diagnosis (underdiagnosis), and hence – insufficient coverage of therapy (hypotherapy) for patients with ocular allergies. Classification of ocular allergies Ocular allergies include a whole group of diseases that have a common pathophysiological mechanism (hypersensitivity), but different clinical manifestations. The following are the most common clinical forms of ocular lesions (small changes have been made to our previously published classification [2, 3]): – seasonal pollinous conjunctivitis; – drug allergies; – vernal keratoconjunctivitis; – large papillary conjunctivitis; – chronic allergic conjunctivitis; – allergies with keratoconjunctivitis sicca; – allergies when wearing contact lenses; – eye damage due to systemic immune diseases; – allergic reactions in infectious eye diseases. Although it has been repeatedly emphasized that both acute and chronic allergic eye diseases have a common immunopathogenesis [36], we attach the main importance in choosing therapy to the clinical course of the disease. Accordingly, the course of the disease is acute, subacute and chronic, but the causative factor is also taken into account [4]. Basic means of local antiallergic therapy Considering the central role of histamine and histamine H1 receptors in the pathogenesis of AK, it is generally accepted that H1 receptor antagonists are the first choice drugs. The advantages of local therapy for allergic conjunctivitis compared to oral administration are a rapid therapeutic effect, since the medicine enters directly onto the affected mucous membrane, and a lower likelihood of systemic side effects. The main basic therapy is provided by two groups of drugs - antihistamine eye drops and those that inhibit mast cell degranulation. Antihistamine eye drops, which additionally often contain a vasoconstrictor, have the fastest effect. In acute allergic conjunctivitis, these drugs block H1 receptors, reduce the tissue response to histamine, and thereby provide an antiallergic effect within a few minutes: itching and swelling of the eyelids, lacrimation, hyperemia and swelling of the conjunctiva are reduced. Among the drugs in this group, the most widely recognized in Russia are Spersaller eye drops, containing antazoline hydrochloride and tetrizaline hydrochloride (Novartis, Switzerland), and Polinadim eye drops, created in our country, containing diphenhydramine and naphazoline [5]. Eye drops that inhibit mast cell degranulation. The most used is Lecrolin (Santen, Finland) - eye drops containing a 2% solution of disodium cromoglycate. It is believed that cromoglycate inhibits IgE-dependent allergic reactions by preventing the release of histamine and other mediators by mast cells. This prevents the development of such clinical manifestations of allergies as eye irritation, swelling, itching, and lacrimation. Lecrolin, like other cromoglycates, has not only a therapeutic, but also a preventive effect, which is the main feature of these drugs. In accordance with the mechanism of antiallergic action, the therapeutic effect develops gradually, but lasts longer than with the use of antihistamine eye drops. Lecrolin without a preservative in tube droppers is the first eye drops to arrive in Russia that do not contain a preservative, which means that it excludes an allergic reaction to the preservative. Opatanol - antiallergic eye drops with a dual mechanism of action Opatanol® is the trade name of a 0.1% solution of olopatadine hydrochlorate, developed in the form of eye drops by the Alcon laboratory (USA) and released in Belgium. The drops are registered in Russia - No. LS-000661 dated August 28, 2005 [6]. Chemical name (z)-11-[(Dimethylamino)propylidene]-6, 11-dihydrodibenz(b,e)-oxepin-2-acetic acid hydrochloride [12]. The eye drops contain olopatadine hydrochloride and excipients: benzalkonium chloride, sodium chloride, disodium phosphate dodecahydrate, concentrated hydrochloric acid and/or sodium hydroxide (to adjust the pH), purified water. Pharmacological group – antiallergic drug. Pharmacological action: olopatadine is a selective