Pharmacodynamics
Trazodone, being a triazolopyridine derivative, has a predominantly antidepressant effect, with some sedative and anxiolytic effects. Trazodone has no effect on MAO, which distinguishes it from MAO inhibitors and tricyclic antidepressants.
Quickly affects mental (affective tension, irritability, fear, insomnia) and somatic symptoms of anxiety (palpitations, headache, muscle pain, frequent urination, sweating, hyperventilation).
Trazodone is effective for sleep disorders in patients with depression, increases the depth and duration of sleep, and restores its physiological structure and quality.
Trazodone stabilizes the emotional state, improves mood, reduces pathological craving for alcohol in patients suffering from chronic alcoholism during the period of alcohol withdrawal syndrome, also in remission. For withdrawal symptoms in patients suffering from dependence on benzodiazepine derivatives, trazodone is effective in the treatment of anxiety, depression and sleep disorders. During remission, benzodiazepines can be completely replaced by trazodone.
The drug is not addictive.
Helps restore libido and potency, both in patients with depression and in people who do not suffer from depression.
The mechanism of action of trazodone is associated with the high affinity of the drug for certain subtypes of serotonin receptors, with which trazodone enters into an antagonistic or agonistic interaction depending on the subtype, as well as with the specific ability to cause inhibition of serotonin reuptake.
The neutral uptake of norepinephrine and dopamine has little effect.
The drug does not affect body weight.
Pharmacokinetics
Absorption of the drug from the gastrointestinal tract after oral administration is high. Taking trazodone during or immediately after a meal slows the rate of absorption, reduces Cmax of the drug in blood plasma and increases Tmax. Tmax of the drug is achieved 1/2–2 hours after oral administration.
The drug penetrates histohematic barriers, as well as into tissues and fluids (bile, saliva, breast milk).
Plasma protein binding is 89–95%.
Trazodone is metabolized in the liver, the active metabolite is 1-m-chlorophenylpiperazine. T1/2 in the first phase is 3-6 hours, in the second phase - 5-9 hours. The majority of the metabolized drug is excreted through the kidneys, in the urine (about 75%) and is completely completed 98 hours after taking the drug, excreted in the bile about 20%.
In vitro studies on human microsomes have shown that trazodone is primarily metabolized by cytochrome P450 CYP3A4.
Buy Trittico tablets with control. release 150mg No. 20 in pharmacies
Trade name:
Trittico
International nonproprietary name:
trazodone
Dosage form:
extended release tablets
Compound
One extended-release tablet contains:
active ingredient: trazodone hydrochloride 150.0 mg; excipients: sucrose (pressed sugar), carnauba wax, povidone K25 (polyvinylpyrrolidone), magnesium stearate.
Description
Biconvex tablets of white or white-yellowish oval shape with two parallel marks on both sides.
Pharmacotherapeutic group:
antidepressant
Pharmacological properties
Pharmacodynamics
Trazodone is a triazolopyridine derivative effective for the treatment of depressive disorders, including depression associated with anxiety and sleep disorders, and is characterized by a short latency period (about a week).
Trazodone is a serotonin reuptake inhibitor and 5-HT2 receptor antagonist, the activation of which is mainly associated with insomnia, anxiety, psychomotor agitation and changes in sexual function.
Unlike other psychotropic drugs, trazodone is not contraindicated in glaucoma, urinary tract diseases, does not have extrapyramidal effects, and does not potentiate adrenergic transmission; Due to the lack of anticholinergic activity, trazodone does not have the typical effects of tricyclic antidepressants on cardiac activity.
Pharmacokinetics
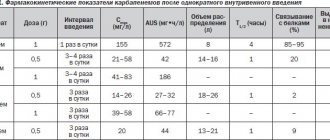
Following oral administration of a single dose of 75 mg extended-release trazodone, a Cmax of approximately 0.7 mcg/mL is achieved through a tmax of 4 hours after dosing and an AUC of approximately 8 mcg/mL/h. After oral administration of a single dose of 150 mg trazodone extended release, Cmax of about 1.2 mcg/ml is achieved after a tmax of 4 hours after administration, AUC is about 18 mcg/ml/h. The half-life is approximately 12 hours.
A food interaction study did not reveal significant changes in Cmax and AUC when Trittico 150 mg extended-release tablets were administered before or after meals.
In vitro studies on human liver microsomes have shown that trazodone is primarily metabolized by cytochrome P4503A4 (CYP3A4).
Indications
Depressive disorder with or without anxiety.
Contraindications
- known hypersensitivity to trazodone or any excipient; — alcohol intoxication and intoxication with sleeping pills; - acute myocardial infarction; - sucrose/isomaltose deficiency, fructose intolerance, glucose-galactose malabsorption, since the drug contains sucrose; - under 18 years of age (safety has not been established).
Carefully
Caution in dosing and regular monitoring is recommended in patients with the following conditions:
- epilepsy, especially a sharp increase or decrease in dose; - patients with liver and/or kidney failure, especially severe; - patients with heart diseases such as angina pectoris, conduction disorders or atrioventricular (AV) block of varying degrees, myocardial infarction (early recovery period); - hyperthyroidism; - urinary disorders, such as prostatic hypertrophy, despite the slight anticholinergic effect of trazodone; - acute angle-closure glaucoma, increased intraocular pressure, although due to the slight anticholinergic effect of trazodone, no significant changes are expected.
Use during pregnancy and breastfeeding Pregnancy
Data from a limited number of pregnant women who took the drug during pregnancy (<200) did not reveal any adverse effects of trazodone on pregnancy or fetal/newborn health. There are no other epidemiological data available.
Animal studies have not revealed direct or indirect adverse effects on pregnancy, embryonic/fetal development, delivery or postnatal development at therapeutic doses.
Teratogenicity studies in rats revealed increased embryo-lethality when the drug was used only in doses toxic to the mother's body (300-450 mg/kg/day). Embryo-lethal effects and rare cases of congenital anomalies were observed in rabbits when the drug was used only in doses toxic to the mother's body (150-450 mg/kg/day). The absence of a direct effect on the embryo is confirmed by studies of the penetration of trazodone through the placental barrier in rats: only minor concentrations of trazodone were found in embryonic tissues and amniotic fluid.
