Pharmacological properties
The drug contains biogenic stimulants, which are found in the aqueous extract from aloe leaves, as well as in substances contained in the juice of chokeberry fruits, including vitamins and microelements.
An aqueous extract from aloe leaves stimulates granulocytopoiesis, enhances the ability of absorption, degradation and recognition of antigens. Strengthens, first of all, the humoral immune response; stimulates an increase in the number of B lymphocytes in peripheral blood; at the same time having a weaker effect on the protective response of the cell type. In addition, it slightly increases resistance to contact infection. The immunostimulating effect of the drug is manifested in supporting the balance of the immune system, which is especially important for children who do not yet have a fully formed protective mechanism. Clinical studies conducted on children showed that the drug stimulates development, as well as weight gain in study participants.
Research methods
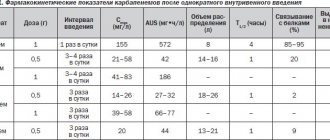
The study was conducted in accordance with the design presented in Table. 1.
For each patient, an examination card was filled out, which included the following data:
— individual patient code (patient serial number);
- last name, first name, patronymic of the patient;
— diagnosis of the underlying disease;
- accompanying illnesses;
— information about therapy;
— the nature of the course of the disease, information about the characteristics and duration of respiratory diseases for a 6-month calendar period of the previous year similar to the observed one, with calculation of the morbidity index;
— clinical and laboratory examination data (general clinical blood and urine tests, immune status, blood biochemistry);
— reason for leaving the study (if necessary).
To assess the condition of patients, the concept of “morbidity index” was introduced into the program (calculated for a 6-month calendar period similar to the observed one prior to treatment with Bioaron C), which is calculated using the following formula: the number of days of illness (for inpatient treatment with a coefficient of 2) + the number of days of antibacterial therapy . The average morbidity index in the study group before the start of treatment with Bioaron C was 57.10 ± 14.12.
All patients underwent clinical and immunological examination before and after a course of Bioaron S, which included:
- taking anamnesis;
— general clinical blood test;
— general clinical urine analysis;
- biochemical blood test (total protein, ALT, AST, urea, creatinine, glucose);
— determination of serum immunoglobulins (IgA, IgM, IgG);
— study of the main subpopulations of lymphocytes in peripheral blood (CD3+, CD4+, CD8+, CD19+ cells).
The children's condition was assessed on days 0 and 15–17 after inclusion in the study, as well as 6 months after inclusion in the study. Anamnesis was collected at the time of inclusion of the child in the study, on days 15–17 and 6 months after inclusion in the study. Clinical examination was carried out on days 0 and 15–17 from the moment the child was included in the study.
The day after inclusion and examination, children began to take Bioaron S in the following doses: patients 3–6 years old - 5 ml 2 times a day, patients 6–12 years old - 5 ml 3 times a day orally for 14 days.
The safety assessment criteria were:
— subjective feelings of patients while taking the study drug;
- unexpected adverse events associated with taking Bioaron S;
— data from laboratory and instrumental studies.
special instructions
During pregnancy and breastfeeding. no negative effects were identified. the drug is intended for children.
Children. The drug is intended for children aged 3 years and older.
The ability to influence reaction speed when driving vehicles or working with other mechanisms. The drug is intended for children.
Safety precautions for use. A single dose of the drug (5 ml) contains 3.3 g of sucrose (0.33 XE), which must be taken into account by patients with diabetes mellitus.
The drug may distort the results of a number of laboratory tests (blood glucose, bilirubin, transaminase activity, LDH).
Do not change the dose of the drug without the doctor’s instructions or expand the indications for use.
conclusions
The study demonstrated the high clinical effectiveness of Bioaron S, which indicates its obvious bioimmunological effect in children with frequently recurrent bacterial and viral infections of the upper respiratory tract. However, there were initially no deviations from the norm in key indicators of immune status (immunoglobulins A, M, G and the main subpopulations of lymphocytes CD3+, CD4+, CD8+, CD19+), that is, clinically effective therapy with Bioaron C did not affect the initial normal indicators of immune status. The use of Bioaron S in the doses used in the study in children is safe and does not cause side effects. Thus, this drug can be recommended in clinical practice for the treatment of recurrent bacterial and viral infections of the upper respiratory tract in children.
Note!
Description of the drug Bioaron S syrup fl. 100ml on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
results
In 3 children, while taking Bioaron S, an exacerbation of underlying diseases occurred and their legal representatives refused further participation in the study. The legal representatives of 4 children refused further participation in the study without explanation. The legal representatives of 2 children refused re-examination (blood sampling).
The drug Bioaron S was well tolerated; no patients experienced side effects when taking it.
When studying hematological parameters before and after taking Bioaron S, there was no significant dynamics in the number of erythrocytes, leukocytes, lymphocytes, eosinophils, band and segmented neutrophils and ESR (Table 2), a biochemical blood test also did not show significant changes in the amount of total protein, ALT, AST , urea, creatinine and glucose while taking the drug (Table 3).
As a result of a urine test during the use of Bioaron S, there were no significant changes in the specific gravity and number of leukocytes and erythrocytes (Table 4).