inhibitor of H1-histamine receptors and also inhibits the release of inflammatory mediators from mast cells. Has a pronounced antiallergic effect. It has no effect on α-adrenergic, dopamine, muscarinic types 1 and 2, as well as serotonin receptors. Pharmacokinetic studies have shown that when applied topically, systemic absorption is low. The maximum concentration (Cmax) of olopatadine in blood plasma is achieved within 2 hours after topical application and ranges from 0.5 ng/ml or less to 1.3 ng/ml. It is excreted primarily by the kidneys, 60–70% unchanged. Numerous experimental studies and clinical observations have shown a unique dual mechanism of action - an antihistamine effect and inhibition of mast cell degranulation. Opatanol as an antihistamine In experiments in vitro and in vivo, it was shown that olopatadine is highly effective in inhibiting H1 receptors and has no effect on H2 and H3 receptors [12], which is due to the peculiarities of the chemical structure [39]. It has been shown that the selective effect of olopatadine on H1 receptors is more pronounced than that of antihistamines such as levocabastine, pheniramine, and antazoline [43]. Already the first experimental studies showed that olopatadine has a high selective effectiveness against H1 receptors, while at the same time it has no effect on α-adrenergic, dopamine, muscarinic and serotonin receptors, which is an important advantage compared to other antiallergic drugs [12, 43]. Studies of olopatadine have revealed another important mechanism of antiallergic effects. In recent years, conjunctival epithelial cells have attracted increasing interest as a potential site of drug action. It was found that epithelial cells are capable of producing cytokines under various influences [32] and have functional H1 receptors [42]. The effect of histamine on conjunctival epithelial cells stimulates the production of interleukins IL-6 and IL-8, and treatment with antagonists prevents this production [49]. Research suggests that blocking histamine effects on the epithelium provides, broadly speaking, some of the anti-inflammatory effects of many antihistamines [48,49]. Opatanol as an inhibitor of the release of mast cell mediators In the experiment, olopatadine inhibits the release of histamine, tryptase and the pro-inflammatory cytokine TNF2 from conjunctival mast cells [47]. The complex mechanism by which olopatadine inhibits mast cell degranulation is not fully understood, but the drug is thought to limit the migration of cells such as eosinofins to the site of the allergic reaction [24]. The effect on the conjunctival epithelium leads to a polarized suppression of the migration of inflammatory cells. This additional anti-inflammatory effect provides a rationale for the use of olopatadine in combination with corticosteroids in the treatment of chronic inflammatory diseases such as allergic keratoconjunctivitis and vernal catarrh [24]. One of the problems with antihistamines is the negative side effect on mast cell membranes (lysis). Among them, only olopatadine does not have a negative effect on mast cell membranes. In one study, the negative effects of antihistamines on membranes were distributed from highest to lowest as follows: desloratadine > clemastine > azelastine = ketotifen > diphenhydramine > pyrilamine > emedastine > epipastine > olopatadine [20]. The explanation given for the therapeutic effect of these drugs is that the drugs exert their clinical effect through antihistamine activity, although at the same time they promote mast cell degranulation after initial stabilization [41]. Olopatadine in a clinical experiment To assess antiallergic efficacy, the conjunctival allergen-induced model (CMEA) is considered ideal. This model is recognized in the USA as the most significant for the registration of new antiallergic drugs [8]. CMVA is an allergic reaction that can be standardized according to criteria including itching, redness and swelling of the eyelids, conjunctival hyperemia and chemosis. In addition to the severity of clinical signs, the time