The drug should be prescribed to pregnant women only if the potential benefit to the woman justifies the possible risks to the fetus. When used before delivery, newborns should be monitored for signs of withdrawal syndrome.
Breastfeeding period
Limited data indicate that small amounts of trazodone are excreted in breast milk, but the level of the active metabolite is unknown. Due to insufficient data, the decision to continue/discontinue taking the drug or continue/discontinue breastfeeding should be made taking into account the benefits of breastfeeding for the child and the benefits of therapy for the mother.
Directions for use and doses
The tablets should be taken whole, without chewing, and with plenty of water.
Therapy should begin with an evening dose and gradually increase daily doses.
The drug can be taken regardless of meals.
The drug should be taken in cycles of at least one month.
Adults
75-150 mg/day as a single dose in the evening before bed. The dose can be increased to 300 mg/day, divided into two doses. In hospitalized patients, the dose may be gradually increased to 600 mg/day in repeated doses.
Elderly patients
For elderly or debilitated patients, the recommended daily dose should be reduced to 100 mg/day in divided doses or as a single dose in the evening before bedtime. This dose can be gradually increased under medical supervision depending on the effectiveness and tolerability of the drug. In most cases, a single dose of more than 100 mg should be avoided in these patients. A dose exceeding 300 mg/day is usually not required.
Side effect
Cases of suicidal ideation and suicidal behavior have been reported during trazodone therapy or in the early period after completion of therapy.
The following symptoms, some of which have also been reported in cases of untreated depression, have been reported in patients taking trazodone.
| Organ System Class (MedDRA) | Frequency unknown (frequency cannot be determined from available data) |
| Blood and lymphatic system disorders | Agranulocytosis, thrombocytopenia, eosinophilia, leukopenia, anemia |
| Immune system disorders | Allergic reactions |
| Endocrine system disorders | Syndrome of inappropriate antidiuretic hormone secretion (SIADH) |
| Metabolic and nutritional disorders | Hyponatremia1, weight loss, anorexia, increased appetite |
| Mental disorders | Suicidal thoughts or behavior2, confusion, insomnia, disorientation, mania, anxiety, nervousness, agitation (very rarely worsening to episodes of delirium), delirium, aggressive reaction, hallucinations, nightmares, decreased libido, withdrawal symptoms |
| Nervous system disorders | Serotonin syndrome, seizures, neuroleptic malignant syndrome, dizziness, vertigo, headache, drowsiness3, restlessness, decreased vigilance, tremor, visual impairment, memory impairment, myoclonic seizures, severe aphasia, paresthesia, dystonia, taste changes |
| Heart disorders | Cardiac arrhythmia4 (including polymorphic ventricular tachycardia), palpitations, premature ventricular contractions, two consecutive complexes of premature ventricular contractions, ventricular tachycardia, bradycardia, tachycardia, QT prolongation |
| Vascular disorders | Orthostatic hypotension, arterial hypertension, syncope |
| Respiratory, thoracic and mediastinal disorders | Nasal congestion, shortness of breath |
| Gastrointestinal disorders | Nausea, vomiting, dry mouth, constipation, diarrhea, dyspepsia, abdominal pain, gastroenteritis, increased salivation, paralytic ileus |
| Disorders of the liver and biliary tract | Liver dysfunction (including jaundice and liver cell damage)5, intrahepatic cholestasis |
| Skin and subcutaneous tissue disorders | Skin rash, itching, hyperhidrosis |
| Musculoskeletal and connective tissue disorders | Limb pain, back pain, myalgia, arthralgia |
| Renal and urinary tract disorders | Urination disorder |
| Genital and breast disorders | Priapism6 |
| General and administration site disorders | Weakness, swelling, flu-like symptoms, fatigue, chest pain, fever |
| Laboratory and instrumental data | Elevated liver enzyme levels |
1 Fluids and electrolytes should be monitored in symptomatic patients. 2 See the “Special Instructions” section. 3 Trazodone is an antidepressant with sedative properties, and lethargy is sometimes observed in the first days of therapy, which usually subsequently disappears. 4 Animal studies have shown that trazodone is less cardiotoxic than tricyclic antidepressants, and clinical studies suggest that the drug has a low potential for causing cardiac arrhythmias in humans. Clinical studies in patients with a history of heart disease have shown that trazodone may be arrhythmogenic in some patients in the population. 5 Adverse effects on liver function have been reported in rare cases. 6 See the “Special Instructions” section.
Overdose
Symptoms: drowsiness, dizziness, nausea, vomiting. In more severe cases, coma, tachycardia, hypotension, hyponatremia, seizures and respiratory arrest have been reported. In relation to cardiac activity, these may be bradycardia, prolongation of the QT interval and polymorphic ventricular tachycardia.
Symptoms may appear within 24 hours or later after an overdose. Overdose of trazodone in combination with other antidepressants can cause serotonin syndrome.
Treatment: There is no specific antidote for trazodone. Activated charcoal may be used in adults who have taken more than 1 g of trazodone or in children who have taken more than 150 mg of trazodone within 1 hour of the onset of symptoms. As an alternative therapy, adults may undergo gastric lavage within 1 hour of taking a potentially life-threatening dose.
Observation is necessary for at least 12 hours after taking the drug, as well as monitoring blood pressure, pulse and Glasgow Coma Scale scores. If Glasgow Coma Scale scores are reduced, blood oxygen saturation is monitored. Monitoring of cardiac function is necessary in symptomatic patients.
Single short seizures do not require therapy. Frequent or prolonged seizures are corrected with intravenous diazepam (0.1-0.3 mg/kg body weight) or lorazepam (4 mg for adults or 0.05 mg/kg body weight for children).
If these measures do not control seizures, intravenous phenytoin may be recommended. To correct the acid-base balance and metabolic disorders, it is necessary to use oxygen by inhalation.