The results of a study of immunological parameters in children taking Bioaron S are presented in table. 5 and 6. When studying serum IgM, IgG, IgA, as well as subpopulations of lymphocytes CD3+, CD4+, CD8+, CD19+, no significant dynamics in their levels were noted.
Additionally, it can be noted that the children took the drug with pleasure, since it did not cause them any unpleasant taste sensations.
The effectiveness of treatment was assessed by changes in the morbidity index within 6 months after the end of taking Bioaron S in comparison with the same calendar period of the previous year:
- no negative effect (increase in exacerbation severity index by more than 25%) was noted;
- no effect (exacerbation severity index did not change or decreased by 25%) in 15 patients - 25.8%;
— a satisfactory effect (decrease in the severity index of exacerbations by 25–50%) was noted in 31 patients — 53.5%;
— a good effect (decrease in the exacerbation severity index by more than 50%) was observed in 12 patients — 20.7%.
The morbidity index in children before the course of treatment with Bioaron C was 57.10 ± 14.12, and after the course - 38.15 ± 13.92, thus, its significant positive dynamics were noted.
Good treatment effectiveness was noted in 12 patients (20.7%), the frequency of relapses of bacterial and viral infections of the upper respiratory tract decreased by 3 times. Satisfactory - in 31 patients (53.5%), the frequency of relapses of bacterial and viral infections of the upper respiratory tract decreased by 2.5 times.
Thus, 74.2% of patients had a positive (satisfactory or good) effect. In 25.8% of patients, there was no significant effect of Bioaron S administration on the frequency of exacerbations of bacterial and viral infections of the upper respiratory tract. In 8 children, the examination revealed sensitization to household and/or epidermal allergens, which indicates the allergic nature of the disease and causes the low effectiveness of the Bioaron S drug; in 2 children, siblings began to attend kindergarten during the study period, and therefore the degree of contact with carriers of respiratory infections increased, which could affect the incidence of the disease in children included in the study group; In 4 children, the morbidity index decreased by 18–23%, which is an insufficiently satisfactory effect, and they were included in the “no effect” group in accordance with the criteria for assessing the effectiveness of the study.
Data were obtained that the frequency of exacerbations of bacterial and viral infections of the upper respiratory tract when prescribing Bioaron S in 74.2% of patients decreases by 2.5–3 times within 6 months after a course of Bioaron S. In the remaining 25.8% of patients, the frequency diseases remained the same or decreased by less than 25%. None of the children taking Bioaron S experienced an increase in the frequency or severity of diseases.
BIOARON S (syrup)
There is a two-week course for children from six years old, you need at least two of these bottles!
It turns out that the same cosmic cost comes out. See for yourself, the bottle contains 100 ml, the dose for children over six years old is 5 ml 3 times a day. It turns out that you need 15 ml for every day, and 105 ml for a week, that is, it’s barely enough even for seven days! So, regarding the affordability of the price, I can immediately say NO! What will we get?
For 150-200 rubles we will get a standard set.
The bottle has a convenient dispenser, which is literally “plugged” with a lid.
There is also an interestingly shaped measuring cup with divisions reaching up to 20 milliliters.
This original form does not bring any special convenience, except that it looks unusual on the bottle.
The taste of the syrup is pleasant, seemingly sweet, but at the same time with sourness, the smell is kind of caramel-herbal (but more sweet), the color is bright red, in order to achieve this, the manufacturers put chokeberry juice in the composition.
It’s convenient that the method of use and dosage are written both on the box and on the bottle itself, and, of course, in the instructions.
They also decided to place the composition everywhere, although this is the weakest point of the drug. I would like to draw your attention to the fact that the composition contains ONE OF THE MOST DANGEROUS PRESERVATIVES - SODIUM BENZOATE, which the manufacturer perfectly hid under the veil of natural ingredients. In addition, in combination with ascorbic acid, which is abundant in syrup, this preservative can form benzene, which is a strong carcinogen. Let's go further, according to research by British scientist Peter Piper, a professor at the University of Sheffield, such a compound can cause destructive DNA damage in mitochondria, which can cause a number of serious diseases, such as neurodegenerative diseases, liver cirrhosis, Parkinson's disease and others. The effect of sodium benzoate supplementation on hyperactivity in children has been seriously debated. Moreover, this additive is prohibited in some countries, but in Europe and the CIS countries it “lives and rejoices.” “Yes, it’s a small dose,” you say, but the child needs to drink two whole bottles of these to complete the full course! By the way, I don’t understand how this preservative could end up in the composition before water!?
To be fair, it is worth saying that when we did not know these terrible details and calmly drank the syrup, it helped us, and we began to get sick less often. But the product does not have such super-effectiveness. But I can’t say anything about increasing appetite, because it’s already excellent for us.
In general, even if you see already familiar preservatives that have been found more than once in other products and “nothing has happened to you,” then I still advise you to go online and check if this is true. The sooner you do this, the better it will be for both your body and your psyche. I think I described Bioaron C in detail in order to have a complete understanding of the advantages and disadvantages of the drug. So it’s “the boss’s business.” Thank you for reading my review, I hope it helped you and made you think about all sorts of “foods” there.”