of onset and duration of the allergic reaction are also taken into account. This model was also used by us in evaluating the antiallergic eye drops Polinadim, created at the Moscow Research Institute of Eye Diseases named after. Helmholtz [5]. It was this model with placebo control that made it possible to evaluate the effectiveness, safety, optimal drug concentration, onset and duration of action of olopatadine. The onset of action of olopatadine was noted at 5 minutes, and the duration was 8 hours. A concentration of olopatadine of 0.1% significantly suppresses the peak of the allergic reaction [10]. In another study, pruritus and hyperemia were significantly reduced in eyes treated with olopatadine compared with control eyes [38]. It was noted that after 5 hours the level of eosinophils, neutrophils, lymphocytes, and the total number of cells significantly decreased compared to control eyes. Tear histamine levels were also significantly lower at 5 hours compared to samples at 30 minutes. Thus, olopatadine in these randomized, placebo-controlled experiments significantly reduced the clinical and morphological indicators of conjunctival mast cell degranulation. The significant effect of olopatadine on such signs of allergy as itching and hyperemia was also confirmed in studies in comparison with other drugs: artificial tears [10], ketorolac [29], nedocril [22], ketotifen [15], cromolyn [33], azelastine [35], lateprednol etabonate [16], epipastine [35]. The use of the same model in our observations showed a higher antiallergic effectiveness of Opatanol eye drops compared to azelastine. Opatanol in the treatment of allergic conjunctivitis One of the first clinical observations was a comparative study of olopatadine eye drops and ketorolac 0.5%, a non-steroidal anti-inflammatory drug, in the treatment of seasonal AK [29]. Olopatadine significantly reduced the development of an allergic reaction and was well tolerated compared to ketorolac, the latter increasing hyperemia (apparently due to an irritant effect). In clinical observations covering 100 patients with AK, two drugs were used – olopatadine and ketotifen for 2 weeks [37]. Based on comfort and effectiveness, 81% of patients preferred olopatadine. 80 patients with AK were divided into two groups: one received olopatadine 0.1%, the other received ketotifen 0.05% 2 times a day [13]. The condition was assessed after 30 minutes, 48 hours, 7 and 14 days. Olopatadine had a more pronounced therapeutic effect, acted faster and lasted longer. In another series of studies involving 66 patients with seasonal AK [31], ketotifen (0.025%) or olopatadine (0.1%) was used for 3 weeks. Although the authors noted some benefit of ketotifen in reducing eyelid itching and conjunctival hyperemia, tolerance of both drugs was similar. Comparative studies of olopatadine and cromolyn in two parallel groups of patients (n=185) with seasonal conjunctivitis showed advantages of olopatadine in terms of swelling, hyperemia and tolerance [33]. Moreover, in children under 11 years of age, the rate of hyperemia was 2 times lower in the group receiving olopatadine. Interesting results were obtained in a comparative double-blind study of olopatadine and azelastine in the treatment of AK (n=111) [44]. Olopatadine was more effective, possibly due to the undesirable nonspecific degranulation effect of azelastine on cell membranes. In a comparative study, one instillation of olopatadine was more effective than 57 instillations over 2 weeks of the corticosteroid loteprenol for eyelid itching (n=50) [16]. Olopatadine was more effective in suppressing pruritus, hyperemia and chemosis compared with epinastine (n=53) [35]. According to the authors, epinastine was equivalent to placebo in terms of hyperemia, possibly due to the fact that the initial stabilization of mast cells is followed by degranulation with the release of histamine. This is confirmed by studies showing that ketotifen, azelastine, and epinastine cause lysis of the membranes of conjunctival mast cells and corneal epithelial cells with subsequent