In case of hypotension and excessive sedation, symptomatic and supportive therapy should be provided. If severe hypotension persists, dopamine or dobutamine should be considered.
Interaction with other drugs
General
The sedative effect of antipsychotics, hypnotics, sedatives, anxiolytics and antihistamines may be enhanced; in such cases, a dose reduction is recommended.
The metabolism of antidepressants is accelerated due to the hepatic effects of oral contraceptives, phenytoin, carbamazepine and barbiturates. The metabolism of antidepressants is inhibited by cimetidine and some other antipsychotic drugs.
CYP3A4 inhibitors
In vitro drug metabolism studies indicate that there is a potential for drug interaction when trazodone is administered with cytochrome P4503A4 (CYP3A4) inhibitors such as erythromycin, ketoconazole, itraconazole, ritonavir, indinavir, and nefazodone. If trazodone is used with a strong CYP3A4 inhibitor, a lower dose of trazodone is necessary.
The use of CYP3A4 inhibitors should be avoided whenever possible.
Carbamazepine
The results of combined use are expressed in a decrease in the concentration of trazodone in plasma. Co-administration of carbamazepine at a daily dose of 400 mg led to a decrease in the plasma concentrations of trazodone and its active metabolite m-chlorophenylpiperazine to 76% and 60%, respectively. For this reason, patients taking trazodone in combination with carbamazepine should be closely monitored for the need to increase their trazodone dosage.
Tricyclic antidepressants
Concomitant use with trazodone should be avoided due to the risk of interaction. Be wary of serotonin syndrome and cardiac side effects.
Fluoxetine
Rare cases of elevated trazodone plasma levels and adverse effects have been reported when trazodone was combined with fluoxetine, a CYP1A2/2D6 inhibitor. The mechanism of their pharmacokinetic interaction has not been studied. A pharmacodynamic interaction (serotonin syndrome) cannot be excluded.
MAO inhibitors
Possible interactions with monoamine oxidase inhibitors (MAOIs) have been reported. Although some doctors prescribe the two drugs at the same time, using trazodone concomitantly with MAO inhibitors or within two weeks of stopping MAO inhibitors is not recommended. The use of MAO inhibitors within one week of trazodone therapy is also not recommended.
Phenothiazines
Cases of serious orthostatic hypotension have been reported when used concomitantly with phenothiazines, for example, chlorpromazine, fluphenazine, perphenazine.
Anesthetics/muscle relaxants
Trazodone hydrochloride may enhance the effects of muscle relaxants and volatile anesthetics and should be used with caution.
Alcohol
Trazodone enhances the sedative effect of alcohol. Alcohol should be avoided during trazodone therapy.
Levodopa
Antidepressants may speed up the metabolism of levodopa.
Other
Concomitant use of trazodone with drugs that prolong the QT interval may increase the risk of ventricular arrhythmias, including torsades de pointes. Caution is required when used in combination with trazodone.
Because trazodone is a weak norepinephrine reuptake inhibitor and does not alter the blood pressure response to tyramine, it is unlikely that compounds like guanethidine would interfere with the hypotensive effects. However, animal studies suggest that trazodone may inhibit most of the acute effects of clonidine. With other antihypertensive drugs, the possibility of potentiation should be considered, although interaction has not been clinically proven.
Side effects may occur more frequently when trazodone is used concomitantly with medications containing St. John's wort (Hypericum perforatum).
Cases of changes in prothrombin time have been reported in patients taking trazodone and warfarin concomitantly.
Concomitant use of digoxin or phenytoin with trazodone may result in increased serum levels of digoxin and phenytoin. Serum digoxin and phenytoin levels should be monitored in such patients.
special instructions
Use in children and adolescents (up to 18 years of age)
Trazodone should not be used in children and adolescents under 18 years of age.
Suicide/suicidal ideation or worsening clinical symptoms
People with depression have an increased risk of suicidal thoughts, self-harm, or suicide. This risk persists until significant remission occurs. Since improvement may not occur for the first few weeks of treatment or more, patients should be closely monitored until improvement occurs. It is common clinical experience that the risk of suicide may increase in the early stages of recovery.
It is known that patients with a history of suicidal events, or patients who exhibit a significant degree of suicidal ideation even before treatment, have a higher risk of suicidal ideation or suicide attempts, and should be closely monitored during treatment. A meta-analysis of placebo-controlled clinical trials of antidepressants used in adults with mental disorders showed an increased risk of suicidal behavior in patients under 25 years of age with antidepressants compared with placebo.
Careful monitoring of patients, especially those at high risk, should accompany drug therapy, especially in its early stages and after dose changes. Patients (and their caregivers) should be warned to monitor for any clinical deterioration, suicidal behavior or thoughts, or unusual changes in behavior, and to immediately seek professional advice if such symptoms occur. To minimize the potential risk of suicide attempts, especially at the beginning of treatment, the minimum required dose should be prescribed in each specific case.
If jaundice develops, trazodone therapy should be discontinued.
The use of antidepressants in patients with schizophrenia or other mental disorders may result in possible worsening of psychiatric symptoms. Paranoid thoughts may get worse. When treated with trazodone, depressive episodes can range from manic depression to manic psychosis. In this case, trazodone should be discontinued.
Interactions with the development of serotonin syndrome or neuroleptic malignant syndrome. Fatal neuroleptic malignant syndrome has been reported in cases of concomitant use with antipsychotics for which this syndrome was a known possible adverse drug reaction.
Since agranulocytosis can manifest itself as a flu-like syndrome, sore throat and fever, it is recommended to monitor the blood count when these symptoms appear.
Cases of hypotension, including orthostatic hypotension and syncope, have been reported in patients receiving trazodone. Concomitant use with antihypertensive drugs may require a reduction in the dose of the antihypertensive drug.
Elderly patients
Elderly patients are often more responsive to antidepressants, and orthostatic hypotension, somnolence, and other anticholinergic effects of trazodone are more commonly reported.