release of histamine [20]. In the treatment of AK, olopatadine was more effective than levocabastine [36]. In the treatment of 20 patients (40 eyes) with spring catarrh (average age 26.6 years), olopatadine was used twice a day for 2 months [26]. There was a significant improvement in all symptoms of the disease: lacrimation, itching of the eyelids, hyperemia, foreign body sensation, mucous discharge, conjunctival hyperemia. There was no effect on the limbal papillae. The intensity of goblet cells in the conjunctiva decreased markedly. Patients with AK while wearing contact lenses noted the effectiveness of olopatadine (n=20) and better tolerability compared to placebo [21]. Drops were instilled 15 minutes before lens insertion. Moreover, patients receiving olopatadine could wear lenses 2.1 hours longer. However, special studies have shown that olopatadine does not accumulate in contact lenses [27]. It is known that local antiallergic therapy with AK is more effective than the systemic use of antihistamines [2]. Also, local use of olopatadine for AK turned out to be more effective than systemic use of loratadine, but is even more effective with joint complex therapy [11,12]. In another study of seasonal conjunctivitis (n=94), the addition of topical olopatadine to oral loratadine increased treatment efficacy and improved quality of life [34]. However, systemic use of loratadine, like other antihistamines, leads to dry eye syndrome, which worsens the course of allergic inflammation [40, 45]. Olopatadine (0.1%), cromolyn (2%) or levobastine (0.05%) were used in the treatment of AK in children (mean age 7.2 to 8.8 years). All three drugs were well tolerated, but olopatadine was more effective (p < 0.05) [17,23]. For seasonal conjunctivitis in children (n=50) aged 4 to 11 years, olopatadine was more effective than cromoglyquine or levocabastine and was well tolerated when instilled twice daily for 6 weeks [23]. It was shown that the instillation of olopatadin in the eyes with rhinoconjunctivitis itself has a therapeutic effect not only on the course of conjunctivitis, but also of rinite (n = 131) [34]. This effect is associated with the ingress of the drug from the conjunctival bag to the nose through the tear system. In other studies, the additional use of olopatadin is locally to the antihistamine drug in the form of a spray in the nose or systematically significantly increased the effectiveness of the treatment of rhinoconjunctivitis (n = 200) and improved the quality of life of patients [14]. The conclusion of numerous pre -line and clinical studies have shown that Olopatadin, who entered an ophthalmic practice called Opatanol, eye drops (Alcon), is a drug with a double mechanism of action: antihistamine and stabilizing fat cells of conjunctiva. Thus, Opatanol combines the two main directions of basic therapy of allergic eyes of the eyes: selectively blocks H1 - hystamine receptors and inhibits the release of histamine and other inflammation from conjunctivatic mast cells. Clinical studies have shown a higher effanne efficiency in the treatment of allergic conjunctivitis compared to antihistamine drugs, fat cell membranes and non -steroidal anti -inflammatory drugs. Opatanol eye drops are well tolerated, can be used for a long time (up to 4 months with seasonal conjunctivitis) and is allowed for use in children's practice (from 3 years and older). Thus, an ophthalmological practice has entered a new anti -allergic drug of a double mechanism of action. At the same time, further clinical observations can show the predominant place of use of opatanol in various clinical forms of allergic eyes. The article was taken into print on December 14, 2006