Particular attention should be paid to potential additive effects due to concomitant use of other psychotropic or antihypertensive drugs or in the presence of risk factors, such as concomitant diseases, that may exacerbate these reactions.
It is recommended that the patient or caregiver be informed of the possible occurrence of such effects. It is necessary to carefully monitor their manifestation in the patient after initiation of therapy, before and after titration in an increasing dose.
During long-term therapy with trazodone, a gradual dose reduction is recommended before discontinuation of the drug to minimize withdrawal symptoms such as nausea, headache, and malaise.
There is no evidence that trazodone hydrochloride has any addictive properties.
As with other antidepressants, QT prolongation has been reported very rarely with trazodone. Caution should be exercised when administering trazodone with drugs that prolong the QT interval.
Trazodone should be used with caution in patients with known cardiovascular disease, including QT prolongation. Strong CYP3A4 inhibitors may result in increased serum levels of trazodone.
As with other drugs with alpha-adrenolytic activity, trazodone is very rarely associated with priapism. This can be corrected by intracavernosal injection of an alpha-adrenergic agent such as epinephrine or metaraminol. However, there are reports of trazodone-induced priapism that required surgery or resulted in permanent sexual dysfunction. Patients who develop this suspected adverse reaction should stop taking trazodone immediately.
Impact on urine test results
When immunoassays are used to monitor drugs in urine, the reactivity of the trazodone metabolite meta-chlorophenylpiperazine (m-CPP), which is structurally similar to methylenedioxymethamphetamine (MDMA, ecstasy), may cause a false-positive reaction to amphetamine. In these cases, confirmatory analysis using mass spectrometry is recommended.
Impact on the ability to drive vehicles and machinery
Trazodone has little or no effect on the ability to drive vehicles or use machinery. The patient should be warned of the risks when driving or operating machinery until he is sure that he is not experiencing drowsiness, lethargy, dizziness, confusion, or blurred vision.
Release form
Extended release tablets, 150 mg.
10 tablets per blister made of PVC / aluminum foil.
2 or 6 blisters along with instructions for use in a cardboard box.
Storage conditions
At a temperature not higher than 25 ºС. Keep out of the reach of children!
Best before date
3 years. Do not use after the expiration date.
Vacation conditions
Dispensed by prescription.
Indications for the drug Trittico
anxiety-depressive states of endogenous nature (including involutional depression);
psychogenic depression (including reactive and neurotic depression);
anxiety-depressive states against the background of organic diseases of the central nervous system (dementia, Alzheimer's disease, cerebral atherosclerosis);
depressive states with prolonged pain syndrome;
alcoholic depression;
benzodiazepine dependence;
libido and potency disorders (including erectile dysfunction in depressive conditions).
Contraindications
hypersensitivity to the drug;
pregnancy period;
lactation period;
children under 6 years of age.
The drug should be prescribed with caution to patients with AV block, myocardial infarction (early recovery period), arterial hypertension (dose adjustment of antihypertensive drugs may be required), ventricular arrhythmia, a history of priapism, renal and/or liver failure. Patients under the age of 18 years, due to the possibility of developing the risk of suicidal behavior (suicidal plans, aggressiveness, tendency to contradict, anger).
Side effects
From the side of the central nervous system: increased fatigue, drowsiness, agitation, headache, dizziness, weakness, myalgia, incoordination, paresthesia, disorientation, tremor.
From the cardiovascular system and hematopoietic system: decreased blood pressure, orthostatic hypotension caused by adrenolytic action (especially in persons with vasomotor lability), arrhythmia, conduction disturbances, bradycardia; leukopenia and neutropenia (usually minor)..
From the gastrointestinal tract: dryness and bitterness in the mouth, nausea, vomiting, diarrhea, loss of appetite.
Other: allergic reactions, eye irritation, priapism (you should immediately stop taking the drug and consult a doctor).
Interaction
Trazodone may enhance the effect of some antihypertensive drugs and usually requires a dose reduction.
Simultaneous administration with drugs that depress the central nervous system (including clonidine, methyldopa) enhances the effect of the latter.
Antihistamines and drugs with anticholinergic activity enhance the anticholinergic effect of trazodone.
Trazodone enhances and prolongs the sedative and anticholinergic effects of tricyclic antidepressants, haloperidol, loxapine, maprotiline, phenothiazine, pimozidane and thioxanthine.
When tricyclic antidepressants and trazodone are prescribed simultaneously, cardiovascular side effects may occur.
MAO inhibitors increase the risk of side effects.
When used together, it increases the concentration of digoxin and phenytoin in the blood plasma.
In vitro drug metabolism studies indicate the potential for pharmacological interaction of trazodone with cytochrome P450 CYP3A4 inhibitors such as ketoconazole, ritonavir, indinavir and fluoxetine. Inhibitors of CYP3A4 may lead to significant increases in plasma concentrations of trazodone, thereby increasing the likelihood of adverse events. Therefore, when used in combination with potent CYP3A4 inhibitors, the dose of trazodone should be reduced.
When trazodone is taken in combination with carbamazepine, the plasma concentration of trazodone is reduced. Therefore, patients taking trazodone and carbamazepine concomitantly should be closely monitored.
Trittico 150 mg 20 pcs. tablets aziende chimiche riunite angelini france
pharmachologic effect
Antidepressant.
Composition and release form Trittiko 150 mg 20 pcs. tablets aziende chimiche riunite angelini france
Tablets - 1 tablet:
- Active ingredient: trazodone hydrochloride 150.0 mg;
- Excipients: sucrose 84.0 mg; carnauba wax 24.0 mg; povidone 24.0 mg; magnesium stearate 6.0 mg.
10 tablets in a PVC/aluminum foil blister.
2 or 6 blisters along with instructions for use in a cardboard box.
Description of the dosage form
Biconvex tablets, white or white with a yellowish tint, oval in color with two parallel lines on both sides.
Characteristic
Triazolopyridine derivative.
Directions for use and doses
Tablets should be taken orally 30 minutes before meals or 2 to 4 hours after meals. The tablets should be taken whole, without chewing, and with plenty of water.
Initial dose of the drug: 100 mg, taken once before bed after meals. On the 4th day you can increase the dose to 150 mg. Further increases in the dose in order to achieve the optimal therapeutic effect should be made by 50 mg/day every 3-4 days until the optimal dose is reached. A daily dose of more than 150 mg should be divided into 2 doses, with the smaller dose taken after lunch and the main dose before bed.
The maximum daily dose for outpatients is 450 mg.
Maximum daily dose for inpatients. 600 mg.
For elderly and debilitated patients, the initial dose is up to 100 mg/day in divided doses or once before bedtime. It can be increased under medical supervision, depending on the effectiveness and tolerability of the drug. A dose exceeding 300 mg/day is usually not required.
Pharmacodynamics
Pharmacodynamics: trazodone inhibits neuronal reuptake of serotonin, is an antagonist of 5-HT2A/2c-serotonin receptors and an α1-adrenergic receptor blocker and has an antidepressant effect.
Pharmacokinetics
Absorption of trazodone from the gastrointestinal tract after oral administration is high. Taking trazodone during or immediately after a meal slows the rate of absorption, reduces the maximum plasma concentration of trazodone, and increases the time to reach maximum concentration (TCmax).
TCmax is achieved 1/2-2 hours after oral administration.
Trazodone penetrates through histohematic barriers into tissues and fluids (bile, saliva, breast milk).
Communication with plasma proteins is 89 - 95%.
Trazodone is metabolized in the liver, the active metabolite is 1-m-chlorophenylpiperazine. The half-life is 3-6 hours, in the second phase 5-9 hours. Elimination of most of the metabolized trazodone is carried out by the kidneys with urine - about 75%, and is completely completed 98 hours after administration; About 20% is administered with bile. In vitro studies on human microsomes have shown that trazodone is primarily metabolized by the cytochrome P450 isoenzyme (CYP3A4 isoenzyme).
Indications for use Trittico 150 mg 20 pcs. tablets aziende chimiche riunite angelini france
Depression with or without anxiety.
Contraindications
- Hypersensitivity to the active substance or any excipient;
- pregnancy period;
- lactation period;
- alcohol intoxication and intoxication with sleeping pills;
- The safety of trazodone for children under 18 years of age has not been established, therefore the use of the drug in children and adolescents is not recommended;
- sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption, since the drug contains sucrose.
With caution: the drug should be prescribed with caution to patients with AV block, myocardial infarction (early recovery period), arterial hypertension (dose adjustment of antihypertensive drugs may be required), ventricular arrhythmia, a history of priapism, and renal and/or liver failure.
Application of Trittico 150 mg 20 pcs. tablets aziende chimiche riunite angelini france during pregnancy and breastfeeding
The drug is not recommended for use by pregnant women. The drug is not recommended for use during breastfeeding.
special instructions
Suicide/suicidal ideation or worsening clinical symptoms: Depressive conditions have an increased risk of suicidal ideation, self-harm, or suicide. The risk may last until significant remission occurs. Since improvement may not occur for the first few weeks of treatment or more, patients should be closely monitored until such improvement occurs. It is common clinical experience that the risk of suicide may increase in the early stages of recovery. It is known that patients with a history of suicidal events, or patients who exhibit a significant degree of suicidal ideation even before treatment, have a higher risk of suicidal ideation or suicide attempts, and should be closely monitored during treatment. A meta-analysis of placebo-controlled clinical trials of antidepressants used in adults with mental disorders showed an increased risk of suicidal behavior in patients under 24 years of age when taking antidepressants compared with placebo. Careful monitoring of patients, especially those at high risk, should accompany drug therapy, especially in its early stages and after dose changes. Patients (and their caregivers) should be warned to monitor for any clinical deterioration, suicidal behavior or thoughts, or unusual changes in behavior, and to immediately seek professional advice if such symptoms occur.
Since the drug has some adrenergic blocking activity, bradycardia and a decrease in blood pressure may develop. Therefore, caution should be exercised when prescribing the drug to patients with a tendency to prolong the QT interval, atrioventricular block of varying severity, and patients with a recent myocardial infarction. When treated with trazodone in patients with bipolar disorder, depressive episodes can range from manic depression to manic psychosis. In these cases, it is necessary to interrupt treatment.
If you have epilepsy, use trazodone with caution, in particular avoiding sudden increases or decreases in dose. With simultaneous use of trazodone with drugs with serotonergic activity (tricyclic antidepressants, selective serotonin reuptake inhibitors, norepinephrine and serotonin reuptake inhibitors and monoamine oxidase inhibitors) and antipsychotics, serotonin syndrome may occur. When trazodone is used simultaneously with drugs containing St. John's wort, side effects may be more frequent.
When using trazodone, agranulocytosis may develop, so it is recommended to conduct peripheral blood tests, especially if there is a sore throat when swallowing and fever.
Trazodone is effective for sleep disorders in patients with depression, increases the depth and duration of sleep, and restores its physiological structure and quality. The use of the drug does not affect body weight.
The drug is not addictive.
Impact on the ability to drive vehicles and operate machinery
During the period of use of the drug Tritgiko, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms: drowsiness, dizziness, nausea, vomiting. In more severe cases, coma, tachycardia, hypotension, hyponatremia, seizures and respiratory failure. Impaired function of the cardiovascular system (prolongation of the QT interval, bradycardia). After an overdose, symptoms may take 24 hours or more to appear.
Treatment: There is no specific antidote for trazodone. In cases of overdose, gastric lavage and administration of activated charcoal are necessary within 1 hour after taking the overdose. Symptomatic and supportive treatment is carried out.
Side effects Trittico 150 mg 20 pcs. tablets aziende chimiche riunite angelini france
Trittico may cause side effects, although not all patients experience them.
From the blood and lymphatic system: agranulocytosis, thrombocytopenia, eosinophilia, leukopenia and anemia.
From the immune system: allergic reactions.