Literature 1. Johansson SGO, Haahtela T.// Allergy and immunology. – 2005. – N1. – pp. 81–91. 2. Maychuk Yu.F. //Bulletin of Ophthalmology. – 2000. – No. 5. – P.10–14. 3. Maychuk Yu.F. // Wedge. ophthalmol. – 2002. – No. 1. – P. 6–9. 4. Maychuk Yu.F. // Attending doctor. – 2006. – No. 8. – pp. 61–64. 5. Maychuk Yu.F., Pozdnyakov V.I., Pozdnyakova V.V., Yakushina L.N. // Bulletin of ophthalmology. – 2006. – No. 5. – pp. 35–38. 6. Opatanol // Instructions for use, “s.a. Alkon-Couvreur n.v., Belgium. – 2005. 7. Abelson MB Allergic diseases of the eye. W. B. Saunders Co., Philadelphia. 2001. 8. Abelson MB, Chambers WA, Smith LM//Arch. Ophthalmol. – 1990/ – v.108. – P84–88. 9. Abelson MB, Pratt S, Mussoline JF, et al. Clin. Ther. – 2003. – v.25. – P.2070–2084. 10. Abelson MB, Spitalny L.//Am. J. Ophthalmol. 1998. – v.125. – P.797–804. 11. Abelson MB, Welch DL // Acta. Ophthalmol. Scand. Suppl. 2000. – No. 230. – R.60–63. 12. Sharif NA, Xu SX, Yanni JM//J. Ocular Pharmacol. and Ther. – 1996. – N4. – P. 401–407. 13. Aguilar AJ //Acta Ophthalmol. Scand. Suppl. 2000. – N230. – P.52–55. 14. Berger WE, Beck M., Kimura S. et al.//Ann. All Asth. Immunol. – 2005. – P.95. 15. Bergy GJ, Spangler DL, Bensch G., et al.//Clin. Ther. – 2000. – v.22. – P.826–833. 16. Berdy GJ, Stoppel JO, Epstein AB//Clin. Ther. – 2002. – v.24. – P.918–929. 17. Bertin D., Fedrigo A., Canno-Parra J.//Ophthalm. Res. – 2001. – v.33 (suppl. 1). 18. Billory L.//Med. Clin. N. Am. – 2006. – v.90. – P.129–148. 19. Bogacka E. // Pol. Merkurius Lec. – 2003. – 84. – P.714–715. 20. Brockman HL, Momsen MM, Knudtston JR, et al.//Ocular Immunol. Inflamm. – 2003. – v.2. – P.247–268. 21. Brodsky M., Berger WE, Butrus S., et al.//Eye Contact Lens. – 2003. – v29. – P.113–116. 22. Butrus S., Greiner JV, Discepola M., et al.//Clin. Ther. – 2000. – v.22. – P.1462–1472. 23. Ciprandi G, Turner D, Cross RD et al. //Cur. Ther. Res. – 2004. – v.65. – No. 2. – P.186–199. 24. Cook EB, Stahl JL, Barney NP, et al.//Ann Allergy Asthma Immunol. – 2001. – v.87. – P.424–9. 25. Cornea and external eye disease. Ed. T. Reinhard, F. Larkin. Springer. – 2006. – 229P. 26. Corum I., Yeniad B., Bilgin LK et al.//J. Ocular Pharm. Therap. – 2005. – v.21. – No. 5. – P.400–405. 27. Dassanayake NL, Carey TC, Owen GR//Acta. Ophthalmil. Scand. – 2000. – v.78. – P.16–17. 28. Davas RG // Chemical Immunology and Allergy. Ed. Johannes Ring et al. Carger. – 2006. – P. 110–120. 29. Deschenes J., Discepola M., Abelson MB//Acta Ophthalmol. Scand. Suppl. – 1999. – No. 228. – P.47–52. 30. Friendlander MN//In: Current ocular therapy. Ed. F. T. Frannfeder, F. H. Roy, J. W. B. Randall. Saunders Comp. – 2000. – 323P. 31. Ganz M., Koll E., Detjen P. et al.//Advanced in Therapy. – 2003. – v.20. – No. 2. – P.79–91. 32. Hingorani M., Calder V., Buckley RJ, et al.//Exp. Eye Res. – 1998. – v.67. – P.491–500. 33. Katelaris CH, Ciprandi G., Missotten L., et al.//Clin. Ther. – 2002. – v.24. – P.1561–1575. 34. Lanier BQ, Gross RD, Marks BB et al.//Ann. Allergy, Asthma, Immunology. – 2001. – v.86. – P.641–648. 35. Lanier BQ, Finegold I., D'Arienzo P., et al.//Curr. Med. Res. Opinion. – 2004. – v.20. – P.1227–1233. 36. Leonardi A.//Emerging drugs. – 2005. – v.10. - No. 3. – P.505–520. 37. Leonardi A., Zafirakis P.//Curr. Med. Res. Opinion. – 2004. – v.20. – P.1167–1173. 38. Leonardi AA, Abelson MB//Clin. Ther. – 2003. – v.25. – P.2539–52. 39. Nonaka H., Otaki S., Jhshima E., et al.//Eur. J. Pharmacol. – 1998. – v.345. – P.111–117. 40. Ousler GW, Wilcox KA, Gupta G., et al.//Ann. All Asth. Immunol. – 2004. – v.93. – P.460–464. 41. Rosen–Wasser LJ, O'Brien T., Weyne J.//Current medical research and opinion. – 2005. – v.21. – No. 9. – P.1377–1387. 42. Sharif NA, Xu SX, Magnino PE, et al.// Exp. Eye Res. – 1996. – v.63. – P.169–178. 43. Sharif NA, Xu SX, Miller ST, et al.//J. Pharmacol. Exp. Ther. – 1996. – v.278. – P.1252–1261. 44. Sprangler DL, Bensch G., Berdy GJ // Clin. Ther. – 2001. – v.23. – P.1272–1280. 45. Welch D., Ousler GW, Nally LA, et al.//Adv. Exp. Med. Biol. – 2002. –№506. – P.1051–1055. 46. World Health Organization. Prevention of allergy and allergic asthma. WHO. Geneva. WHO/NMH/MNC/CRA/03.2.2003. 47. Yanni JM, Gamache DA, Spellman JM et al.//Ann Allergy Asthma Immunol. – 1997. – v.79. – P.541–545. 48. Yanni JM, Weimer LK, Sharif NA, et al.//Arch Ophthalmol. –1999. –v.117. – P.643–647. 49. Yanni JM, Sharif NA, Gamache DA, et al.//Acta Ophthalmol. Scand. –1999. – v.77. – P.33–37.