From the endocrine system: syndrome of inappropriate secretion of antidiuretic hormone (SNA ADH).
Mental disorders: suicidal thoughts or behavior, confusion, mania, phobias, emotional instability, delirium, hallucinations.
From the nervous system: epileptic seizures, dizziness, headache, insomnia or drowsiness, amnesia, tremor, convulsions, paresthesia, impaired taste.
From the cardiovascular system: palpitations, tachycardia, bradycardia, ventricular extrasystoles, ventricular paroxysmal tachycardia, prolongation of the QT interval, increased blood pressure (BP), decreased blood pressure, fainting.
From the gastrointestinal tract: nausea, vomiting, dry mouth, dyspepsia; abdominal pain, diarrhea, increased salivation, paralytic ileus.
From the skin and subcutaneous tissues: itching, erythematous rash, sweating.
Musculoskeletal and connective tissue disorders: myalgia, arthralgia.
From the kidneys and urinary tract: urinary disorders.
On the part of the genital organs and breast: priapism (patients who experience this side effect should immediately stop taking the drug and consult a doctor).
Other: increased fatigue, weakness, increased body temperature, flu-like syndrome.
Drug interactions
Trazodone may enhance the effect of some antihypertensive drugs and usually requires a dose reduction.
Simultaneous use with drugs that depress the central nervous system (including clonidine, methyldopa) enhances the effect of the latter.
H1-histamine receptor blockers and drugs with m-anticholinergic activity enhance the m-anticholinergic effect of trazodone.
Trazodone enhances and prolongs the sedative and m-anticholinergic effects of tricyclic antidepressants, haloperidol, loxapine, maprotiline, phenothiazine, pimozidan and thioxanthine.
With the simultaneous use of tricyclic antidepressants and trazodone, cardiovascular side effects may occur.
MAO inhibitors increase the risk of side effects.
When used together, it increases the concentration of digoxin and phenytoin in the blood plasma.
In vitro drug metabolism studies indicate the possibility of pharmacological interaction of trazodone with inhibitors of the cytochrome P450 isoenzyme (CYP3A4 isoenzyme), such as ketoconazole, ritonavir, indinavir and fluoxetine. Inhibitors of the CYP3A4 isoenzyme may lead to a significant increase in plasma concentrations of trazodone, thereby increasing the likelihood of adverse events. Therefore, when taken in combination with potent inhibitors of the CYP3A4 isoenzyme, the dose of trazodone should be reduced. When trazodone is taken in combination with carbamazepine, the plasma concentration of trazodone is reduced. Therefore, patients taking trazodone and carbamazepine concomitantly should be closely monitored.
Directions for use and doses
Orally, 30 minutes before or 2–4 hours after meals. The tablets should be taken whole, without chewing and with plenty of water.
The initial dose is 50–100 mg, once before bedtime. On the 4th day it is possible to increase the dose to 150 mg. Further increases in dosage should be made by 50 mg/day every 3–4 days until the optimal dose is reached. A daily dose of more than 150 mg should be divided into 2 doses, with the smaller dose taken after lunch and the main dose before bed.
The maximum daily dose for outpatients is 450 mg, for inpatients - 600 mg.
Children 6–18 years old: initial daily dose of 1.5–2 mg/kg/day, divided into several doses. If necessary, the dose is gradually (with an interval of 3-4 days) increased to 6 mg/kg/day.
Elderly and debilitated patients: initial dose up to 100 mg/day in several doses or once before bedtime. If necessary, the dose can be increased (usually no more than 300 mg/day).
Treatment of libido disorders: recommended daily dose of 50 mg.
Treatment of erectile dysfunction: monotherapy - recommended daily dose - 150-200 mg, combination therapy - 50 mg.
Treatment of benzodiazepine dependence: The recommended treatment regimen is based on a gradual, sometimes over several months, reduction of the benzodiazepine dose. Each time, reducing the benzodiazepine dose by 1/4 or 1/2 tablet, 50 mg of trazodone is added at the same time. This ratio is left unchanged for 3 weeks, then a further gradual reduction in the dose of benzodiazepines is started until their complete withdrawal. After this, reduce the daily dose of trazodone by 50 mg every 3 weeks.
Antidepressant Trittico - reviews
Larisa
Trittico was prescribed to me by a neurologist due to insomnia, extreme fatigue and depression. Honestly, I believed that I would rest and all the problems would go away, but alas, a miracle did not happen and I decided to take pills. Although Trittico’s tablets are large, they are easily divided; I was prescribed a dosage slightly less than the tablet itself, so I divided it without any problems. A pronounced effect from taking it occurs after about a week. The depressed state went away, my mood improved, in general, I cheered up and came to life. But sleep returned from the first pill, I take it and after 2 hours I fall asleep without problems or pain. I took it for a month, but here the doctor regulates the duration, in fact, as well as the dosage itself, so you should not rely on someone’s reviews, but listen to the doctor. The big advantage of trittico is that it has no withdrawal symptoms. Even after long-term use, you should not worry that a rebound will occur after discontinuation. There is nothing like that, after the withdrawal, I also sleep well and feel great. Now, the most important thing is to live and enjoy life. Trittiko helped me a lot!
Zhanna
I suffered from sleep disorder for quite a long time. At first I took light sedatives in the evening, at first they helped me, then they stopped. I fell asleep quickly, but in the middle of the night I woke up five or six times, which is why I felt exhausted and very tired in the morning.
After six months of such torment, I finally went to the doctor, and he prescribed me trittiko. This was my first time encountering such a drug; I had never taken it before. I bought it at the pharmacy, the price is normal. Not high and not low, average, I would say.
The doctor prescribed me to take 50 mg of the drug an hour before bed. One tablet is 150 mg, so my dosage is 1/3 of the tablet. It is easy to break: the manufacturer specially applies two break strips, so the tablet can be divided into three parts and the pieces can be placed in the cells of the medicine box.
After the first pill, I fell asleep within an hour and a penny. I took it around 21:30, and by 22:40 I was already asleep. Passed out like dead. Although at first there was no effect for the first half hour, then weakness set in, my body became weak and began to sag, so I quickly crawled to the bed and settled comfortably under the blanket. I slept soundly. In the morning I got up with a little difficulty. There was a slight fog in my head, then I wanted to sleep for another two hours. By lunchtime this feeling had passed.
For the next two days, it was also difficult to get up in the morning, then gradually this effect went away. Maybe the body is just accustomed to the drug. Throughout the course of treatment, I slept well. When I finished, the insomnia did not return. Although I don’t fall asleep right away, at the appointed time, when the pill starts to work, my body automatically relaxes, and I want to go to bed.
Sergey
I am a very suspicious person)) I got upset from the zherovik, under my arm, I talked about it every day for 3 months)) the depression became terrible, I could not sleep and eat, I turned to a psychotherapist, listened to me, calmly explained and gave treatment injections 2 times a day for 10 days, and ,,the drug Trittiko,, I felt the effect immediately, at first 1/3 then 2/3, then completely good sleep, appetite and mood, side effects, headache in the morning, and low blood pressure)) you drink coffee and after 1 hour everything is normal but, Good drug
Victoria
I have my own phobias, or an increased feeling of anxiety, as one doctor said. And it all started with an unsuccessful flight, there was severe turbulence, in short, then I was saying goodbye to life... Then I tried to go to a psychologist, because I still had to fly for work. As a result, I drove myself to the point where, in addition to airplanes, I also became afraid of cars. It is impossible to live fully in such psychosis and eternal anxiety, so the course was taken on doctors. Those. I hope you understand the state I was in, that I was ready to do anything to let me go. The doctor liked me, calmed me down, and said that there are many people with increased anxiety and I am no exception. He'll prescribe some pills and everything will be fine. I was prescribed Trittico, these are tablets with thymoleptic, anxiolytic, sedative and muscle relaxant effects. The dosage is selected individually, I was prescribed to take 100 mg. When I took the first pill, I probably didn’t really understand the effect, but I slept as calmly as possible that day and without waking up. After 2 weeks of taking the second and third tablets, I caught myself thinking that life is beautiful, the sun is shining. In general, the flight was coming up in a while, I calmly endured it, I was even able to fall asleep during the flight. When I got off the plane, I realized that Trittiko had worked perfectly and I had conquered my fears. I have no side effects from it. Is it worth taking? It is worth it if prescribed by a doctor and there is such a need.
Marina
The end of last year turned out to be very difficult for me. It seemed like nothing serious was happening, but some minor troubles constantly arose at work, at home, conflicts, and so on every day. As a result of all this hassle, insomnia appeared. I generally don’t sleep well, I can’t fall asleep for a long time, or I fall asleep quickly, but then wake up three to five times at night. And if earlier motherwort, soothing tea and similar mild remedies helped me with varying success, then gradually they turned out to be powerless. I went to the doctor and he prescribed Trittico for me. To be honest, I didn’t really want to resort to such drugs, but the doctor insisted. After all, long-term sleep problems have a very bad effect on the body and gradually the consequences can be even more destructive. Plus he reassured me that he prescribed the minimum dosage and said that they don’t get hooked on Trittico and that he doesn’t have withdrawal symptoms, so everything will be fine. Well, what can I say, the doctor did not deceive me, indeed, from the first pill I fell asleep like a normal person. During the course, sleep returned to normal as much as possible, and during the withdrawal period there were no problems, everything went as calmly as possible.
Volodina
I take Trittico for insomnia, it works just perfectly - I fall asleep quickly, have a restful sleep, and have a fresh head in the morning.
Olga
I started sleeping well with Trittiko. I suffered from insomnia due to mild depression and increased anxiety. I haven't slept properly for over two months. I tried folk remedies, but it didn’t help. Trittico was prescribed by a doctor. The drug is available with a prescription, but there are no fees, even the local police officer can prescribe it. I found no side effects with Trittiko. I fall asleep a little over an hour after taking 1/3 of the tablet. The sleep at night is deep, calm, without dreams, I really get enough sleep with it during the night. Trittiko has no withdrawal syndrome and no addiction, this is a big plus. By the way, the tablets have three notches on the tablets, so dividing them is very convenient.
Oleg
I don’t suffer from depression, but I often experience anxiety, which greatly disturbs my sleep. The doctor advised me to drink trittiko in order to sleep better and reduce anxiety, and it helped me. I sleep peacefully all night, negative thoughts no longer enter my head, I don’t worry about trifles. In my case, the drug was perfect.
Yana
I drink trittiko for insomnia, it helps me. Without it, I slept 3 hours a night in total and was useless all day. With it, I began to get enough sleep, and my overall quality of life improved.
Plato
After trittiko I sleep very well. It begins to act in an hour, sometimes plus 10-20 minutes. In the mornings, well, for the first few days it was difficult to get up, but then everything returned to normal. Of course, there is no “cucumber” cheerfulness, but I always wake up without much enthusiasm. I didn't find any side effects. I was afraid that there would be a withdrawal syndrome, I finished the course gradually, as the doctor said, there were no signs of addiction to the drug.
+7-928-05XXXXX
https://protabletky.ru/trittico/
This drug pulled me out of severe masked depression. From the shelves there was a headache, dizziness, nausea, decreased blood pressure, but the benefits and results were worth the patience. Sleep returned to normal, the nervous system came into balance, metabolism was restored, I lost 9 kg in 4 months, blood sugar dropped to normal, I had previously been diagnosed with type 2 diabetes, libido was restored, which I had forgotten about for 7 years.
Guest
https://www.medcentre.com.ua/review/46829-trittiko
It’s a very good drug, if it doesn’t cause any side effects for you, you forget your anxieties and fears_ you just gradually come to life. But I want to warn anyone who has low blood pressure - this is fraught. My blood pressure is 100/55 (+,- 10) units of the upper level. Well. as always, ah, nothing will happen. But at night I got up, didn’t have time to get there... My eyes darkened, I grabbed a can of coffee. So I woke up with a can in my hand, I couldn’t understand anything, where I was and what was wrong with me - after lying down for a while, everything came to me normal - the pressure drops so quickly! Now I started with amitroptyline, it’s still better than others. I’m thinking of switching to tritico - I don’t know the doctor’s reaction. Taking tritico constant blood pressure control And if they don’t get out of bed right away - sit for a while Good luck!
Maksim
https://www.medcentre.com.ua/review/31022-trittiko
I am 30 years old, since childhood I have been tormented by a constant feeling of anxiety, and sometimes it is impossible to do anything and the presence of fear and from this hopelessness depression sets in, I tried many antidepressants, tranquilizers, but then the doctor prescribed me Trittico I drink for 4 days 50 mg and the effect is amazing, I feel like a person again, I will continue to drink it, as much as I need and it is not addictive, as it is written in the instructions, there was not a single side effect, except for dry mouth, then I plan to do an MRI of the brain and undergo psychotherapy, to find out why I have been suffering from anxiety since childhood, I give it a 5+ trittiko!!
Alexander Alexander
https://health.mail.ru/drug/trittiko/comments/?page=4#comment-3275
An excellent drug. I began to feel better - I had a restful sleep, the muscle pain in my legs went away, my legs became lighter, my gait even changed. I wake up like a child, smooth deep breathing after taking it, the effect of the drugs I take for asthma has improved, expectoration of sputum has improved and this process itself has become under my control. Side effects include a slight ringing in the ears, but these are minor compared to the positive results.
Zhenya
https://www.otzyvua.net/trittiko/review-349273
At one time I took Trittico + Nerofulol, and to no avail. The drugs significantly improved the condition. The only negative, as far as I remember, is drowsiness. Regarding the combination of these drugs, it affects everyone differently and is probably more effective than the advertised afobazole. In general, if you have the desire (and, most importantly, the financial opportunity), then it makes sense to drink them in a 2 or 3 week course
Smsv
https://otzovik.com/review_1117365.html
Advantages:
Effective
Flaws:
Expensive
Due to the fact that I was forced to take the exam at the beginning of this year, I began to have trouble sleeping. It was absolutely impossible to sleep because of bad thoughts and bad expectations. In the morning I walked around lethargic and sleep-deprived, I didn’t want to eat, I was too lazy to do anything, my mood was low. I have never experienced such severe stress and depression as then. Some time later, a friend advised me to see a neurologist and I did so. The doctor told me that I shouldn’t kill myself like that, I need to pull myself together and act. He advised me two options: make an appointment with a psychologist or take prescription pills. I liked the second option, because I was not in the mood to go to a psychologist. Ultimately, I was prescribed Angelini "TRITTICO" and I started drinking them. The instructions stated that the tablets should be taken 30 minutes before meals, the initial dose is 50-100 mg, then the dose can be increased to 150 mg. In general, the pills helped me, although the effect was not felt immediately. About a week later I felt more or less well, and then it went from there. I read reviews on the Internet that the pills don’t help, that you shouldn’t listen to doctors, etc. I think that these people simply don’t have enough patience or they overdid it with these pills, because you need to follow the instructions. Thank you for your attention.
haardath
https://irecommend.ru/content/uluchsheniya-zhizni-dazhe-cherez-mesyats-priema
I have been diagnosed with depression, and for the last month I have been taking Trittico as prescribed by a doctor in combination with the tranquilizers Grandaxin. I had insomnia, lack of appetite, apathy, inability to force myself to do the simplest things, depression. The course began with a third of the tablet in the first week, after which it was increased to 2/3.
What can I note from the advantages:
— the drug works, I feel noticeably better
— the problem with insomnia has been solved. I drink trittico an hour and a half before bed, and I get a strong feeling of drowsiness, I fall asleep without problems
- there were no side effects
- relatively inexpensive
- lasts a long time with my course of treatment
- each tablet is neatly marked with stripes into three parts, it is convenient to cut off both a third and 2/3 of the tablet. To avoid losing pieces, I cut up a certain amount of tablets in advance and store them in a small box
— the intensity of migraines softened, this was not the main purpose of taking antidepressants, but nevertheless a good improvement in the quality of life
Of the minuses:
— drowsiness continues in the first half of the day, sometimes it’s very difficult, especially when you have to get up early in the morning.
- hard to wake up
special instructions
Since the drug has some adrenolytic activity, bradycardia and a decrease in blood pressure may develop. Therefore, caution should be exercised when prescribing the drug to patients with cardiac conduction disorders, AV block of varying severity, or recent myocardial infarction. When using trazodone, a slight decrease in the number of leukocytes is possible, which does not require specific treatment, except in cases of severe leukopenia. Therefore, it is recommended to conduct peripheral blood tests, especially if there is a sore throat when swallowing and fever.
The drug does not have an anticholinergic effect, so it can be prescribed to elderly patients suffering from prostatic hypertrophy, angle-closure glaucoma, and cognitive impairment.
If you experience prolonged and inadequate erections, you should consult a doctor.
There have been no relevant studies on the effectiveness of the drug in pediatrics, so the drug should be used with caution in persons under 18 years of age. Doses for children under 6 years of age have not been established.
During treatment you should refrain from drinking alcohol.
Influence on the ability to drive vehicles and machinery. Since the drug has anxiolytic and sedative activity, a decrease in attention and reaction speed is possible. During treatment, you should refrain from engaging in potentially hazardous activities that require concentration and rapid psychomotor reactions.
Precautions for the substance Trazodone
During treatment, a general blood test should be performed regularly (for timely detection of leuko- and neutropenia); ECG monitoring is desirable in patients with cardiovascular diseases. Careful monitoring of patients with suicidal tendencies is required, especially in the first weeks of treatment. Treatment is immediately stopped with the development of priapism, severe neutro- and leukopenia; in other cases, drug withdrawal should be carried out gradually. During treatment you should avoid drinking alcohol.
Use with caution during work for vehicle drivers and people whose activities involve increased concentration.